Strategies for large-scale analysis of non-histone protein methylation by LC-MS/MS†
Abstract
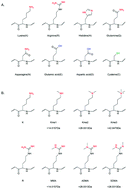
Protein methylation is an important post-translational modification (PTM) that plays crucial roles in the regulation of diverse biological processes. Though many efforts have been devoted to the investigation of protein methylation, the analysis of non-histone methylation at the proteome level is still a great challenge. The alteration of the protein/peptide physicochemical properties caused by methylation is very small, thus it is difficult to develop highly efficient enrichment approaches to separate methylated peptides from a pool of diverse background peptides. The mass shifts caused by methylations are identical to the substitutions of some amino acids, thus it is difficult to confidently identify methylated peptides. In this review, we report on recent advances in the development of methods for large-scale analysis of non-histone protein methylation. Especially the methods for efficient enrichment and the approaches for controlling identification confidence have been covered.


 Please wait while we load your content...
Please wait while we load your content...