Flexible and rechargeable Zn–air batteries based on green feedstocks with 75% round-trip efficiency†
Chao
Lin
a,
Sambhaji S.
Shinde
a,
Yong
Wang
b,
Yu
Sun
a,
Si
Chen
b,
Haojie
Zhang
c,
Xiaopeng
Li
 *c and
Jung-Ho
Lee
*c and
Jung-Ho
Lee
 *a
*a
aDepartment of Materials and Chemical Engineering, Hanyang University, Ansan, Kyunggido 15588, Republic of Korea. E-mail: jungho@hanyang.ac.kr
bDepartment of Chemical Engineering, School of Environmental and Chemical Engineering, Shanghai University, 99 Shangda Road, 200444, Shanghai, P. R. China
cCAS Key Laboratory of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute (SARI), Chinese Academy of Sciences (CAS), Shanghai 201210, China. E-mail: lixp@sari.ac.cn
First published on 15th August 2017
Abstract
Here, we report a flexible solid-state Zn–air battery (SZAB) that achieves a record round-trip efficiency of 75%. The major components of the SZAB are based on biomass derivatives. Glucose acts as the carbon source to construct the bifunctional oxygen electrocatalyst. Methylcellulose simultaneously enhances the ionic conductivity and water retention of the gel electrolyte, endowing the SZAB with enhanced flexibility and efficiency.
Driven by the explosive increase in portable and wearable electronics in the past few decades, the demand for advanced solid flexible batteries has increased considerably. Although the market is currently dominated by lithium-ion batteries, there is a growing interest in the development of alternative batteries that are environmentally friendly, cheap, and safe. Zn–air batteries (ZABs) are ideal candidates because zinc is abundant, cheap, and environmentally benign. Furthermore, they have a high theoretical energy density of 1086 W h kg−1.1 In 2015, Chen, Peng, and Cho2–4 pioneered the development of solid-state ZABs (SZABs) in different configurations, such as fiber-, planar- and cable-type, and showed that these devices have the potential for commercialization. However, SZABs suffer from low efficiencies. To date, the highest achieved round-trip efficiency for a SZAB is ∼66%; moreover, this device uses precious RuO2 as the oxygen evolution catalyst.5
Substantial improvements in the efficiency of SZABs rely on the novel design of electrocatalysts that can effectively catalyze the oxygen reduction/evolution reaction (ORR/OER), as well as the use of a stable solid-state electrolyte with high ionic conductivity. Meng et al.6 demonstrated an active bifunctional 3D electrocatalyst derived from metal–organic frameworks (MOFs) consisting of OER-active metallic Co4N and ORR active Co–N–C centers, and the resultant SZABs showed a round-trip efficiency of 59%. Fu et al.7 adopted a delicate 3D nano-architectured hair-like catalyst array constructed of 1D nitrogen-doped carbon nanotubes (NCNTs) and 2D mesoporous Co3O4 nanopetals, and the assembled SZABs demonstrated excellent cycling stability and encouraging round-trip efficiencies of up to 62%. Su et al.8 prepared highly active bimetal FeCo nanoparticles encapsulated in in situ grown NCNTs with a bamboo-like structure, which permit the SZABs to cycle at high current densities. In addition, several breakthroughs concerning solid-state electrolytes have been made. Zhang et al.9 reported a laminate-structured nanocellulose/graphene oxide composite membrane coordinated with quaternary ammonium groups that uses dimethyl octadecyl[3-(trimethoxysilyl)-propyl]ammonium chloride (DMAOP) as the functional precursor. This membrane showed a superior ionic conductivity of 58.8 mS cm−1 at 70 °C. Kim et al.10 prepared an electrospun polyvinyl alcohol (PVA) and polyacrylic acid (PAA) nanofiber membrane impregnated with Nafion that had an ionic conductivity of 6.6 mS cm−1 and showed selective hydroxide and Zn(OH)42− ion transport. These recent results highlight the importance of the creation of dual active phases/sites and 3D microstructure design for bifunctional electrocatalysts, as well as the functionalization of the polymer skeleton of the solid electrolyte. Considering previous reports, which have mostly focused on the optimization of a single SZAB component, it is necessary to engineer electrocatalysts and solid electrolytes simultaneously to achieve high efficiencies.
