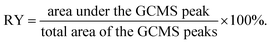Laccase–natural mediator systems for “green” synthesis of phenolic monomers from alkali lignin†
Bin
Yao
 a,
Praveen
Kolla
b,
Ranjit
Koodali
a,
Praveen
Kolla
b,
Ranjit
Koodali
 c,
Selvaratn
Balaranjan
c,
Sunav
Shrestha
c and
Alevtina
Smirnova
*ab
c,
Selvaratn
Balaranjan
c,
Sunav
Shrestha
c and
Alevtina
Smirnova
*ab
aMaterials Engineering and Science Program, South Dakota School of Mines and Technology, Rapid City, SD 57701, USA. E-mail: Alevtina.Smirnova@sdsmt.edu
bChemistry and Applied Biological Sciences Department, South Dakota School of Mines and Technology, Rapid City, SD 57701, USA
cChemistry Department, University of South Dakota, Vermillion, SD 57069, USA
First published on 10th July 2017
Abstract
Being a major byproduct of pulp and paper industry, lignin has attracted attention as a source of high-value organic chemicals, e.g. phenolic monomers that can be produced by lignin enzymatic treatment. In this study, the alkali lignin was treated by the laccase of Trametes versicolor (LTV) and laccase of Myceliophthora thermophila (LMT) laccases with or without natural mediator methyl syringate (MS). After treatment, the lignin pore volume has been increased by 66% and 167% for LTV–MS and LMT–MS systems, respectively. The mass balance analysis confirms that the 5 wt% LTV–5 wt% MS (LTV–MS (5, 5)) system produces 40 wt% more of the organic compounds than the 5 wt% LMT–5 wt% MS (LMT–MS (5, 5)) system, thus demonstrating higher efficiency of the LTV–MS toward lignin decomposition. The gas chromatography-mass spectroscopy (GCMS) analysis indicates that after lignin treatment with LTV–MS more phenolic products are produced in comparison to LMT–MS, among them 3-hydroxy-4-methoxy-benzaldehyde has the highest relative yield of 34.7 and 23.8 wt% for LTV–MS and LMT–MS systems, respectively. Pyrolysis-GCMS (pyr-GCMS) of the solid-state lignin residue after its treatment with laccase–mediator system (LMS) confirms significant enrichment of the solid-state lignin surface with phenolic groups. The total organic carbon (TOC) analysis shows that 38.76 mg L−1 of the organic carbon is produced by the LTV–MS (5, 5) system. A decrease in the decomposition temperature by 4 °C for the lignin sample treated by the LTV–MS (in comparison to LMT–MS) obtained from the thermogravimetric (TGA) analysis confirms superior performance of the LTV–MS vs. LMT–MS system. A comparative study of the enzymatically active systems with two laccases and a natural mediator applied to the alkali lignin has been performed. This study highlights an important role of the laccase in combination with a mediator methyl syringate for production of high-value phenolic monomers.
