Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Implementation of MALDI MS profiling and imaging methods for the analysis of real crime scene fingermarks
R.
Bradshaw
a,
N.
Denison
b and
S.
Francese
 *a
*a
aCentre for Mass Spectrometry Imaging, Biomolecular Research Centre, Sheffield Hallam University, Sheffield, S1 1WB, UK. E-mail: s.francese@shu.ac.uk
bIdentification Services Yorkshire and the Humber Region, West Yorkshire Police, Wakefield, UK WF27UA
First published on 7th April 2017
Abstract
In the study presented here, four examples of crime scene fingermarks analysed by Matrix Assisted Laser Desorption Ionisation Mass Spectrometry Imaging (MALDI MSI), in collaboration with the local Police Force, are reported. These marks are associated to high profile crimes such as drug dealing, murder and harassment. Following the application of forensic enhancement techniques by the CSI or the crime lab, one of the marks could be directly analysed on the surface of deposition and the others were analysed as primary lifts. In one case, no physical or molecular information was obtained whereas in two cases both ridge detail (albeit not useful for identification purposes) and molecular information could be retrieved. In one case, the intelligence gathered around the suspect's state of mind is, to date the most accomplished demonstration of the benefits and the operational feasibility MALDI MS based methods. These four casework examples are to be considered a first insight into the limitations, benefits and feasibility of MALDI MS based methods in the field; due to the extreme variability in the state of the mark, comprehensive evaluation of these aspects can only be undertaken upon the analysis of a large number of crime scene marks. However, this work does represent a significant advancement, compared to previous published work, as it demonstrates operational feasibility, with different levels of success, despite uncontrollable, unknown and unaccountable environmental and donor variability.
Introduction
Since 2008, several examples of chemical imaging techniques have been reported in the literature for application to latent fingermark analysis. The interest of the analytical community in developing methods to detect and map the molecular make up of latent fingermarks has the potential to be a real game changer with respect to more informed investigations and judicial debates. Amongst the analytical techniques, Desorption Electrospray Ionisation (DESI) was the first mass spectrometric technique to be reported as suitable for latent fingermark imaging,1 followed by Surface Assisted Laser Desorption Imaging (SALDI),2 Matrix Assisted Laser Desorption Ionisation Mass Spectrometry Profiling and Imaging (MALDI MSP and MSI),3 Secondary Ion Mass Spectrometry (SIMS)4 and Direct Analysis in Real Time (DART),5 with SALDI and DART being mostly used for chemical analysis rather than imaging purposes. Amongst the spectroscopic techniques, Raman Imaging6 and FT-IR7 or ATR FT-IR Imaging8 have also been reported to be suitable for latent fingermark analysis in laboratory settings. Out of the above mentioned techniques, only MALDI, SIMS and ATR FT-IR were selected by the Centre of Applied Science and Technology, Home Office UK, to be included within the Fingermark Visualisation Manual9 as techniques having potential to be used in casework. Of these technologies, MALDI MSP and MSP have arguably demonstrated superiority with regards to integration and compatibility with the fingermark enhancement workflow. Furthermore, in the present paper, it will be demonstrated for the first time that these techniques have reached a level of maturity like no other mass spectrometric or spectroscopic techniques, enabling them to be transitioned to casework. This is unprecedented and represents a significant advancement with respect to previous work published by our group. It is, in fact, the culmination of nine years of developments including recent protocol developments actually enabling fingermarks enhanced at crime scene or at crime labs to be recovered and prepared for MALDI MS based analyses.Up to this point, methods designed and refined in laboratory settings had been proposed to detect a large number of forensically relevant substances, such as those employing Ion Mobility Separation for the detection of drugs and metabolites,10 and/or to map them directly on the fingermark ridges thus potentially providing the link between the biometric information and corpus delicti.11,12 Potential was also shown to detect and visualise explosives and pharmaceuticals;13–15 studies were also published reporting on the opportunity to use MALDI MSI to separate overlapping ridge impressions16 and to integrate it with other spectroscopic techniques.12 This body of work has opened up an avenue to what these authors have named “chemical criminal profiling”, that is, the opportunity to infer information on suspects’ lifestyle and identity through the chemical species detected in their fingermark residue.
The development of MALDI MSP and MSI has come a long way since their first reported use for the analysis of latent fingermarks in 20093 and, already in 2011, there was a drive to transition these methods to the field. One study reported on an alternative method to powder development (one of the most common fingermark enhancement techniques (FET) used by crime scene investigators (CSI)), to enable visualisation and MALDI MSP/MSI analysis of fingermarks,17 thus demonstrating the operational capability of these techniques/methods. However, arguably, current FET will still be applied to fingermarks in the first instance for many years to come; therefore, subsequent studies investigated the compatibility between FET and MALDI MSP/MSI (“tandem analysis”) to generate a workflow which showed a promising potential for translation into the field.18
On occasion, samples were obtained from actual crime scenes and methods were applied crudely as developed in the laboratory;19 though successful, these “one off” method application to real crime scene marks had not been further refined. The vast majority of the marks successfully analysed in tandem were mock samples prepared in laboratory settings.18,20 Whilst operational feasibility has now been more largely investigated with a clear opportunity for implementation in the field, only limited predictions can be made on methods’ robustness and versatility in operational circumstances. In the authors’ view, given the extreme variability of fingermark donors’ characteristics and of the environmental conditions the marks could be exposed to, it will take the analysis of a large number of actual crime scene marks to make a suitable evaluation of this particular aspect.