Biomass is an environmentally benign and economically viable carbon source. Biomass derived porous carbon materials display high electrochemical storage properties,11 while appealing for energy storage devices, such as lithium/sodium ion batteries.12,13 Of note, there are few reports utilizing biomass in SZABs. Park et al. used a gelatin based solid state electrolyte for SZABs; however, the electrolyte suffered from poor ionic conductivity (3.1 × 10−3 S cm−1) and the battery did not possess rechargeability.14 Adopting biomass in the preparation of bifunctional electrocatalysts and solid-state electrolytes for SZABs is recommended for more eco-friendly energy applications in the future.
Glucose and methylcellulose are two kinds of biomass derivatives that can be produced in abundance via chemical conversion or enzymatic hydrolysis processes.15,16 In this work, glucose acts as a carbon source to construct a 3D nitrogen-doped carbon framework integrated with Co/Co3O4 nanoparticles (Co/Co3O4/3D-NC) via a simple pyrolysis method. The Co/Co3O4/3D-NC catalyst possesses electrochemically favorable hierarchical pores and homogeneously distributed Co/Co3O4 nanoparticles, and thus it is a promising catalyst with high activity for the ORR and OER, as well as excellent stability in an alkaline electrolyte. Methylcellulose is used to enhance the ionic conductivity and water retention of the PVA gel electrolyte. The assembled SZABs show high flexibility and excellent discharge/charge performances at current densities ranging from 0.1 to 1 mA cm−2.
Results and discussion
The bifunctional electrocatalyst was prepared by a simple thermal pyrolysis step (700 °C) under a N2 atmosphere, as shown in Fig. 1a. Upon the pyrolysis process at relatively low temperature (350–550 °C), urea is converted to 2D graphitic carbon nitride (C3N4),17–19 whereas glucose converts into the form of graphitic carbon.20,21 With the pyrolysis temperature ramping above 700 °C, C3N4 decomposed into NH3 and CxNy, resulting in the formation of thin nitrogen-doped carbon nanosheets (Fig. S1†).22,23 Meanwhile, Co ions are reduced in situ in the form of metallic Co nanoparticles (NPs) uniformly distributed on the surface of carbon nanosheets. The reticulated carbon chains generated on the surface of Co NPs are immediately diffused at the interface between carbon nanosheets and Co NPs.24 Subsequently, the Co NPs lifted off the carbon nanosheets while catalyzing the growth of carbon nanotubes (CNTs) via a ‘‘tip-growth’’ mechanism (Fig. 1b).25 Thus, the 3D nitrogen-doped carbon microstructure was obtained from a one-step pyrolysis process. Finally, the as-prepared Co/3D-NC was partially oxidized in air at 250 °C, resulting in a Co/Co3O4 Schottky junction.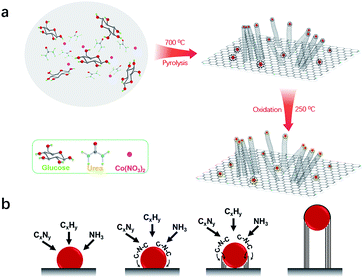 | ||
| Fig. 1 (a) Schematic illustration of the fabrication of the Co/Co3O4/3D-NC catalyst. (b) The growth mechanism of multi-walled carbon nanotubes. | ||
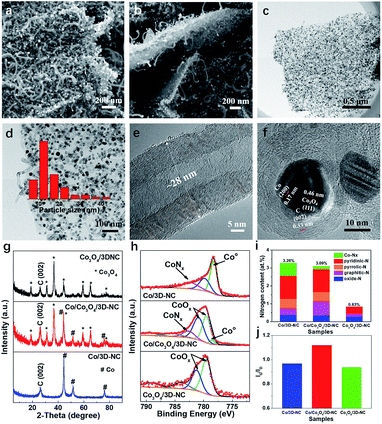
