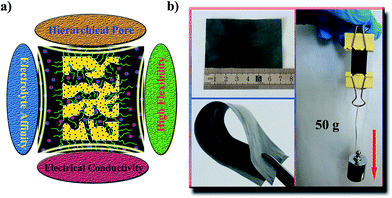
A polymer-supported electrolyte-affinity hybrid membrane and modification of the amphiphilic block copolymer for use as a super-high flexible and high-performance supercapacitor†
Xiaoning
Zhao
,
Yunlong
Yang
,
Jiayu
Wu
,
Yongtao
Tan
,
Ying
Liu
,
Lingbin
Kong
 ,
Long
Kang
and
Fen
Ran
,
Long
Kang
and
Fen
Ran
 *
*
State Key Laboratory of Advanced Processing and Recycling of Non-ferrous Metals, Lanzhou University of Technology, Lanzhou 730050, P. R. China. E-mail: ranfen@163.com; ranfen@lut.cn
First published on 10th April 2017
Abstract
In this study, a super-high flexible membrane electrode (FME) was developed via a facile method based on liquid–liquid phase separation involving the migration and self-assembly of the components. Note that the surface segregation and chain orientation of the amphiphilic block copolymer PAA-b-PAN-b-PAA on the membrane surface during the phase separation process provide the hierarchical porous structure and electrolyte-affinity electrode surface; this hierarchical porous structure provides pathways for the electrolyte ions into and from the electrolyte/solution interface for further contact and reaction of the electrochemically active materials with the electrolyte ions. In a three-electrode system, the specific capacitance of FME-Ni(OH)2 can reach up to 2198.6 F g−1 (769.5 C g−1) at the current density of 0.5 A g−1 from 0 to 0.35 V as compared to that for non-flexible Ni(OH)2 (1588.6 F g−1; 556.0 C g−1). Moreover, a flexible asymmetric supercapacitor with FME-Ni(OH)2 as the positive electrode and FME-AC (commercial activated carbon) as the negative electrode showed the high specific capacitance of 102.2 F g−1 (163.5 C g−1) and the maximum energy density of 36.3 W h kg−1 at the power density of 400 W kg−1; moreover, it retained the energy density of 20.6 W h kg−1 at the high power density of 4000 W kg−1 in the potential window ranging from 0 to 1.6 V in a 6 M KOH aqueous solution.
With the rapid development of portable and wearable electric devices such as mobile phones and flexible displays,1,2 there is an increasing demand for reliable power sources and high performance energy storage devices that have excellent flexibility and are lightweight and safe.3–5 Flexible supercapacitors are one type of important energy storage devices used for smart and wearable electronics that have been successfully prepared in earlier studies.6–8 The greatest difference between flexible and conventional supercapacitors is that the components (such as electrodes, packing shell, current collector, and electrolyte) inside the flexible supercapacitors are flexible.9 In addition, the fabrication of flexible supercapacitors depends on the preparation of highly flexible electrode materials. Recently, flexible electrode materials in thin and flexible films have attracted extensive attention due to their high power density and long cycle life, resulting from their excellent electrical properties and ultra-thinness.10 Particularly, Niu et al. fabricated high-performance SWNT films on stretched polydimethylsiloxane and obtained flexible film electrodes.11 Yu et al. prepared flexible graphene films that could be supported on PET substrates and glass.12 Furthermore, some freestanding carbon films have also been reported to be directly used as flexible supercapacitor electrodes.13–15
To further manufacture supercapacitors with excellent energy output, porosity, and good conductivity flexible electrodes were developed.16,17 A porous structure makes it possible to fabricate high-performance electrodes with a large loading site for the electrochemical materials and fillers in the bulk materials; moreover, the porosity allows fast movement of the electrolyte ions inside the electrodes, which contribute to high power density.9 For instance, Wang et al. deposited NiCo2O4 nanowire arrays on Ni foams via a hydrothermal process. The prepared electrode materials show a super-high capacitance of 2618 F g−1 at the discharging current density of 2 A g−1.18 The high conductivity of the electrodes materials can promote effective rapid transmission of electrons in the bulk materials, which ensures the electrodes with a good rate capability and cycle stability. Particularly, Ghosh et al. synthesized CF-supported Ni(OH)2 composites by growing unique hierarchical flowery Ni(OH)2 on carbon fibers. The CF-Ni(OH)2 composites exhibited a capacitance of up to 789 F g−1 at the current density of 2 A g−1.19,20 Besides the pore structure and good conductivity, electrolyte affinity is another key feature for flexible supercapacitors. In fact, electrolyte affinity on the electrode material surface is quite important for all the electrode processes, and the electrochemical reactions will fail if the electrolyte cannot effectively infiltrate onto the electrode surface, especially the porous surface. To date, it has been reported that the incorporation of heterogeneous atoms,21–23 such as N, O, and P, and surface oxygen-containing acid groups,24 such as –OH and –COOH, are conductive to improving the electrolyte affinity on the electrode material surface, further improving the electrochemical performance.