1. Introduction
Lignin, as highly cross-linked and three-dimensional organic polymer, is the second most abundant in nature following cellulose.1 Due to its complex and rigid structure, lignin is hard to decompose.2 Over 50–60 million tons of lignin are produced annually in the world, most of which come from the black liquor of the paper industry.3 Lignin waste is usually burned resulting in low heat and power energy generation efficiency. Therefore, new approaches for lignin utilization,4 for example production of high value-added products from lignin, are required.5Microbes are known to naturally decompose lignin.6 Among them, white rot fungi is known as the most efficient microbe for lignin degradation that produces three peroxidases7 and laccase enzymes.8 The copper-containing laccase (p-diphenol oxygen oxidoreductase, EC 1.10.3.2) is able to catalyze the one-electron oxidation of phenolic substrates9 that supply electrons to the laccase molecule for the four electron reduction of oxygen to water.10 This process takes place at the four copper centers which contain three kinds of copper, such as T1, T2 and T3.11 One of them, Type I (T1) Cu, plays the dominant role in the lignin oxidation processes and shows a characteristic transfer absorption band of sulfur to Cu(II) at 610 nm.12 However, besides well-known catalytic activity of laccases toward oxidation of the phenolic groups, they cannot oxidize lignin completely.5 One of the reasons is in large size of the laccase molecules, that cannot penetrate lignin pores and oxidize its internal surfaces.13 The second reason is in the redox potential (0.4–0.8 V) of laccases which is not sufficiently high for oxidation of the non-phenolic groups of lignin.14 Consequently, a mediator as an electron carrier, assisting laccase in lignin depolymerization, is considered as a key player in enhancing the system efficiency15 toward synthesis of phenolic compounds.16
Most mediators tested earlier for lignin degradation are artificial compounds based on nitrogen heterocycles.17 For example, the artificial mediator 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)18 was used in combination with laccase for evaluation of its activity in presence of two lignin model compounds (3,4-dimethoxyphenyl)-2-phenylethanediol and 1-(3,4-dimethoxy-phenyl)-2-(2-methoxyphenoxy)-propane-1,3-diol.19 The LMS with artificial 5,5′-hydrazotetrazole (HBT) mediator was investigated for paper pulp delignification and ethanol production from lignocellulose.20 Moreover, the LMS system based on Pycnoporus cinnabarinus laccase and HBT mediator was applied to a delignification bleaching processes in the pulp and paper industry.21
At present, significant attention is paid to the natural mediators which in comparison to the artificial mediators are economically viable and environmentally friendly.22 Among them, methyl syringate as a derivative of guaiacol is considered as a potential natural mediator for lignin degradation.23
Though numerous articles have been published in regard to the enzymatic degradation of lignin by natural24 and artificial16 mediators, this work has a novelty in using two different laccases and detailed analysis of their decomposition products produced in presence of natural mediator methyl syringate.23 For comparison of the lignin decomposition products, two different laccases, specifically LMT25 and LTV26 with the corresponding oxidation potentials of 0.48 V and 0.78 V have been studied. The difference between the two laccases was in their enzymatic activity pertinent to different temperature ranges and pH values, as well as in their electrochemical behavior that was investigated in our previous studies.27,28 Recently, the laccase from Myceliophthora thermophila in combination with methyl syringate was tested for removal of lignin and was demonstrated to enhance saccharification from Eucalyptus feedstock.29
The purpose of this study is in comparison of the ratios of the laccase and mediator and evaluation of the nature of the laccase that can assist in synthesis of the high-value phenolic products from lignin. Specifically, the analysis of the lignin decomposition products in presence of two laccases and a natural mediator methyl syringate is considered. In this study, the alkali lignin degradation efficiency and the reaction products, such as solid and organic liquid phases have been characterized by different techniques, such as SEM, BET, TGA, TOC, GCMS, and pyr-GCMS.
2. Experimental
2.1. Chemicals
In all the experiments, alkali (Kraft) lignin from Sigma-Aldrich (USA) (product # 370959) has been used for enzymatic degradation. Since the company does not provide specific information regarding the lignin properties, NMR and FTIR analysis has been performed (ESI Fig. S1–S2†). Sodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), sodium acetate (NaAc), acetic acid (HAc), and laccase from Trametes versicolor (LTV) (12.9 U per mg) were purchased from Sigma-Aldrich (ESI Fig. S4†). Methyl syringate (MS) mediator and laccase from Myceliophthora thermophila (LMT) (2.8 U per mg) were provided by Novozyme Corp. (Denmark) (ESI Fig. S3†).2.2. Alkali lignin enzymatic treatment and phase separation
The alkali lignin (50 g L−1) was treated with the laccase–MS mediator system at 1, 3, and 5 wt% of each of the two components (laccase and MS) in 37 mL of the buffer solutions, specifically sodium phosphate (pH 6.5) and sodium acetate (pH 4.5) for LMT and LTV, respectively. The enzymatic treatment of the lignin samples was performed in 250 mL three-neck flasks with oxygen gas bubbling through the solution at a constant stirring rate of 800 rpm.The lignin samples were treated in buffer solutions for 72 h at 40 °C and 70 °C for LTV and LMT, respectively. The results of the lignin decomposition in presence of the LMS system were compared with a baseline experiments when the lignin sample was treated under the same reaction conditions, but without mediator.