Scanning electron microscopy (SEM) images indicate that the Co/Co3O4/3D-NC has a 3D structure, in which 1D multiwall carbon nanotubes (MCNTs) grew on both sides of thin 2D carbon nanosheets (Fig. 2a and b). Furthermore, transmission electron microscopy (TEM) images (Fig. 2c and d) reveal that abundant Co-related nanoparticles with an average particle size of ∼16 nm were embedded in the nanosheets or the tips of the nanotubes. The MCNTs had 25–30 graphitic layers with a diameter of about 28 nm (Fig. 2e). Moreover, the lattice spacings of 0.17 and 0.46 nm can be ascribed to the (200) and (111) planes of Co and Co3O4, respectively,26,27 confirming the co-existence of metallic Co and Co3O4 NPs (Fig. 2f). Furthermore, the X-ray diffraction (XRD) patterns (Fig. 2g) clearly show the presence of both metallic Co and Co3O4 in the Co/Co3O4/3D-NC catalyst, indicating the partial oxidation of the Co nanoparticles into Co3O4. Increasing the oxidation temperature to 300 °C led to the complete conversion of Co to Co3O4 NPs, as in the Co3O4/3D-NC catalyst. N2 adsorption/desorption measurements were performed to determine the pore diameter distribution of all the samples. A typical type-I pattern with a steep increase at low relative pressures (P/P0 < 0.1) confirms the microporous structure of Co/3D-NC (Fig. S2a†).28 In contrast, a type-IV isotherm with an H3-type hysteresis was observed for the Co/Co3O4/3D-NC and Co3O4/3D-NC samples.29 The Barrett–Joyner–Halenda (BJH) pore distribution revealed the presence of hierarchical meso/macropores of Co/Co3O4/3D-NC, spanning from 5 to 140 nm, as well as diminished microporous features, suggesting the removal of amorphous carbon after air oxidation (Fig. S2b†). Wide-scan X-ray photoelectron spectroscopy (XPS) survey spectra (Fig. S3a†) revealed the existence of carbon, oxygen, nitrogen, and cobalt species in all samples. The atomic percentages of N and C decreased with increasing oxidation temperature from 2 to 0.8 at% and 96.7 to 78.7 at%, respectively, indicating the consumption of carbon upon air oxidation. The high-resolution Co 2p XPS spectrum of Co/Co3O4/3D-NC shows the presence of Co0, Co2+, and Co3+ at binding energies of 778.5, 779.5, and 780.9 eV, respectively, confirming the co-existence of metallic Co and Co3O4 phases (Fig. 2h).30,31 The O 1s spectrum of Co/3D-NC has four peaks at 531, 532.5, 533.8, and 536.3 eV corresponding to –OH, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O
O, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N species, respectively (Fig. S3b†).32,33 The Co/Co3O4/3D-NC and Co3O4/3D-NC catalysts showed an additional peak of metal–oxygen bonding at 530 eV, confirming the formation of Co3O4.26 The high-resolution N 1s XPS spectra of the Co/3D-NC and Co/Co3O4/3D-NC catalysts are divided into five peaks at 398.7 ± 0.3, 399.8 ± 0.1, 400.8 ± 0.3, 401.6 ± 0.1, and 403.9 ± 0.1 eV, attributed to pyridinic-N, Co-Nx, pyrrolic-N, graphitic-N, and oxide-N, respectively (Fig. S3c†).34,35 Note that all N species, except the oxide-N, are potential active sites for oxygen electrocatalysis in the prepared catalysts (Fig. 2i).36–38 The Co/Co3O4/3D-NC sample has the highest graphitic degree (i.e., the ratio of graphitic carbon/disordered carbon (Ig/Id)), 1.12, compared to those of Co/3D-NC and Co3O4/3D-NC (Fig. 2j), which verifies the removal of amorphous carbon during air oxidation. The above characterization results indicate the inherent structural features of Co/Co3O4/3D-NC, including uniformly distributed Co/Co3O4 nanoparticles strongly intercalated with nitrogen-doped carbon, a high graphitization degree, and electrochemically favorable hierarchical pores, which make it an ideal bifunctional electrocatalyst for rechargeable ZABs.
