An evenly distributed sulfur-doped nickel zinc hydroxyl carbonate dispersed structure for all-solid-state asymmetric supercapacitors with enhanced performance†
Abstract
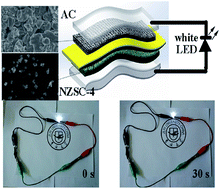
Herein, rational evenly distributed sulfur-doped nickel zinc hydroxyl carbonate (NZSC) nanoparticles are prepared by a simple two-step hydrothermal method, getting a high degree of dispersion of the nickel zinc hydroxyl carbonate (NZC) precursor and then sulfidizing to increase the conductivity. Meanwhile, its large specific surface area of 226 m2 g−1, resulting in a large number of redox sites, can effectively reduce volume change during the charge–discharge process. As a positive electrode material, NZSC-4 shows a high capacitance value of 1634 F g−1 at 1 A g−1 and outstanding rate performance (73% rate retention at 20 A g−1). What's more, an all-solid-state asymmetric supercapacitor (ASC) was assembled, using NZSC-4 as the positive electrode and activated carbon as the negative electrode. The ASC exhibits a high energy density of 36.17 W h kg−1 from a high voltage range of 0 to 1.7 V at a power density of 0.85 kW kg−1, appropriate leakage current and self-discharge. Interestingly, two fully charged ASCs (in series) can power the white light-emitting diodes (LED, working voltage greater than 3 V) effectively, and subsequently light yellow LED and then red LED after the white bulb consumes part of the energy. Hence, the NZSC-4 as a pseudocapacitive material looks promising.


 Please wait while we load your content...
Please wait while we load your content...