After treatment, the samples were filtered using a Büchner funnel and washed with 10 mL of dichloromethane (DCM). The pH values of the liquid fractions, controlled by a pH meter, were adjusted to ∼2.5 by addition of 1 wt% HCl. The liquid organic fractions were extracted by DCM (3 times, 20 mL each). For evaluation of the mass balance, the organic liquid phases from three extractions were combined and dried completely by using a rotary evaporator. For GCMS analysis, 1.0 mL DCM and 10 μL of the internal standard were added to each of the dried samples. For pyrolysis-GCMS (pyr-GCMS), the solid lignin residues were dried in the oven at 50 °C for 24 h.
2.3. Scanning electron microscope (SEM) characterization
Before and after LMS treatment, the lignin samples were tested with a Zeiss Supra 40VP variable-pressure field emission SEM. The scanning electron micrographs were recorded under an electron beam acceleration of 1 keV at a working distance of 5 mm using an in-lens detector.2.4. Brunauer–Emmett–Teller (BET) analysis
The BET analysis of the alkali lignin samples was conducted before and after contact with the laccase–mediator systems (5 wt% laccase and 5 wt% MS). The surface morphology of the materials was characterized using multi point N2-physisorption isotherms measured at 77 K by an automated Quantachrome Nova2200e analyzer. Prior to analysis, the degassing of the lignin samples was conducted at 80 °C in N2 overnight.2.5. Gas chromatography-mass spectrometry (GCMS) analysis
After extraction, the samples were tested by GCMS-QP2010 from Shimadzu. The weight of each sample was 1.0 mg and the split mode was applied for all injections with a sampling time of 1 min. Ultra-pure helium (99.999%) was used as a carrier gas with a constant flow rate of 5.8 mL min−1. A 30 m Rxi®-5Sil MS fused silica column (Restek Corporation, Bellefonte, PA, USA) with a 0.25 mm I.D. and a 0.25 μm film thickness was used for experiments. The initial oven temperature at 50 °C was held for 1.0 min. Then it was ramped to 280 °C with a heating rate of 10 °C min−1, ramped again to 320 °C with a heating rate of 20 °C min−1, and held for 5 min. The transfer line and the injector temperatures were 250 and 300 °C, respectively. The total ion current (TIC) mode was applied for MS data with a mass range of 45–500 m/z. The relative yield (RY) of the phenolic products was defined as follows:2.6. Pyrolysis-gas chromatography-mass spectrometry (pyr-GCMS)
pyr-GCMS Shimadzu QP2010 Ultra was equipped with an automatic sampler provided by Frontier. The injection port temperature was set at 250 °C and the furnace operating was at 500 °C. For each sample, 0.1 g of a solid phase was weighed and placed in a sample holder provided by Frontier. For identification of the solid phase products, the National Institute of Standards and Technology (NIST) mass spectral library with a search program was used.2.7. Total organic carbon (TOC) analysis
A total organic carbon (TOC) analyzer from Shimadzu was used for the analysis of the total amount of the organic carbon in a liquid sample containing a mixture of aqueous and organic phases. The TOC analyzer was calibrated using a potassium hydrogen phthalate aqueous solution from 0 mg L−1 to 1000 mg L−1. The original liquid samples were diluted 30 times prior to measurements to obtain the TOC values in the calibration range.2.8. Thermogravimetric analysis (TGA)
The thermogravimetric analysis (TGA) of the samples (0.1 g each) was performed using an SDT Q600 (TA Instruments) analyzer. The measurements were performed in the temperature range from 25 to 800 °C at the heating rate of 10 °C min−1 in air.3. Results and discussion
3.1. Morphological study
The morphological study of lignin oxidation in presence of the LTV–MS and LMT–MS systems was performed by SEM and BET. The SEM analysis reveals the changes in the lignin surface morphology in presence of both LTV–MS and LMT–MS systems (Fig. 1). The surface of the original lignin (Fig. 1A) becomes more porous after treatment with both LTV–MS (Fig. 1B) and LMT–MS (Fig. 1C) systems that correlates with the previous studies.30 However, the difference between the lignin samples treated with LTV–MS (Fig. 1B) or LMT–MS (Fig. 1C) is not visible. The morphological study of lignin after treatment by LTV + MS and LMT + MS systems is similar to the results of lignin after treatment by immobilized laccase–mediator system (ILMS).31 However, after treatment by ILMS the lignin structure is more porous, which correlates with the fact that the TGA-derived temperature of the kraft lignin in this study is higher.The distinction between the morphology of the LTV–MS and LMT–MS treated samples of lignin was confirmed by the comparative BET analysis (Fig. 2, Table 1). The recorded type IV nitrogen adsorption–desorption isotherms (Fig. 2a) is an indication of an interconnected porous mesostructured. The observed hysteresis is due to the nitrogen capillary-condensation phenomenon which is typical for mesoporous materials.