N species, respectively (Fig. S3b†).32,33 The Co/Co3O4/3D-NC and Co3O4/3D-NC catalysts showed an additional peak of metal–oxygen bonding at 530 eV, confirming the formation of Co3O4.26 The high-resolution N 1s XPS spectra of the Co/3D-NC and Co/Co3O4/3D-NC catalysts are divided into five peaks at 398.7 ± 0.3, 399.8 ± 0.1, 400.8 ± 0.3, 401.6 ± 0.1, and 403.9 ± 0.1 eV, attributed to pyridinic-N, Co-Nx, pyrrolic-N, graphitic-N, and oxide-N, respectively (Fig. S3c†).34,35 Note that all N species, except the oxide-N, are potential active sites for oxygen electrocatalysis in the prepared catalysts (Fig. 2i).36–38 The Co/Co3O4/3D-NC sample has the highest graphitic degree (i.e., the ratio of graphitic carbon/disordered carbon (Ig/Id)), 1.12, compared to those of Co/3D-NC and Co3O4/3D-NC (Fig. 2j), which verifies the removal of amorphous carbon during air oxidation. The above characterization results indicate the inherent structural features of Co/Co3O4/3D-NC, including uniformly distributed Co/Co3O4 nanoparticles strongly intercalated with nitrogen-doped carbon, a high graphitization degree, and electrochemically favorable hierarchical pores, which make it an ideal bifunctional electrocatalyst for rechargeable ZABs.
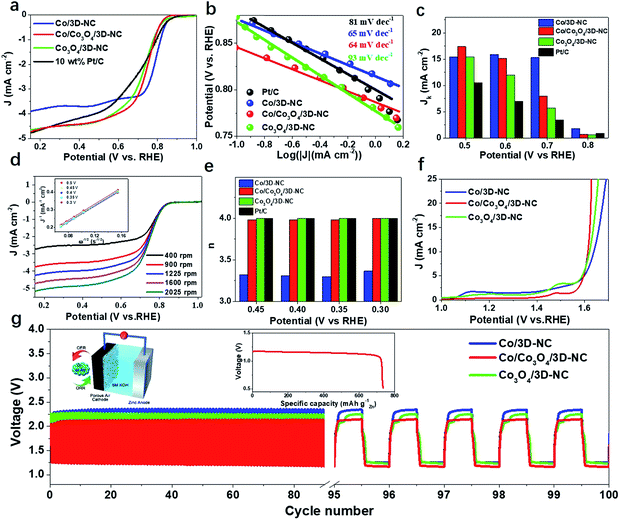
The catalytic activity for the ORR was first explored by linear sweep voltammetry (LSV) in 0.1 M KOH electrolyte with a rotating disk electrode (RDE) setup (Fig. 3a). The Co/Co3O4/3D-NC demonstrated an onset potential of Eonset = 0.86 V (i.e., at 0.1 mA cm−2), a half-wave potential of E1/2 = 0.73 V, and a diffusion-limited current density of JL = 4.71 mA cm−2, better than those of Co/3D-NC (Eonset = 0.88 V, E1/2 = 0.78 V, and JL = 3.90 mA cm−2) and Co3O4/3D-NC (Eonset = 0.87 V, E1/2 = 0.72 V, and JL = 4.65 mA cm−2). The Co/Co3O4/3D-NC sample also shows a better catalytic performance than the commercial benchmark, a 10 wt% Pt/C catalyst, but this is still inferior to that of the 20 wt% Pt/C catalyst (Fig. S4†). The Co/Co3O4/3D-NC exhibited rapid reaction kinetics with the lowest Tafel slope, 64 mV dec−1, compared to those of Co/3D-NC, Co3O4/3D-NC, and 10 wt% Pt/C (Fig. 3b). Fig. 3c confirms that Co/Co3O4/3D-NC exhibits a superior intrinsic kinetic current density of 17.4 mA cm−2 at 0.5 V, which is almost 1.7 times greater than that of the 10 wt% Pt/C catalyst (10.5 mA cm−2). Furthermore, the ORR electron transfer process of Co/Co3O4/3D-NC was studied by RDE experiments at different rotating speeds, from 400 to 2025 rpm, under O2-saturated conditions (Fig. 3d). The corresponding Koutecky–Levich (K–L) plots over the potential range of 0.3–0.5 V exhibit excellent linearity and very similar slopes, suggesting first-order reaction kinetics (Fig. 3d, inset).27 The calculated