Recently, a study was published covering investigations undertaken under the Home Office Innovation Fund, enabling a collaboration between Sheffield Hallam University's Fingermark Research Group (FRG) and West Yorkshire Police (WYP), UK.21 These investigations specifically concerned the development of MALDI MSP and MSI methodology for operational use. From this collaboration, a step forward has been taken by proposing a method to pinpoint the best sample treatment for marks enhanced using the powders most often employed by WYP (carbon black, aluminium and TiO2); by best sample treatment, it is meant the treatment enabling the most chemical information and ridge detail to be obtained upon MALDI MSI analysis. Sample treatment methods concerned the modalities of matrix application in combination with the use of the initial enhancing forensic powder. This is especially important for secondary lifts, which are the residual mark material lifted after a first cycle of enhancement and tape lifting (primary lift). Secondary lifts may be all it is granted to analyse by the Police as primary lifts are normally kept as evidence; therefore establishing the most efficient sample treatment prior to analysis was paramount in order to avoid loss of information, which is already minute in the remaining residue. The report indicated suitability of any of the trialed sample preparation methodologies (except the Dry-Wet method17) for marks preliminarily enhanced using aluminium or carbon powders, whereas in the case of preliminary TiO2 powder enhanced marks, re-enhancing the mark using the initial powder and spray-coating with the MALDI matrix was recommended. Contextually, the report also demonstrated and highlighted the importance to analyse primary lifts instead, which were demonstrated to outperform secondary lifts in terms of maximizing forensic opportunities; in the case of primary lifts, MALDI matrix spray coating and matrix spotting are the sample preparation methods to be applied prior to imaging and profiling analyses respectively.
The natural evolution of the study briefly described above was the investigation of the translational feasibility to real crime scene samples. As illustrated further in this paper, in preparation for the analysis of real forensic evidence, preliminary consideration (followed by protocol development) was given to the collection, transport and storage of the crime scene marks under operational conditions; this also represents an advancement compared to previously published work and it is a significant one as lack of compliance with CSI requirements prevents any analysis carried out using new and developing technologies. For primary lifts, normally a CSI would seal them in acetate backing sheets for transport and storage of the evidence. Therefore a prior study was undertaken to assess the possibility to obtain both ridge detail and chemical information from fingermark lifts removed from acetate sheets which proved to be successful (data not shown). In the event that: (a) only secondary lifts were accessible, (b) the mark had been suitably photographed and the photo kept as primary evidence by the police or (c) the primary lifted mark was deemed to have no evidential value by the CSI who granted access to the mass spectrometrist, a system was devised to transport and store the lifts in a non contactless manner, avoiding contamination with debris, in an in house customised box, as shown in Fig. 1.
Once a way to store and transport the evidence was established, police casework could be investigated. In particular, four examples of casework are reported here to illustrate the first and more in depth insights into the limitations and benefits of applying MALDI MSP and MSI under operational conditions. Given that the benefits of analysing primary lifts were demonstrated,18 the authors were granted the opportunity to only analyse primary lifted marks or marks directly analysed on the surface of deposition.
In particular, two marks (i) and (ii) were originally located on a textured light frame and a plug socket respectively; they were stored in the customised box (Fig. 1) and subsequently analysed as primary lifts. Another mark (iii) was analysed directly on the original surface of deposition, a drug packet, following initial development by the crime lab. The last mark discussed in this paper (iv) was located on the interior side of a window frame and was tape lifted at the crime scene following enhancement with carbon black powder before being sealed in acetate for storage.
The marks under consideration in this paper are classed as belonging to the high profile crimes category; marks (i)–(ii) were collected at a cannabis farm during an investigation of drug dealing, while mark (iii) was found at a crime scene of a murder; finally mark (iv) was from an harassment case.
