Electrochemical deposition of carbon nanotubes from CO2 in CaCl2–NaCl-based melts†
Abstract
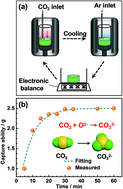
As part of the efforts to address global climate change, the identification of methods for the capture of carbon dioxide and its selective electrochemical conversion into value-added carbonaceous materials in molten salt electrolytes is a research topic of scientific and technological significance. In most cases, metal electrodes such as nickel and stainless steel are used as the cathode to investigate the nucleation and growth of a variety of carbon nanostructures. In this study, the electrochemical deposition of carbon nanotubes (CNTs) and carbon microstructures was performed in molten CaCl2–NaCl–CaO using glassy carbon and graphite rod as the cathode and RuO2–TiO2 as the anode. The capture formula was established and the capture coefficient was defined and calculated to be 1.8 s−1. Cyclic voltammetry, constant voltage electrolysis, as well as on-line outlet gas analysis were conducted to investigate the electrode reactions, and the results indicated that the captured CO2 can be electrochemically converted to carbon and environmentally-friendly oxygen as the only by-product. SEM and TEM images showed that quasi-spherical and nanotubular carbon were deposited on the graphite and glassy carbon cathodes at 750 °C. However, by regulating the temperature, quasi-spheres and nanosheets were observed at the glassy carbon cathode.


 Please wait while we load your content...
Please wait while we load your content...