electron transfer number (n) based on the K–L plots is ∼4.0 (Fig. 3e), suggesting an ideal four-electron ORR pathway.39,40Fig. 3f presents the OER polarization curves obtained from RDE measurements of Co/3D-NC, Co/Co3O4/3D-NC, and Co3O4/3D-NC. Co/Co3O4/3D-NC has a low overpotential (0.39 V@20 mA cm−2) compared to those of Co/3D-NC (0.44 V) and Co3O4/3D-NC (0.41 V). In addition, the smallest Tafel slope (63 mV dec−1) was observed for Co/Co3O4/3D-NC compared to those of Co/3D-NC (128 mV dec−1) and Co3O4/3D-NC (94 mV dec−1) (Fig. S5†). To scrutinize and evaluate the practicability of Co/Co3O4/3D-NC, we assembled a conventional aqueous ZAB using 6 M KOH as it possesses excellent charge–discharge performance with a small voltage gap of 0.82 V, which is lower than those of Co/3D-NC (0.97 V) and Co3O4/3D-NC (0.99 V) samples with no notable change after continuous cycling for 100 cycles (equivalent to 17 h, Fig. 3g). The galvanostatic discharge curves (inset of Fig. 3g) show that the Co/Co3O4/3D-NC based ZAB has a high-energy density of 850 W h kg−1.
A flexible and rechargeable SZAB was fabricated using a Co/Co3O4/3D-NC coated carbon cloth as the air electrode (Fig. 4a). However, besides the ORR/OER performance of the bifunctional catalysts, the chemical and physical properties, especially the ionic conductivities of the solid-state electrolytes are crucial for the round-trip efficiency of SZABs. Therefore, a solid-state elastic polymer electrolyte composed of poly(vinyl alcohol) (PVA) and methylcellulose (Fig. S6†) was developed. Methylcellulose is suitable for use as a synthetic electrolyte membrane because of its large content of hydrophilic hydroxyl groups.41 The hydrophilic phase of the membrane is the ion transport highway and can significantly affect the ionic conductivity.9,41 As shown in Fig. 4b and c, the methylcellulose-modified PVA (M-PVA) membrane displayed better water retention and an enhanced ionic conductivity of 0.39 S cm−1 compared to the conventional PVA electrolyte (0.24 S cm−1). The Co/Co3O4/3D-NC based battery had a high capacity of 8.5 mA h cm−2 with an average voltage of 1.21 V at a current density of 1.0 mA cm−2 (Fig. 4d). The charge–discharge polarization curves and peak power density shown in Fig. 4e confirm the rechargeability and high power density of this battery (37.8 mW cm−2). To investigate the flexibility of the SZAB, it was intentionally bent and twisted into different shapes while connected to a miniature bulb. The lightbulb remained powered during the twisting of the battery, demonstrating its excellent flexibility and durability. In addition, four batteries were connected, and these exhibited a high open-circuit voltage of 5.57 V and could be used to light an LED device (more