Intramolecular non-covalent interactions as a strategy towards controlled photoluminescence in copper(i) complexes†
Abstract
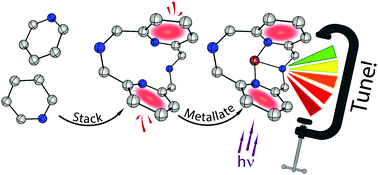
In this work, we describe a new strategy for designing photoluminescent Cu(I) complexes. At its core are simple cyclophane inspired N-donor ligands featuring intramolecular interactions between aromatic units within a single molecule. Variation of the steric bulk inflicted a change in intramolecular stacking distances that in turn affected the emission colour of copper(I) complexes tunable in a 0.5 eV range from green to red. As the interactions driving emission are confined to the single molecule, no intermolecular aggregation is required to enable photoluminescence in solution, pristine crystals, or solution-cast polymer films. A crystallographic study provides a link between the spatial proximity of the aromatic rings of the ligands (ranging from 3.349 to 3.731 Å) and the enhancement of emission efficiency, which increases dramatically from 0.02 to 0.78 at 296 K as the ring spacing contracts. Photophysical and theoretical analyses confirm the involvement of intramolecular interactions in the formation of the emissive state and describe the observed phenomena at the molecular level.


 Please wait while we load your content...
Please wait while we load your content...