Herein, we fabricated a flexible membrane electrode (FME) with an electrolyte affinity surface, abundant pores, and good electrical conductivity via a facile method based on the liquid–liquid separation of polyethersulfone (PES), which is commonly used in engineering plastics,25,26 that was used as the substrate material for flexibility. The membrane structure is shown in Fig. 1a. Flower-like Ni(OH)2 particles were used as the active substance for the supercapacitors; acetylene black and conducting graphite were used as the conductive additives, whereas the block copolymer PAA-b-PAN-b-PAA was used as an additive to provide a high electrolyte affinity. For supercapacitors, the FME offers several distinct benefits: (i) the unique porous structure can allow abundant adsorption of electrolyte ions on the surface of the electrode materials during the charge–discharge process; (ii) the electrolyte affinity can greatly decrease the ion transport resistance in the bulk materials, rendering a good rate capability; and (iii) the outstanding flexibility ensures the application of FME as a practical device or cell. Based on these benefits, a super-high flexible asymmetric supercapacitor (FASC) was assembled using a polymer–nickel hydroxide membrane as the positive electrode and a polymer–carbon membrane as the negative electrode. The FASC exhibited super-high electrochemical performance such as high specific capacitance, high energy density, and long cycle stability.
FME involving Ni(OH)2 (FME-Ni(OH)2) was prepared using the polymer phase-separation mechanism, reported in our earlier work,27 with some modifications, and the details can be found in the ESI.† In the casting solution, Ni(OH)2, PES, acetylene black, and conducting graphite were the hydrophobic components, whereas the block copolymer PAA-b-PAN-b-PAA was the hydrophilic component. During phase separation, the hydrophobic components were surrounded by the PES macromolecules. Note that the amphiphilic PAA-b-PAN-b-PAA underwent a migration and self-assembly process during phase separation. With the fast solvent exchange of DMAC and water, PAA-b-PAN-b-PAA migrated to the membrane surface; on the membrane surface, it self-assembled with the hydrophilic block of –PAA directed on the surface of the membrane, whereas the hydrophobic block of –PAN embedded in the membrane substrate after the phase-separation process. Note that this type of membrane surface assembled with an amphiphilic block copolymer showed a high stability during its practical application. In addition, solvent exchange and migration of the block copolymer led to the generation of a porous structure. The prepared FME, as shown in Fig. 1b, was smooth, flat, and flexible with a uniform thickness of 56 μm. The FME membrane could be bent over a large angle, cut into any required shape, and could withstand a large weight of 50 g, demonstrating good mechanical strength (Video 1†). Its outstanding flexibility and mechanical strength can be attributed to the use of PES as the membrane substrate or the so-called polymer binder.