than 100 small LED lamps) (Fig. 4f). In addition, typical charge–discharge cycling measurements were conducted at different current densities to evaluate the cycling stability (Fig. 4g). The Co/Co3O4/3D-NC based SZAB had a small voltage gap of 0.6 V at a current density of 0.25 mA cm−2. Moreover, the charge–discharge voltage was maintained when the current density increased to 1 mA cm−2. This flexible SZAB showed a superior round-trip efficiency of 75% at a current density of 0.25 mA cm−2 compared to reported SZABs (Fig. 4h).3–6,9,42,43
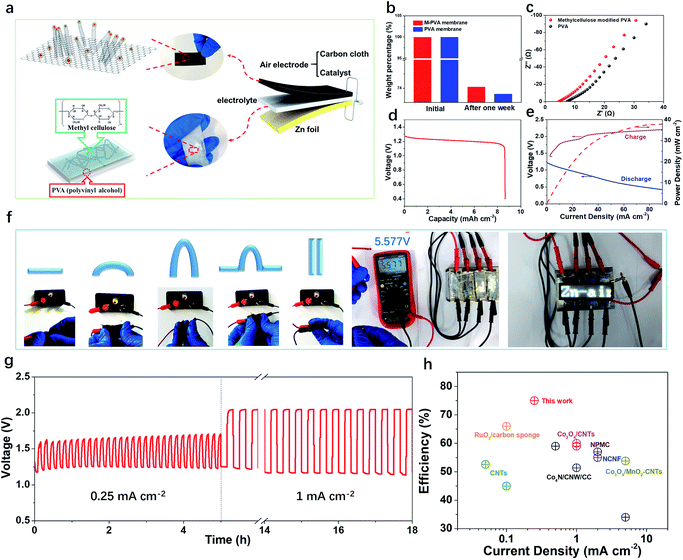 | ||
| Fig. 4 (a) Schematics showing the structure of a SZAB based on Co/Co3O4/3D-NC and M-PVA. (b) The weight percentage of the PVA and M-PVA membrane before and after one week of storage. (c) Alternating current (AC) impedance spectra of the PVA and methylcellulose-modified PVA membranes in the frequency range of 1 MHz to 10 Hz. (d) Discharge capacity of the Co/Co3O4/3D-NC based SZAB. (e) Charge and discharge polarization curves, and power density plots of the Co/Co3O4/3D-NC based SZAB. (f) Photograph of the SZAB powering a miniature bulb under various bending and twisting conditions, and series connected SZABs powering the white LED panel with an open-circuit voltage of 5.57 V. (g) Cycling performance of the SZAB at current densities of 0.25 mA cm−2 and 1 mA cm−2. (h) The charge/discharge efficiency vs. current density of the Co/Co3O4/3D-NC-based SZAB and recently reported SZABs.3–6,9,42,43 | ||
Conclusions
In summary, we developed a scalable and environmentally benign 3D Co/Co3O4/3D-NC oxygen electrode and an M-PVA solid-state polymer electrolyte by facile pyrolysis and polymerization processes. A flexible Zn–air battery assembled using Co/Co3O4/3D-NC oxygen electrodes and M-PVA electrolytes showed outstanding rechargeable performance with a charge–discharge efficiency of 75%. This high round-trip efficiency has been obtained mainly because of the improved oxygen electrochemistry of the catalyst and the enhanced ionic conductivity of the M-PVA. Although the round-trip efficiency at large current densities still requires further improvement, our work highlights the importance of simultaneous engineering of the bifunctional electrocatalyst and polymer electrolyte, and demonstrates the great potential of applying biomass derivatives to SZABs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2017R1A2B3006941). Financial support from the National Natural Science Foundation of China (Grant No. 21403280) is acknowledged.References
- J. Fu, Z. P. Cano, M. G. Park, A. Yu, M. Fowler and Z. Chen, Adv. Mater., 2017, 29, 1604685 CrossRef PubMed.
- J. Park, M. Park, G. Nam, J.-s. Lee and J. Cho, Adv. Mater., 2015, 27, 1396–1401 CrossRef CAS PubMed.
