Optimization of subcritical water extraction of phenolic antioxidants from pomegranate (Punica granatum L.) peel by response surface methodology†
Abstract
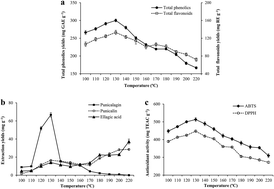
Subcritical water extraction (SWE), a ‘green’ and efficient extraction technology, was applied to extract phenolic antioxidants from pomegranate peel in this study. In single-factor experiments, the effects of extraction temperature (100–220 °C), time (5–80 min) and the water/solid ratio (20–60 mL g−1) on the extraction yields of total phenolics (TP), total flavonoids (TF), and major phenolic compounds (punicalagin, punicalin, and ellagic acid) from pomegranate peel were investigated, as well as their antioxidant capacities. The results showed that the yields of phenolic compounds and antioxidant activities were significantly affected by these parameters. The highest levels of TP (314.65 mg GAE per g), TF (153.66 mg RE per g), and antioxidant activities (DPPH and ABTS radical-scavenging abilities) were observed at 130 °C for 20 min with a solid/water ratio of 50 mL g−1. The highest yield of punicalagin (81.04 mg g−1) from pomegranate peel was also observed under these conditions. However, punicalin and ellagic acid yields both continued to increase with increasing temperature and extension of time. Subsequently, on the basis of the single-factor experiments, three independent variables, temperature (X1, 110–150 °C), time (X2, 10–30 min), and the water/solid ratio (X3, 40–60 mL g−1), were optimized to maximize the total phenolic yields (Y1) and DPPH antioxidant activities (Y2) by a response surface methodology with a three-factor, three-level Box–Behnken design. The optimized conditions for phenolic antioxidant extraction were 126.1 °C, 18.5 min, and a water/solid ratio of 54.8 mL g−1, and the corresponding predicted values of Y1 and Y2 were 323.10 mg GAE per g and 476.81 mg Trolox equivalent antioxidant capacity (TEAC) per g, respectively.


 Please wait while we load your content...
Please wait while we load your content...