 | ||
| Fig. 2 Comparison of the nitrogen adsorption–desorption isotherms (77 K) of the alkali lignin (a) and the pore size distribution (b) for lignin before and after treatment with LMT–MS and LTV–MS. | ||
| Alkali lignin | Specific surface area (m2 g−1) | Pore volume (cc g−1) |
|---|---|---|
| Before treatment | 4.9 | 0.006 |
| After LMT–MS treatment | 6.1 | 0.01 |
| After LTV–MS treatment | 9.8 | 0.016 |
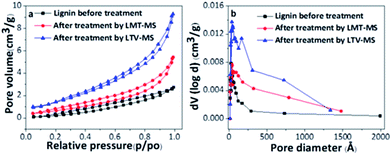
As a result of enzymatic treatment, the specific surface area (SSA) of the lignin was improved to up to 1.2 and 2 times after its treatment with LMT–MS and LTV–MS system, respectively. Compared to the original lignin, the pore volume of the lignin treated with LMT–MS and LVT–MS was 1.6× and 2.6× higher corresponding to the respective ∼66% and ∼167% increase in pore volume.
A comparison of the Pore-Size-Distribution (PSD) for the alkali lignin treated with LMS systems (Fig. 2b) indicates that the pore volume is enhanced due to formation of new mesopores. The results demonstrate that both LMS systems are catalytically active toward lignin degradation, especially the LTV–MS one. This observation is in agreement with the SEM analysis confirming that both LMS systems are involved in the process of lignin degradation.
3.2. GCMS analysis
The GCMS analysis has been used as an analytical tool for quantitative characterization of the phenolic monomers produced from lignin after LMT–MS and LTV–MS treatment. The products detected in the organic phase after lignin degradation are listed in Tables 2 and 3.| Name of the compounda | RTb | m/z | Control sample |
|---|---|---|---|
| a The names of the compounds are based on the identification using the MS NIST library. b Retention time. | |||
| Phenol, 2-methoxy- | 8.88 | 124.00 | |
| 4-Allyl-2-methoxyphenol | 12.85 | 164.08 | |
| 3-Hydroxy-4-methoxy-benzaldehyde | 13.48 | 152.00 | ✓ |
| (E)-2-Methoxy-4-(prop-1-en-1-yl)phenol | 14.13 | 164.08 | |
| Phenol, 2-methoxy-4-propyl- | 14.16 | 166.10 | |
| 1-(4-Hydroxy-3-methoxyphenyl)ethanone | 14.63 | 166.06 | ✓ |
| 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | 15.08 | 180.08 | |
| 2,6-Dimethoxybenzoquinone | 15.46 | 168.04 | |
| Benzeneacetic acid, 4-hydroxy-3-methoxy-, methyl ester | 17.39 | 196.07 | |
| 4-(2-Hydroxyethyl)-2-methoxyphenol | 19.10 | 168.00 | |
| 2-(4-Hydroxy-3-methoxyphenyl)acetic acid | 28.20 | 182.00 | |
| Name of the compounda | RTb | m/z | Control sample |
|---|---|---|---|
| a The names of the compounds are based on the identification using the MS NIST library. b Retention time. | |||
| Phenol, 2-methoxy- | 8.88 | 124.00 | |