- J. Fu, D. U. Lee, F. M. Hassan, L. Yang, Z. Bai, M. G. Park and Z. Chen, Adv. Mater., 2015, 27, 5617–5622 CrossRef CAS PubMed.
- Y. Xu, Y. Zhang, Z. Guo, J. Ren, Y. Wang and H. Peng, Angew. Chem., Int. Ed., 2015, 54, 15390–15394 CrossRef CAS PubMed.
- X. Wang, J. Gao, Z. Cheng, N. Chen and L. Qu, Angew. Chem., Int. Ed., 2016, 55, 14643–14647 CrossRef CAS PubMed.
- F. Meng, H. Zhong, D. Bao, J. Yan and X. Zhang, J. Am. Chem. Soc., 2016, 138, 10226–10231 CrossRef CAS PubMed.
- J. Fu, F. M. Hassan, J. Li, D. U. Lee, A. R. Ghannoum, G. Lui, M. A. Hoque and Z. Chen, Adv. Mater., 2016, 28, 6421–6428 CrossRef CAS PubMed.
- C.-Y. Su, H. Cheng, W. Li, Z.-Q. Liu, N. Li, Z. Hou, F.-Q. Bai, H.-X. Zhang and T.-Y. Ma, Adv. Energy Mater., 2017, 1602420 CrossRef.
- J. Zhang, J. Fu, X. Song, G. Jiang, H. Zarrin, P. Xu, K. Li, A. Yu and Z. Chen, Adv. Energy Mater., 2016, 6, 1600476 CrossRef.
- H.-W. Kim, J.-M. Lim, H.-J. Lee, S.-W. Eom, Y. T. Hong and S.-Y. Lee, J. Mater. Chem. A, 2016, 4, 3711–3720 CAS.
- L. Yan, J. Yu, J. Houston, N. Flores and H. Luo, Green Energy Environ., 2017, 2, 84–99 CrossRef.
- Y. Li, S. Xu, X. Wu, J. Yu, Y. Wang, Y.-S. Hu, H. Li, L. Chen and X. Huang, J. Mater. Chem. A, 2015, 3, 71–77 CAS.
- J. Ou, Y. Zhang, L. Chen, Q. Zhao, Y. Meng, Y. Guo and D. Xiao, J. Mater. Chem. A, 2015, 3, 6534–6541 CAS.
- J. Park, M. Park, G. Nam, J.-S. Lee and J. Cho, Adv. Mater., 2015, 27, 1396–1401 CrossRef CAS PubMed.
- X. Zhang, P. Murria, Y. Jiang, W. Xiao, H. I. Kenttamaa, M. M. Abu-Omar and N. S. Mosier, Green Chem., 2016, 18, 5219–5229 RSC.
- N. Buchtova, A. Guyomard-Lack and J. Le Bideau, Green Chem., 2014, 16, 1149–1152 RSC.
- J. Liu, T. Zhang, Z. Wang, G. Dawson and W. Chen, J. Mater. Chem., 2011, 21, 14398–14401 RSC.
- Y. Zhang, J. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300–5303 RSC.
- F. Pan, J. Jin, X. Fu, Q. Liu and J. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 11108–11114 CAS.
- H. Jiang, Y. Zhu, Y. Su, Y. Yao, Y. Liu, X. Yang and C. Li, J. Mater. Chem. A, 2015, 3, 12642–12645 CAS.
- X. H. Li, S. Kurasch, U. Kaiser and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 9689–9692 CrossRef CAS PubMed.
- T. Chen, Y. Ma, Q. Guo, M. Yang and H. Xia, J. Mater. Chem. A, 2017, 5, 3179–3185 CAS.
- X. Li, Y. Fang, S. Zhao, J. Wu, F. Li, M. Tian, X. Long, J. Jin and J. Ma, J. Mater. Chem. A, 2016, 4, 13133–13141 CAS.
- S. Helveg, C. Lopez-Cartes, J. Sehested, P. L. Hansen, B. S. Clausen, J. R. Rostrup-Nielsen, F. Abild-Pedersen and J. K. Norskov, Nature, 2004, 427, 426–429 CrossRef CAS PubMed.
