Detection of lithium in scalp hair by time-of-flight secondary ion mass spectrometry with high energy (MeV) primary ions
Abstract
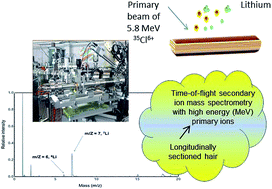
In this exploratory study, the application of secondary ion mass spectrometry with a heavy primary ion beam with an energy of several MeVs (MeV-SIMS) is presented for the simultaneous detection and spatial distribution mapping of lithium in a single, chemically unprocessed strand of scalp hair across a longitudinal profile.


 Please wait while we load your content...
Please wait while we load your content...