Experimental
Materials
Trifluoroacetic acid (TFA), α-cyano-4-hydroxycinnamic acid (α-CHCA) and ALUGRAM1 SIL G/UV254 pre-coated aluminium slides were purchased from Sigma Aldrich (Poole, UK). Standards of cocaine (COC), benzoylecgonine (BZE), norcocaine (NCOC), ecgonine methyl ester (EME) and cocaethylene (CE) were provided by Kings College London (London, UK) through of an ongoing scientific collaboration. Acetonitrile (ACN) and acetone were obtained from Fisher Scientific (Loughborough, UK). MALDI target OPTI TOF spotless inserts were purchased from Applied Biosystems (Foster City, CA, USA). Double-sided conductive carbon tape was purchased from TAAB (Aldermaston, UK). Fingerprint brushes were purchased from Tetra Scene of Crime Ltd (Essex, UK). All fingermark development powders were provided by West Yorkshire Police Forensic laboratory (Wakefield, UK).Instrumentation and instrumental settings
Mass spectrometric analyses in positive mode were conducted using either a modified Applied Biosystems API “Q-Star” Pulsar i hybrid quadrupole time-of-flight (QTOF) instrument (Concord, Ontario, Canada) or a Synapt™ G2 HDMS ion mobility mass spectrometer (Waters Corporation, Manchester, UK). On the Q-star instrument, the orthogonal MALDI source has been modified to incorporate a SPOT 10 kHz Nd:YVO4 solid-state laser (Elforlight Ltd, Daventry, UK),22 operating at 1 kHz, having a wavelength of 355 nm and a pulse duration of 1.5 ns. Images were acquired at a spatial resolution of 150 × 150 μm in raster imaging mode, using ‘oMALDI Server 5.1’ software supplied by MDS Sciex (Concord, Ontario, Canada). MALDI MSI acquisition was performed in positive ion mode in the mass range between m/z 50 and 1000. The declustering potential 2 was set at 25 arbitrary units, with an accumulation time of 0.117 min. The total run time varied between 60–120 minutes depending on the size of the fingermark.The Synapt™ G2 HDMS instrument is equipped with an Nd:YAG laser which was also operated at 1 kHz. All MS spectra were acquired in positive ion ‘sensitivity’ mode in the mass range between m/z 100–1000. For MALDI-IMS-MS/MS analyses, collision induced dissociation (CID) was performed in the T-wave™ transfer cell following initial ion mobility separation. This allowed for all product ions to retain the same ion drift time as their corresponding precursor ion. Collision energy was applied at 26 eV for cocaine and each of the metabolites. In all instances, the MALDI source of the instrument was thoroughly cleaned prior to analyses ensuring no signal arising from potentially forensically relevant molecules were detected in a subsequent blank analysis; furthermore the crime scene samples were analysed before any standards which were used for mass spectral comparison purposes, where appropriate. This ensured that there was no sample carryover which could falsely indicate the presence of the substance of forensic significance in the crime scene samples. Prior to analyses, calibration of the instrument was performed in the range between m/z 50–1000 mass range using Phosphorus Red.
A video spectral comparator (VSC4CX, Foster & Freeman, Evesham, UK) was employed for mark (i–ii) and (iv) to take optical images as a reference for MALDI MSI analyses.
Methods
Data processing
MALDI MS images were processed using Biomap software (Novartis, Basel). All images were normalised against the total ion count (TIC) and specific m/z values were visualised with optimal contrast and intensity to allow for visualisation of the fingermark ridges. Mass spectra were processed using MassLynx 4.1 (Waters Corporation, Manchester, UK), Analyst MDS Sciex (Concord, Ontario, Canada) and the open source multifunctional mass spectrometry software mMass.23,24Collection of fingermarks from crime scenes
Fingermarks (i) and (ii) were recovered from a seized cannabis farm. Visual examination revealed (i) on a textured metal light fitting. Enhancement was performed using TiO2 powder. Mark (ii) was visualised on a plug socket switch following enhancement using aluminium powder. These marks were secured within the storage box shown in Fig. 1 and analysed by MALDI MSI as primary lifts.Mark (iii) was enhanced on a drug packet. Treatment using cyanoacrylate fuming followed by BY40 and image capture were performed according to the Home Office Fingermark Visualisation Manual.9 The fingermark was analysed via MALDI MSI directly on the surface of deposition without any lifting.
Finally, mark (iv) was recovered as a primary lift from an interior window frame using forensic lifting tape following initial development using carbon black powder. The mark was sealed in acetate prior to analysis and analysed after 18 days of storage.
Matrix deposition
For MALDI MSI experiments, fingermarks recovered from crime scenes were spray-coated with α-CHCA using a ‘SunCollect’ autosprayer (SunchromGmbH, Friedrichsdorf, Germany). A solution of 5 mg mL−1 α-CHCA in 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ACN: 0.5% TFA was sprayed onto each of the fingermarks for a total of 4 layers at a rate of 2 μL min−1, using a “slow” raster setting.
30 ACN: 0.5% TFA was sprayed onto each of the fingermarks for a total of 4 layers at a rate of 2 μL min−1, using a “slow” raster setting.
For post-image MALDI MSP and MS/MS analyses, fingermarks were spotted numerous times at different locations (0.5 μL per spot), to generate internal replicates, with the same matrix solution used for spraying. The matrix spots were specifically deposited within the areas of the ridges where the imaging analysis had indicated the presence of components of interest.