| 3-Hydroxy-4-methoxy-benzaldehyde | 13.48 | 152.00 | ✓ |
| 1-(4-Hydroxy-3-methoxyphenyl)ethanone | 14.63 | 166.06 | |
| 4-(2-Hydroxyethyl)-2-methoxyphenol | 19.10 | 168.00 | |
| 2-(4-Hydroxy-3-methoxyphenyl)acetic acid | 28.20 | 182.00 | |
The results of the mass balance (Table 4) demonstrate that the yields of the extractable organics in the aqueous/organic DCM extract increases with the concentrations of laccase and mediator. For example, in case of LTV–MS this increase corresponds to 4.6, 8.4 and 12.3 wt% for LTV–MS (1, 1), LTV–MS (3, 3), and LTV–MS (5, 5) system, respectively.
| LTV | LMT | |||||
|---|---|---|---|---|---|---|
| Laccase (wt%) | 1 | 3 | 5 | 1 | 3 | 5 |
| Yields of extracted organic phase vs. lignin (wt%) | 0.4 | 1.4 | 2.1 | 0.3 | 0.5 | 1.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| LTV–MS ratio (wt%) | LMT–MS ratio (wt%) | |||||
| Laccase–mediator ratio (wt%) | 1–1 | 3–3 | 5–5 | 1–1 | 3–3 | 5–5 |
| Yields of extracted organic phase vs. lignin (wt%) | 4.6 | 8.4 | 12.3 | 3.5 | 5.4 | 8.6 |
The highest amount of the phenolic products was produced from 50 g L−1 of lignin with LTV–MS (5, 5) after the DCM extraction and was 10.2 wt% higher than that for the control sample. The mass balance results also show that for the LTV without MS, only 2.1 wt% of organic compound was extracted by DCM compared to 12.3 wt% when the MS was also present. This result demonstrates an important role of the methyl syringate as a natural mediator in the enzymatic lignin degradation.
The GC-MS chemical analysis of the lignin decomposition products obtained with LTV–MS reveals 11 phenolic monomers, with the four major products being 2-methoxyphenol, 1-(4-hydroxy-3-methoxyphenyl)ethan-1-one, 4-(2-hydroxyethyl)-2-methoxy-phenol, phenol, and 4-methylbenzaldehyde (Table 2). In the control sample, only 1-(4-hydroxy-3-methoxyphenyl)ethanone and 3-hydroxy-4-methoxy-benzaldehyde have been detected. All the detected compounds are in agreement with the internal standard and the NIST library.
In comparison to the LTV–MS, the LMT–MS system produced only five phenolic compounds (Table 3) which is expected from lower enzymatic activity of LMT (2.8 U per mg) vs. LVT (12.9 U per mg). This result of lower LMT enzymatic activity is in correlation with the BET data (Fig. 2).
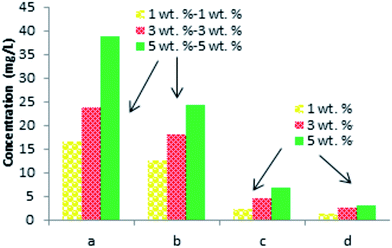
It is necessary to note, that the relative yields of each of the identified phenolic compounds (Tables 2 and 3) increase with the increased weight percent of the laccase–mediator system. The highest yield was observed for the top concentration of the MS mediator (5 wt%) (Fig. 3).
