Simultaneous determination of bisphenol A and tetrabromobisphenol A in tea using a modified QuEChERS sample preparation method coupled with liquid chromatography-tandem mass spectrometry
Abstract
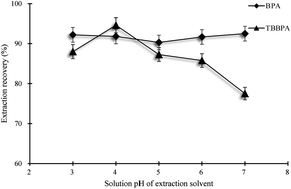
A rapid and sensitive method for the simultaneous determination of bisphenol A (BPA) and tetrabromobisphenol A (TBBPA) in tea using liquid chromatography-tandem mass spectrometry (LC-MS/MS) was developed and validated. Sample preparation was based on a modified QuEChERS procedure through extraction of the target analytes using acetonitrile with 0.7% acetic acid followed by a dispersive solid phase extraction (d-SPE) clean up procedure using C18, MWCNTs and SCX as the adsorbent mixture to eliminate tea co-extracts. The linearity of the method with correlation coefficients (R2) higher than 0.99 was obtained. The limits of detection (LODs), and limits of quantitation (LOQs) were 8.0 and 20.0 μg kg−1 for BPA and 0.4 and 1.0 μg kg−1 for TBBPA, respectively. Satisfactory mean recoveries of BPA and TBBPA at three fortification levels ranged from 88% to 109% and 77% to 99%, respectively, while intra-day and inter-day precisions were below 15%. The developed method was successfully applied to determine BPA and TBPA in 136 tea samples.


 Please wait while we load your content...
Please wait while we load your content...