Results and discussion
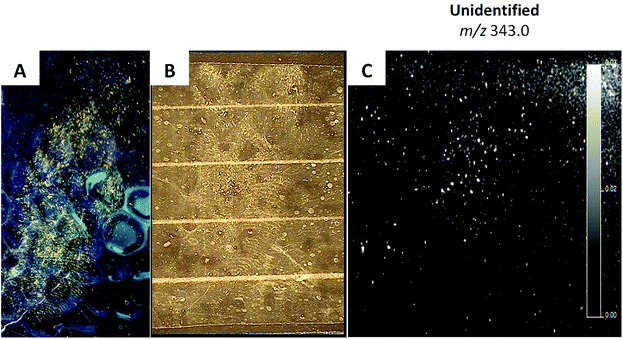
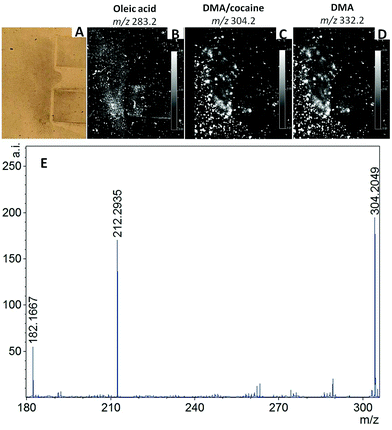
In the study presented here, four examples of the police casework involving fingermarks as main source of forensic evidence are reported. To date, seven casework examples have been investigated through the application of MALDI MSI and MALDI MS based techniques to crime scene marks, but only four will be discussed here as being the most representative of the limitations and benefits of this technology and the methodologies developed. However, a complete evaluation of these aspects can only be undertaken upon the analysis of a large number of crime scene marks. This is due to the extreme variability in the state of the mark such as: “quality of the donor”,25 time since deposition, surface of deposition, environmental factors and prior forensic enhancement treatments; all of these factors can also inter-play and influence directly the result of the application of MALDI MS based methods. Therefore these four case studies are to be considered only a first (though significant) insight into the feasibility and operational character of MALDI MS based methods. Following previous published work, factually demonstrating for the first time the benefits of analysing primary lifted marks as opposed to secondary lifted marks,21 the crime lab and the CSI sent for analysis marks that had been collected as primary lifts in three instances and, in one case, the actual specimen on which the mark was deposited. The four marks were all subjected to MALDI MSI analysis and in some instances to further mass spectrometric characterisation through Tandem MS in order to identify the molecules detected.Marks (i) and (ii) were collected as primary lifts after enhancement with TiO2 and aluminium powder respectively. MALDI MSI analyses were conducted after 1 and 2 days from the collection and the results are shown in Fig. 2. Prior to enhancement by the CSI, an orange colouration was observed within the ridges of mark (i) (Fig. 2A); unfortunately it was not possible to ascertain if the orange compound was pre-existing on the surface, deposited together with the mark or the result of a coincidental association (landed on the mark at a later stage following mark deposition). Further enhancement with TiO2 powder did not yield any useful ridge detail. The primary lift exhibited a poor amount of material by visual inspection and even the faintest hint of ridge detail, observed when the mark was still on the surface, seemed to be absent (Fig. 2B). This could indicate either an inefficient lift, due to the mark being strongly adhered to the surface, or a very old mark. The lifted mark was in any case subjected to MALDI MSI analysis. The analysis confirmed scarcity of fingermark residue material. No ion images of any minutiae could be retrieved and Fig. 2C shows a representative example; this could also be ascribed to the surface being textured, which is an objective problem for conventional enhancement techniques too, due to physical distortion and interruption of the ridge pattern. Also, no useful molecular information could be obtained. It could be speculated that the mark had probably been subjected to high temperature on the light fitting, thus causing molecular thermal degradation. This is an example illustrating the limitations that the MALDI MS based methods may encounter with poor lifts and scarcity of molecular information, possibly due to severely aggressive environmental factors and age. The initial enhancement of mark (ii) was also poor and the CSI deemed the very little ridge detail recovered insufficient for an identification. The mark was therefore lifted, stored in the customised box and transported for mass spectrometric analysis. The acetate was peeled off and an optical image of the lift was taken before and after matrix deposition using a video spectral comparator. Although the ridge detail could not be improved in terms of quantity or quality upon MALDI MSI, probably due to an objective lack of ridge details, molecular information could be this time retrieved (Fig. 3); in particular, endogenous species such as fatty acids, diacyl and tryacylglycerols were detected and identified on the basis of previous work3,25 (data not shown). Fig. 3A shows the molecular image of oleic acid at m/z 283.2 as example of this class of molecules. Interestingly, also a previously detected substance12,16 was observed here at m/z 304.2 and 332.2 and these ions were used to generate molecular images of the mark (Fig. 3B). These ions correspond to n-alkyl dimethylbenzylammonium (DMA) ions having 12 and 14 CH2 repetition units respectively. n-Alkyl DMA is a known antibacterial agent found in toiletry products, wipes and detergents. This is not a forensically relevant substance. However, its detection in operational conditions indicates feasibility of application of MALDI MS based methods for real crime scene marks and therefore it was important to confirm its presence. For this purpose, MALDI MS/MS analyses were performed. Interestingly, not only was the n-alkyl DMA presence confirmed through its ion fragment at m/z 212.3, but also the presence of cocaine could be ascertained through the detection of its characteristic ion fragment at m/z 182.2 (Fig. 3C). DMA (12CH2) (monoisotopic m/z 304.3004) and cocaine (monoisotopic m/z 304.1548) have in fact the same nominal mass; therefore during the MS/MS analysis, the quadrupole in the QTOF instrument would have selected both ions for fragmentation, subsequently yielding ion fragments from both DMA and cocaine.
