In situ investigation on ultrafast oxygen evolution reactions of water splitting in proton exchange membrane electrolyzer cells†
Abstract
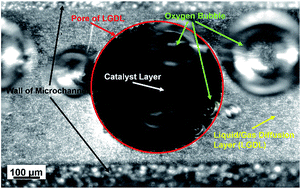
The oxygen evolution reaction (OER) is a half reaction in electrochemical devices, including low-temperature water electrolysis, which is considered as one of the most promising methods to generate hydrogen/oxygen for the storage of energy. It is affected by many factors, and its mechanism is still not completely understood. A proton exchange membrane electrolyzer cell (PEMEC) with optical access to the surface of anode catalyst layer (CL) coupled with a distinguished high-speed and micro-scale visualization system (HMVS) was developed to in situ investigate OERs. It was revealed in real time that OERs only occur on the anode CL adjacent to liquid/gas diffusion layer (LGDL). The CL electrical conductivity plays a crucial role in OERs on CLs. The large in-plane electrical resistance of CLs becomes a threshold of OERs over the entire CL, and causes a lot of catalyst waste in the middle of LGDL pores. Moreover, the oxygen bubble nucleation, growth, and detachment and the effect of current density on those processes were also characterized. This study proposes a new approach for better understanding the mechanisms of OERs and optimizing the design and fabrication of membrane electrode assemblies.


 Please wait while we load your content...
Please wait while we load your content...