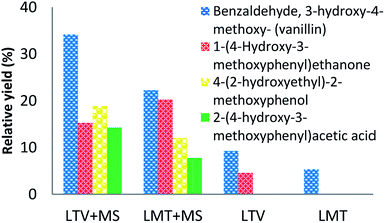 | ||
| Fig. 3 GCMS analysis data of the products formed after lignin treatment with LTV–MS (5, 5) and LMT–MS (5, 5) in comparison to LMT (5 wt%) and LTV (5 wt%) control samples. | ||
Among all the identified phenolic compounds for both laccase–mediator systems and the control samples, the highest relative yield is obtained for 3-hydroxy-4-methoxy-benzaldehyde. Specifically, the LTV–MS and LMT–MS produced 34.7% and 23.8% of the 3-hydroxy-4-methoxy-benzaldehyde, respectively, which is significantly higher in comparison to both LTV (9.3%) and LMT (5.4%) laccase samples without the MS mediator.
The mass balance (Table 4) indicates that the amount of the organic phase in the DCM extract after lignin enzymatic decomposition in presence of LMT–MS increases from 3.5 to 8.6 wt% with the corresponding increase in the concentration of LMT–MS from 1 to 5 wt%. This result demonstrates that the amount of organic compound in DCM phase follows the concentration of both MS mediator and LMT. In case of the LMT without MS, the yield of DCM extractable organics was only 1.3 wt% compared to 8.6 wt% when the LMT–MS was present. In comparison to LMT–MS, the second system showed the highest amount of phenolic compounds produced from the mixture of lignin (50 g L−1) with LTV–MS (5, 5) system. Specifically, 12.3 wt% of phenolic compounds has been produced which is significantly higher than the corresponding value for the control sample without the MS mediator (2.1 wt%).
The chemical analysis of the major lignin depolymerization products confirms that all of them are phenolic monomers (Fig. 3). However, depending on the type of laccase, different number and the amount of the phenolic monomers was produced. Among the identified phenolic derivatives, the highest relative yields are observed for the LTV–MS system. Among the four detected products, the 3-hydroxy-4-methoxy-benzaldehyde was found to be the major compound.
Other minor products are 2-(4-hydroxy-3-methoxyphenyl)acetic acid, 4-(2-hydroxyethyl)-2-methoxyphenol, and 4-hydroxy-benzaldehyde. Among them only 3-hydroxy-4-methoxy-benzaldehyde is observed in the control sample of LMT–MS system.
These results indicate that two laccases (LTV and LMT) have different effects on the number and relative yields of the phenolic monomers. Comparison of the BET and GC-MS results show that LTV has higher efficiency for lignin depolymerization than LMT. In future studies, higher concentration of both laccase and the mediator will be used to obtain higher amount of phenolic compounds.
3.3. Pyrolysis GCMS data of the alkali lignin before and after treatment with LMT–MS and LTV–MS
Pyrolysis of the solid lignin residues produced after lignin treatment with LMT–MS (5, 5) and LTV–MS (5, 5) resulted in a number of phenolic products, such as 2-methoxy-4-methylphenol, 2-methoxy-4-methylphenol, etc. (ESI Table 1†). The highest amount of the phenolic compounds was produced from pyrolysis of the residue of the LTV–MS-treated lignin samples. However, the products for LTV–MS and LMT–MS were different in terms of the concentration and chemical composition of the monomers extracted from the aqueous-organic liquid phase (Tables 2 and 3).3.4. TOC of the liquid samples of alkali lignin after treatment with LMT–MS and LTV–MS systems
The results of the TOC analysis for LTV–MS in comparison to LMT–MS system for different laccase–mediator weight percentages are presented in Fig. 4. It is evident that the total amount of carbon increases with the increased laccase–mediator weight percent. The TOC analysis is in correlation with the GCMS results indicating that the amount of the mediator is important for enhancing lignin decomposition and relative yield of the phenolic compounds. The LTV and LMT in combination with the MS mediator generate significantly higher amounts of the organic carbon in comparison to the control samples without the mediator. The maximum amount of the organic carbon (38.76 mg L−1) was produced for the LTV–MS (5, 5) system which was 70% higher than that for LMT–MS (5, 5).3.5. TGA of the alkali lignin before and after treatment with LMT and LTV