Cocaine is a forensically relevant substance and although in this case its detection may not significantly contribute to the investigation, it does again indicate the operational character of the technologies/methodologies employed. Initially, it seemed puzzling that, while cocaine was detected, THC was not, despite the mark was retrieved in a seized cannabis farm.
The absence of detection could fit within previously encountered instances in which the ionisation efficiency of this molecule in latent marks is poor compared to other drugs of abuse analysed.
Recently a derivatisation method to detect and image THC in hair has been proposed26 and work is in progress to trial and apply this method to THC in fingermarks.
There is also a possibility that THC could have degraded into cannabinol and/or cannabidiol as it is found in aged marijuana samples and it is reasonable to think that heat and light accelerates the aging. However, no evidence for the presence of these species was found by examining the data acquired in MS mode. Detection of these species though, is not to be excluded, if a transfer fragmentation experiment, using an Ion Mobility Mass Spectrometer and the relevant standards had been carried out (as for Mark (iv) further discussed in this paper).
However, presence of THC in the mark, is not necessarily to be expected, even in a cannabis farm. It is in fact highly likely that gloves were used during the manufacturing process, as the frequent retrieval of “marigold” at these scenes indicates; therefore the presence of cocaine could be the result of coincidental and later association.
Mark (iii) was located on a drug packet and the evidence was acquired within a murder case. At the crime lab, cyanoacrylate fuming followed by BY40 was employed to enhance the mark which yielded poor ridge detail (Fig. 4A). The mark was subsequently sent to be subjected to MALDI MSI in the effort to obtain additional ridge detail. No lifting was performed as the mark could be analysed directly on the drug packet due to the surface being flat and thin. The analysis was performed after one month from the collection. Only one ion provided a degree of ridge pattern reconstruction at m/z 104.0 (suspected choline) but the total ion current selection in the m/z range 100–1000 provided more ridge pattern coverage. The choline MS image provided a lower quality image than that obtained by Cyanoacrylate fuming/BY40 while the total ion current image rivalled the amount of ridge detail obtained through conventional enhancement but it did not improve upon it.
Although disappointing, these results are not surprising. While it has been shown that MALDI MSI can be successfully applied to cyanoacrylate fumed marks, the subsequent treatment with BY40 greatly suppresses ionisation;20 this is probably due to the ability of this dye to sequestrate the energy from the laser, thus rendering the MALDI process ineffective for the other molecules present. In previously published work,20 it was shown that while THC and Amphetamine could not yield ridge detail following cyanoacrylate fuming/BY40 treatment, cocaine and heroin provided useful molecular images of the ridge pattern. In that study, fingermarks deposited on aluminium slides were produced after spiking fingertips using a 50 μL aliquot of mixed drug standard solution (4 drugs in total) equivalent to 1.25 μg of pure cocaine and of pure heroin spread over the mark surface. Therefore within this casework, either these drugs were not present in the mark or their amounts were lower than the instrumental detection limit. The MALDI analyst was never told the content of the drug packet and therefore it is difficult to comment beyond these hypotheses.
Casework (iv) was the one that highlighted the most the operational character of the technology developed as well as the complementarity of the intelligence that it is possible to obtain and that can contribute to the overall investigations and court cases. This was an harassment case in which a mark was located on the interior of a window frame. The initial optical examination of the latent fingermark sample showed that it had an oily consistency, similar to what may be observed from a “groomed”5 fingermark. The CSI deemed it appropriate to use carbon black powder to enhance the mark prior to lifting it.
Unfortunately, the result of this enhancement was not conducive to the retrieval of useful ridge detail (Fig. 5A); the primary lift, which was sealed in acetate, was subsequently subjected to MALDI MSI analysis, aiming to the retrieval of more minutiae.
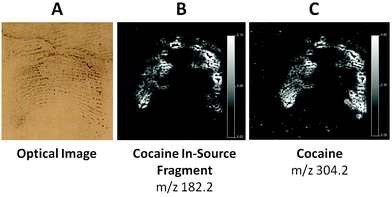
However, the initial observation of the enhanced mark did not show potential for improvement; as for any other technique, ridge detail cannot be generated if it is not originally present. The ridge pattern on the lift appeared to be very smudged. This could be the result of either of the following circumstance or combination thereof (1) enhancement, (2) merging of the ridges upon recovery of an oily mark which was then compressed on acetate (3) original state of the mark after deposition. The MALDI MS imaging analysis confirmed the initial concern as there was no improvement of the ridge detail. Fig. 4B and C show the MALDI molecular images of the mark using the ion signals at m/z 304.1 and 182.1.