- A. Gohier, C. P. Ewels, T. M. Minea and M. A. Djouadi, Carbon, 2008, 46, 1331–1338 CrossRef CAS.
- J. Xiao, C. Chen, J. Xi, Y. Xu, F. Xiao, S. Wang and S. Yang, Nanoscale, 2015, 7, 7056–7064 CAS.
- C. Lin, S. S. Shinde, Z. Jiang, X. Song, Y. Sun, L. Guo, H. Zhang, J.-Y. Jung, X. Li and J.-H. Lee, J. Mater. Chem. A, 2017, 5, 13994–14002 CAS.
- F. Xiaoxiao, Y. Linling, L. Lianghao, Y. Cao, W. Junjie, Y. Xiaokun, C. Jianhua and H. Yongyou, Mater. Res. Express, 2017, 4, 026404 CrossRef.
- G. Fu, Y. Chen, Z. Cui, Y. Li, W. Zhou, S. Xin, Y. Tang and J. B. Goodenough, Nano Lett., 2016, 16, 6516–6522 CrossRef CAS PubMed.
- A. Aijaz, J. Masa, C. Rösler, W. Xia, P. Weide, A. J. R. Botz, R. A. Fischer, W. Schuhmann and M. Muhler, Angew. Chem., Int. Ed., 2016, 55, 4087–4091 CrossRef CAS PubMed.
- W. Xia, R. Zou, L. An, D. Xia and S. Guo, Energy Environ. Sci., 2015, 8, 568–576 CAS.
- V. Shubina, L. Gaillet, S. Ababou-Girard, V. Gaudefroy, T. Chaussadent, F. Farças, T. Meylheuc, C. Dagbert and J. Creus, Appl. Surf. Sci., 2015, 351, 1174–1183 CrossRef CAS.
- Y. Liu, G. Han, X. Zhang, C. Xing, C. Du, H. Cao and B. Li, Nano Res., 2017, 1–14 Search PubMed.
- S. H. Ahn, M. J. Klein and A. Manthiram, Adv. Energy Mater., 2017, 7, 1601979 CrossRef.
- Y.-Z. Chen, C. Wang, Z.-Y. Wu, Y. Xiong, Q. Xu, S.-H. Yu and H.-L. Jiang, Adv. Mater., 2015, 27, 5010–5016 CrossRef CAS PubMed.
- T. Xing, Y. Zheng, L. H. Li, B. C. C. Cowie, D. Gunzelmann, S. Z. Qiao, S. Huang and Y. Chen, ACS Nano, 2014, 8, 6856–6862 CrossRef CAS PubMed.
- W. Xia, A. Mahmood, Z. Liang, R. Zou and S. Guo, Angew. Chem., Int. Ed., 2016, 55, 2650–2676 CrossRef CAS PubMed.
- J. Zhang, Z. Xia and L. Dai, Sci. Adv., 2015, 1, e1500564 Search PubMed.
- Y. Hao, Y. Xu, J. Liu and X. Sun, J. Mater. Chem. A, 2017, 5, 5594–5600 CAS.
- L. Ye, G. Chai and Z. Wen, Adv. Funct. Mater., 2017, 27, 1606190 CrossRef.
- J. Fu, J. Zhang, X. Song, H. Zarrin, X. Tian, J. Qiao, L. Rasen, K. Li and Z. Chen, Energy Environ. Sci., 2016, 9, 663–670 CAS.
- Q. Liu, Y. Wang, L. Dai and J. Yao, Adv. Mater., 2016, 28, 3000–3006 CrossRef CAS PubMed.
- N. Xu, Y. Liu, X. Zhang, X. Li, A. Li, J. Qiao and J. Zhang, Sci. Rep., 2016, 6, 33590 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XRD, SEM, XPS, and N2 sorption isotherms. See DOI: 10.1039/c7se00346c |
| This journal is © The Royal Society of Chemistry 2017 |