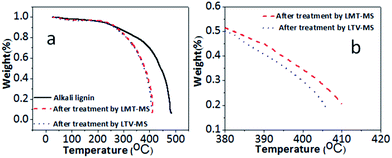
The TGA analysis for LVT–MS and LMT–MS systems in comparison to the original lignin sample are presented in Fig. 5a. According to the TGA analysis, the decomposition of the original lignin starts at 350 °C and at 480 °C almost 80% of lignin is decomposed. Chemical degradation of the lignin after the LMT–MS treatment starts at the same temperature as for the original lignin sample (350 °C), however 80% decomposition is reached at much lower temperature (408 °C).After LTV–MS treatment, the lignin sample has the same start-up thermal decomposition temperature (350 °C), however, 80% decomposition is reached at even lower temperature (395 °C) than for the LMT–MS system (412 °C) (Fig. 5b). This observation is in agreement with the BET, GCMS, and TOC results and confirms that LTV–MS has higher catalytic activity toward lignin degradation in comparison to LMT–MS. Higher degree of lignin enzymatic decomposition leads to a lower thermal decomposition temperature of the solid residue. This decomposition temperature is almost 110 °C higher than the one reported earlier for kraft lignin.32 Since the scan rate, lignin type, and the onset of the decomposition temperature are the same, an assumption was made that these two kinds of kraft lignin have different rigidity at molecular level caused by variation of the synthesis conditions.
4. Conclusion
Lignin degradation by the two laccases from Myceliophthora thermophila and Trametes versicolor in absence and presence of the natural mediator methyl syringate was investigated in terms of morphological, physical, and chemical differences of the liquid organic and solid-state products.An increase in pore volumes and the surface areas of the lignin samples after treatment with LTV–MS and LMT–MS was in correlation with activities of the laccases, being the highest for the LTV.
The GC-MS analysis demonstrates that LTV–MS produces higher amount of 3-hydroxy-4-methoxy-benzaldehyde, 2-(4-hydroxy-3-methoxyphenyl)acetic acid, and 4-(2-hydroxyethyl)-2-methoxyphenol than LMT–MS. The highest amount of organic carbon (38.76 mg L−1) was produced by the LTV–MS (5, 5) emphasizing the important role of the methyl syringe concentration. In order to increase the amount of the phenolic products and the overall system efficiency, more efforts should be committed toward searching for new and more enzymatically active catalytic systems.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the NSF EPSCOR program (Award No. 1330842).Notes and references
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344 Search PubMed.
- F. J. Ruiz-Dueñas and Á. T. Martínez, Microb. Biotechnol., 2009, 2, 164–177 CrossRef PubMed.
- Y. Matsushita, J. Wood Sci., 2015, 61, 230–250 CrossRef CAS.
- G. Cazacu, M. Capraru and V. I. Popa, in Advances in Natural Polymers: Composites and Nanocomposites, ed. S. Thomas, M. P. Visakh and P. A. Mathew, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 255–312, DOI:10.1007/978-3-642-20940-6_8.
- L. P. Christopher, B. Yao and Y. Ji, Front. Energy Res., 2014, 2, 1–13 Search PubMed.
- J. Li, H. Yuan and J. Yang, Front. Biol. China, 2009, 4, 29–38 CrossRef.
- F. Asina, I. Brzonova, K. Voeller, E. Kozliak, A. Kubátová, B. Yao and Y. Ji, Bioresour. Technol., 2016, 220, 414–424 CrossRef CAS PubMed.
- D. S. Wong, Appl. Biochem. Biotechnol., 2009, 157, 174–209 CrossRef CAS PubMed.
- A. M. Mayer and R. C. Staples, Phytochemistry, 2002, 60, 551–565 CrossRef CAS PubMed.
- J.-R. Jeon, P. Baldrian, K. Murugesan and Y.-S. Chang, Microb. Biotechnol., 2012, 5, 318–332 CrossRef PubMed.