While these images were not useful in terms of physical information, they were obtained exploiting the detection of cocaine which, in its intact form, exhibits a m/z at 304.1 as well as of a fragment (in source fragment) at m/z 182.1.
Therefore, once again, cocaine was detected within a mark in operational conditions. Its presence was also confirmed by conventional investigation of the detectives who meanwhile interrogated the suspect. The suspect confessed their crime and was subsequently subjected to a drug test which confirmed cocaine abuse.
Therefore while the MALDI MSI analysis could not be definitive about drug consumption, as the presence of the intact molecule of cocaine in the mark cannot discriminate between drug handling and drug consumption, ordinary investigation could confirm drug abuse.
However, the MALDI analyst was blind to all the details of the casework and of the results of the ongoing investigations and decided to conduct a more in depth analysis searching for metabolites which could prove drug abuse.
It has been previously demonstrated that the use of Ion Mobility Mass Spectrometry separation tandem Mass Spectrometry, MALDI-IMS-MS/MS, enables a sensitive and reliable detection of drug metabolites, distinguishing those sharing the same nominal mass on the basis of their mobility,1 using transfer fragmentation experiments.
In this type of analysis, the ion is subjected to ion mobility first and then fragmentation in the transfer cell of the triwave™ region of the instrument in order for the generated ion fragments to retain the same drift time as their parent ion.
Prior to this analysis the MALDI matrix was preliminarily spotted in 3 different locations of the mark and outside the mark in order to ensure that any detected and forensically relevant species were real and also not present in the matrix.
Transfer fragmentation MALDI IMS-MS/MS analysis was then performed in these locations first, followed by the same analysis applied to standards (and not the other way around to avoid carry over effects in the instrument). The ions preliminarily detected were previously encountered within the drug/metabolite analyses in fingermarks performed by these authors and therefore standards were available to confirm identity. Transfer fragmentation analysis was employed here and confirmed the presence of cocaine (theoretical and experimental m/z 304.1549 and 304.1737 respectively) as well as revealing the presence of benzoylecgonine (theoretical and experimental m/z 290.1392 and 290.1240 respectively), ecgonine methylester (theoretical and experimental m/z 200.1287 and 200.1551 respectively), and norcocaine (theoretical and experimental m/z 290.1240 and 290.1392) (data not shown) confirming cocaine consumption.
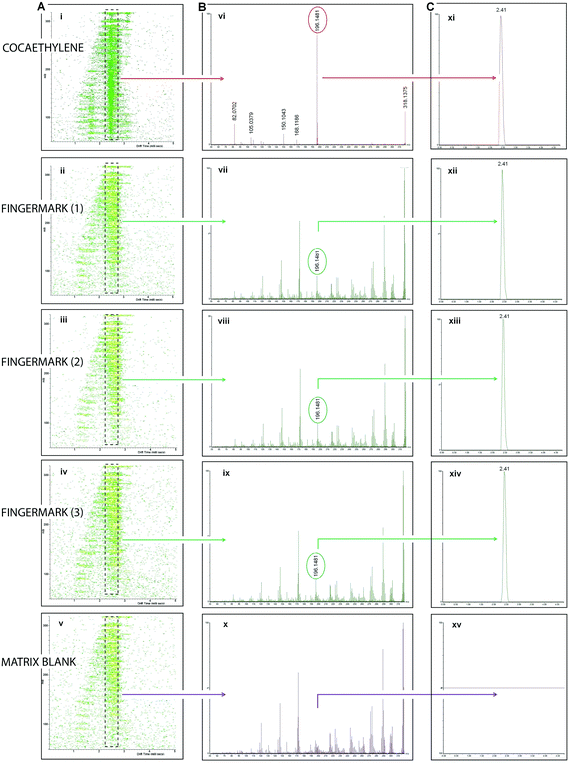
Additionally another interesting ion was revealed, having a m/z at 318.1158 which was not previously detected within MALDI MSI analysis on the less sensitive instrument (MALDI Q-TOF Qstar Pulsar i, Applied Biosystems). MALDI IMS-MS/MS analysis of both the mark and the standard (Fig. 6) allowed for Drift Scope Plots (a graphical representation of the Ion Mobility Separation) to be generated and select DT regions of interest containing both the parent and the corresponding product ions (Fig. 6A). Spectra from these acquisitions were interrogated for a product ion at m/z 196.1481 (Fig. 6B) derived from the collision induced dissociation of the ion at m/z 318.1974 which directly corresponded to that observed in the standard. Drift time chromatograms could then be produced based on the specific DT of this product ion, showing that the product ion in the standard and the mark directly coincided having the same drift time (Fig. 6C). This ion peak was also absent from the matrix blank which indicated that it did not arise from it. This analysis confirmed the identity of this ion as cocaethylene (theoretical m/z 318.1705). This metabolite only forms in the body when cocaine is consumed at the same time as alcohol.27 This information could be very important both in investigations and in a court case as it highlights the state of mind of the individual at the time of committing the crime. While these analyses were being performed, the investigators questioned the individual about alcohol consumption and he firmly denied this instance. Only much later, prior to the case being discussed in Court he confessed to have also been drinking in addition to cocaine consumption. This confirms and highlights once more benefits (current and potential), importance and operational character of the developed methodologies for profiling a suspect, their lifestyle or the circumstances of the case from a mark.