- S. Shleev, A. Christenson, V. Serezhenkov, D. Burbaev, A. Yaropolov, L. Gorton and T. Ruzgas, Biochem. J., 2005, 385, 745–754 CrossRef CAS PubMed.
- L. M. Mirica, X. Ottenwaelder and T. D. P. Stack, Chem. Rev., 2004, 104, 1013–1046 CrossRef CAS PubMed.
- H. Zhu, W. Luo, P. N. Ciesielski, Z. Fang, J. Y. Zhu, G. Henriksson, M. E. Himmel and L. Hu, Chem. Rev., 2016, 116, 9305–9374 CrossRef CAS PubMed.
- K. Li, F. Xu and K.-E. L. Eriksson, Appl. Environ. Microbiol., 1999, 65, 2654–2660 CAS.
- P. K. Chaurasia, S. L. Bharati, M. Sharma, S. K. Singh, R. S. S. Yadav and S. Yadava, Curr. Org. Chem., 2015, 19, 1916–1934 CrossRef CAS.
- O. V. Morozova, G. P. Shumakovich, S. V. Shleev and Y. I. Yaropolov, Appl. Biochem. Microbiol., 2007, 43, 523–535 CrossRef CAS.
- S. Camarero, D. Ibarra, M. J. Martínez and Á. T. Martínez, Appl. Environ. Microbiol., 2005, 71, 1775–1784 CrossRef CAS PubMed.
- R. Bourbonnais and M. G. Paice, FEBS Lett., 1990, 267, 99–102 CrossRef CAS PubMed.
- C. Bohlin, K. Lundquist and L. J. Jönsson, Bioorg. Chem., 2009, 37, 143–148 CrossRef CAS PubMed.
- P. Upadhyay, R. Shrivastava and P. K. Agrawal, 3 Biotech, 2016, 6, 1–12 CrossRef PubMed.
- G. Singh, K. Kaur, S. Puri and P. Sharma, Appl. Microbiol. Biotechnol., 2015, 99, 155–164 CrossRef CAS PubMed.
- A. I. Cañas and S. Camarero, Biotechnol. Adv., 2010, 28, 694–705 CrossRef PubMed.
- T. Rosado, P. Bernardo, K. Koci, A. V. Coelho, M. P. Robalo and L. O. Martins, Bioresour. Technol., 2012, 124, 371–378 CrossRef CAS PubMed.
- A. Calcaterra, C. Galli and P. Gentili, J. Mol. Catal. B: Enzym., 2008, 51, 118–120 CrossRef CAS.
- S. Gouveia, C. Fernández-Costas, M. A. Sanromán and D. Moldes, Bioresour. Technol., 2013, 131, 288–294 CrossRef CAS PubMed.
- R. Bourbonnais, M. G. Paice, I. D. Reid, P. Lanthier and M. Yaguchi, Appl. Environ. Microbiol., 1995, 61, 1876–1880 CAS.
- B. Yao, P. Kolla, R. Koodali, Y. Ding, S. Balaranjan, S. Shrestha and A. Smirnova, New J. Chem., 2017, 41, 958–964 RSC.
- B. Yao, P. Kolla, R. Koodali, C.-M. Wu and A. Smirnova, Int. J. Chem., 2016, 8, 56–63 CrossRef CAS.
- A. Rico, J. Rencoret, J. C. del Río, A. T. Martínez and A. Gutiérrez, Biotechnol. Biofuels, 2014, 7, 1–14 CrossRef PubMed.
- S. A. M. Hamed, Int. Biodeterior. Biodegrad., 2013, 78, 98–102 CrossRef CAS.
- J. Hu, W. Du, X. Ji, B. Yuan, Y. Liu and M. Guo, RSC Adv., 2016, 6, 32572–32579 RSC.
- B. Prieur, M. Meub, M. Wittemann, R. Klein, S. Bellayer, G. Fontaine and S. Bourbigot, RSC Adv., 2017, 7, 16866–16877 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00209b |
| This journal is © The Royal Society of Chemistry 2017 |