Particularly casework example (iv) represents a significant advancement with respect to the work previously published by this group. Transfer fragmentation experiments on an Ion Mobility Mass Spectrometer were already successfully reported, demonstrating the opportunity to detect THC, methadone and cocaine's primary metabolite benzoylecgonine.10 However, in that case, a mark was taken “fresh” in controlled conditions (environmental, ageing and collection wise) from a patient in a rehabilitation clinic. As opposed to mark (iv), the mark analysed in Bailey et al.10 remained undeveloped (no FET were applied), thus avoiding any chemical interference causing ion suppression. Also, differently from the work presented here, in Bailey et al.10 the authors carried out a targeted analysis searching for “commissioned” molecular targets. Finally, differently from the example presented in Bailey et al.,10 in the casework example presented here, transfer fragmentation experiments were successfully carried out after an already performed MALDI MSI analysis. This also highlights the sensitivity of the technology, given that some material would have been ablated already through the MALDI MSI analysis.
A mention must be made to the robustness of these protocols. Mark (iv) was very successfully analysed 17 days after collection yielding important molecular information as well as molecular images. Although mark (i) analysed after one day from collection did not yield any information, this could be due to a number of environmental reasons and/or to inefficient lifting. On the contrary, the analysis of mark (iv) indicates the possibility to “re-visit” crime scene exhibits but may also indicate the opportunity to analyse cold cases involving fingermarks; using this technology, this opportunity was recently demonstrated for a 9 year old blood palm print preliminarily enhanced with acid black 128 and for a 30 year old stain on fabric which was preliminarily enhanced with ninhydrin (data not shown).
Conclusions
For the first time, crime scene marks from high profile cases were analysed in operational conditions by MALDI MS based methodologies. These marks were analysed as primary lifts or directly from the surface of deposition following enhancement by a conventional forensic technique as deemed appropriate by the CSI or the Crime Lab investigator. This paper also reports on the opportunity to cover the analysis of marks stored in acetate (rotine CSI practice), thus increasing the range of type of collected marks that is possible to investigate with these methodologies. Together with the intelligence that it was possible to retrieve, this highlights the improved value scientific of this work, with respect to published work, through the demonstration of a much higher potential to impact forensic practice, forensic investigations and judicial debates. The opportunity to apply MALDI MS based methods, tailored for the analysis of fingermarks within police casework has enabled a preliminary evaluation of the operational feasibility of the methods themselves. The word “preliminary” is very appropriate due to the unpredictable nature of the state of the crime scene mark coupled to a prior physical/chemical treatments. As conditions cannot be controlled like for mock samples prepared in the lab, these factors, together with the unknown but certainly minute amounts of forensically relevant substances in fingermarks, could have well pointed out to inapplicability of MALDI technology/developed methods for the retrieval of chemical and physical information. Instead, while in these four casework examples, the quality of the ridge detail was inferior or only rivalling that provided by forensic enhancement techniques, overall the nature of the intelligence retrieved in operational conditions is useful to chemical profiling the suspect, their state of mind or the circumstances of the crime; this is important in order to steer investigations and/or to contribute towards court case debates. Even the instances in which the detection of cocaine could not significantly contribute to the case, this report demonstrates robustness of the MALDI methods developed to date to stand operational conditions and integrate with the prior application of FET. In all cases, and on the basis of previous developmental work, hypotheses could be made on the quality of the performance of this technology indicating limitations (either deriving from the samples itself or due to the nature of the techniques) as well as benefits. Both in terms of limitations and benefits, this study must be considered only a first insight on the potential of this technology to be used in the field. In order to evaluate its robustness and feasibility fully, a large number of crime scene marks will need to be analysed to cover the wide range of factors making fingermarks an extremely variable specimen in terms of the physical and chemical information that it is possible to retrieve.Acknowledgements
Fingerprint expert John Dixon from West Yorkshire Police is gratefully acknowledged for his concept and making of the latent fingermark storage and transportation box. This work was funded by the Home Office Innovation Fund [2014], UK.References
- D. R. Ifa, N. E. Manicke, A. L. Dill and R. G. Cooks, Science, 2008, 321, 805 CrossRef CAS PubMed
.
- F. Rowell, K. Hudson and J. Seviour, Analyst, 2009, 134, 701 RSC
.
- R. Wolstenholme, R. Bradshaw, M. R. Clench and S. Francese, Rapid Commun. Mass Spectrom., 2009, 23, 3031 CrossRef CAS PubMed
.
- M. I. Szynkowska, K. Czerski, J. Rogowski, T. Paryjczak and A. Parczewski, Surf. Interface Anal., 2010, 42, 393 CrossRef CAS
.
- R. A. Musah, R. B. Cody, A. J. Dane, A. L. Vuong and J. R. E. Shepard, Rapid Commun. Mass Spectrom., 2012, 26, 1039 CrossRef CAS PubMed
.
- A. Tripathi, E. D. Emmons, P. G. Wilcox, J. A. Guicheteau, D. K. Emge, S. D. Christensen and A. W. Fountain III, Appl. Spectrosc., 2011, 65, 611 CrossRef CAS PubMed
.
- K. M. Antoine, B. S. Shirin Mortazavi, A. D. Miller and L. M. Miller, J. Forensic Sci., 2010, 55, 513 CrossRef CAS PubMed
.
- C. Ricci, P. Phiriyavityopas, N. Curum, K. L. A. Chan, S. Jickells and S. G. Kazarian, Appl. Spectrosc., 2007, 61, 514 CrossRef CAS PubMed
.
-
H. Bandey, V. Bowman, S. Bleay, R. Downham and V. H. Sears, Fingermark Visualisation Manual, ed. H. Bandey, CAST, Home Office, Sandridge, UK, 2014 Search PubMed
.
- M. J. Bailey, R. Bradshaw, S. Francese, T. L. Salter, C. Costa, M. Ismail, R. Webb, I. Bosman, K. Wolff and M. de Puit, Analyst, 2015, 396, 6254 RSC
.
- R. Bradshaw, R. Wolstenholme, R. Blackledge, M. R. Clench, L. S. Ferguson and S. Francese, Rapid Commun. Mass Spectrom., 2011, 25, 415 CrossRef CAS PubMed
.
- R. Bradshaw, R. Wolstenholme, L. S. Ferguson, C. Sammon, K. Mader, E. Claude, R. Blackledge, M. R. Clench and S. Francese, Analyst, 2013, 138, 2546 RSC
.
- K. Kaplan-Sandquist, M. A. LeBeau and M. L. Miller, Forensic Sci. Int., 2014, 235, 68 CrossRef CAS PubMed
.
- K. Kaplan-Sandquist, M. A. LeBeau and M. L. Miller, J. Forensic Sci., 2015, 60, 611 CrossRef CAS PubMed
.
- L. Sundar and F. Rowell, Analyst, 2014, 139, 633 RSC
.
- R. Bradshaw, S. Bleay, R. Wolstenholme, M. R. Clench and S. Francese, Forensic Sci. Int., 2012, 222, 318 CrossRef CAS PubMed
.
- L. Ferguson, R. Bradshaw, R. Wolstenholme, M. R. Clench and S. Francese, Anal. Chem., 2011, 83, 5585 CrossRef CAS PubMed
.
- R. Bradshaw, S. Bleay, R. Wolstenholme, M. R. Clench and S. Francese, Forensic Sci. Int., 2013, 232, 111 CrossRef CAS PubMed
.
- R. Bradshaw and S. Francese, Spectrosc. Eur., 2014, 6–8 CAS
, http://212.113.150.109/articles/55-articles/3357-matrixassisted-laser-desorption-ionisation-tandem-mass-spectrometry-imaging-of-small-molecules-from-latent-fingermarks, accessed 6/02/2017.
- G. Groeneveld, M. dePuit, S. Bleay, R. Bradshaw and S. Francese, Sci. Rep., 2015, 5, 11716 CrossRef CAS PubMed
.
- R. Bradshaw, N. Denison and S. Francese, Anal. Methods, 2016, 8, 6795 RSC
.
- P. J. Trim, M. C. Djidja, S. J. Atkinson, K. Oakes, L. M. Cole, D. M. G. Anderson, P. J. Hart, S. Francese and M. R. Clench, Anal. Bioanal. Chem., 2010, 397, 3409 CrossRef CAS PubMed
.
- M. Strohalm, M. Hassman, B. Kosata and M. Kodicek, Rapid Commun. Mass Spectrom., 2008, 22, 905 CrossRef PubMed
.
- M. Strohalm, D. Kavan, P. Novakand and V. Havlicek, Anal. Chem., 2010, 82, 4648 CrossRef CAS PubMed
.
- L. S. Ferguson, S. Creasey, R. Wolstenholme, M. R. Clench and S. Francese, J. Mass Spectrom., 2013, 48, 677 CrossRef CAS PubMed
.
- E. Beasley, S. Francese and T. Bassindale, Anal. Chem., 2016, 20, 10328 CrossRef PubMed
.
- M. R. Brzezinski, B. J. Spink, R. A. Dean, C. E. Berkman, J. R. Cashman and W. F. Bosron, Drug Metab. Dispos., 1997, 25, 1089 CAS
.
- E. Patel, P. Cicatiello, L. Deininger, M. R. Clench, G. Marino, P. Giardina, G. Langenburg, A. West, P. Marshall, V. Sears and S. Francese, Analyst, 2016, 141, 191 RSC
.
| This journal is © The Royal Society of Chemistry 2017 |