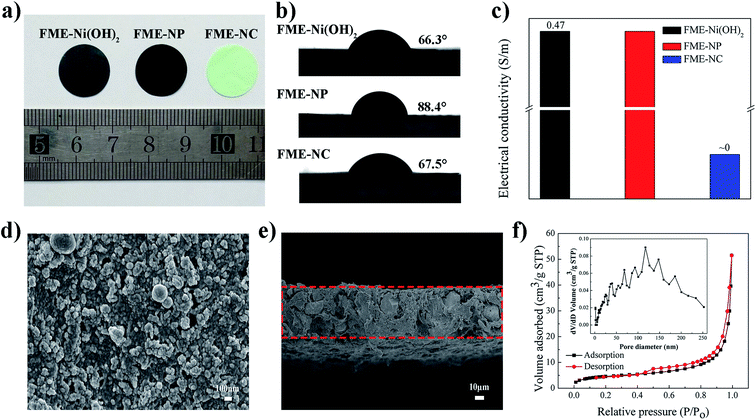
To demonstrate the superiority of the electrode membrane design, we also fabricated an FME without the block copolymer and without the conducting additives, named as flexible membrane electrode no polymer (FME-NP) and flexible membrane electrode no carbon (FME-NC), respectively. As displayed in Fig. 2a, all the designed electrode membranes can be cut into various shapes, such as square, triangular, pentagram, or circular, due to their excellent flexibility; moreover, these membranes can be bent over a large angle and can recover their flat state (Fig. S1 in the ESI†). As shown in Fig. 2b, FME-Ni(OH)2 exhibited a decreased water contact angle value of 66.3° as compared to that of FME-NP (84.4°). It was interesting that FME-NC, in which PAA-b-PAN-b-PAA was blended, also showed a relatively low contact angle of 67.5°, similar to that of FME-Ni(OH)2. These results indicated that the hydrophilicity of the FME-Ni(OH)2 membrane surface was significantly enhanced by the segregation layer of PAA-b-PAN-b-PAA on the surface of the membrane and the orientation arrangement of the –PAA chains, resulted from the migration and self-assembly of the amphiphilic block copolymer. Based on the excellent hydrophilicity of the FME-Ni(OH)2 surface, the electrode membrane of FME-Ni(OH)2 will be easily wetted by the electrolyte during the electrochemical process, showing its good electrolyte affinity. The enrichment of the block copolymer PAA-b-PAN-b-PAA on the membrane surface can be confirmed by X-ray photoelectron spectroscopy (XPS), as shown in Fig. S2 in the ESI.† Moreover, electrical conductivity of the electrode membrane is an important property because PES, as is known, is a type of insulator and, the electrode should be a good conductor. It can be seen that the electrode membrane of FME-NC without blending acetylene black and conducting graphite showed a low value for the electric conductivity, infinitely close to 0. However, both FME-Ni(OH)2 and FME-NP that contain conductive additives in the membranes exhibited an increased electrical conductivity (Fig. 2c). It was apparent that although PES (an insulator) was used to incorporate flexibility into the membrane, the addition of acetylene black and conducting graphite into the flexible membrane composite efficiently enhanced the electrical conductivity.
The structures and properties of FME-Ni(OH)2 were also investigated in detail. The basic structure of the phase-separation membrane of pristine PES was retained in the electrode membrane of FME-Ni(OH)2, as shown in Fig. 2d and e, and S3 in the ESI.† It can also be found that the roughness of the surface of FME increased as compared to that of the pristine membrane due to the migration and self-assembly of the block copolymer and the addition of the active material and conductivity agent (Fig. 2d). Fig. 2e shows the cross-sectional view of FME-Ni(OH)2, the thickness of which was about 56 μm. It should be noted that the thickness of FME-Ni(OH)2 can be easily mediated by adjusting the concentration of the casting solution and the speed of spin coating during the fabrication process of the membranes. Moreover, the active material of Ni(OH)2 particles was uniformly embedded in the electrode membrane, which has been reported in the literature as a type of electrode materials.28,29 The detailed structural characterization of the flower-like Ni(OH)2 nanoparticles can be found in Fig. S4.† Moreover, the structure of Ni(OH)2 in FME-Ni(OH)2 was characterized by TEM (Fig. S5 in the ESI†), which shows the same morphology as that of Ni(OH)2 nanoparticles prepared in the previous step. Note that a large number of pores were formed in the membrane. To further study the porous structure of FME-Ni(OH)2, N2 adsorption–desorption measurements were carried out. From the N2 adsorption–desorption isotherm, it can be seen that the sample exhibited a type-IV isotherm, as shown in Fig. 2f. The pore size distributions were calculated by the BJH method using the adsorption branch of the isotherm, which showed a wide pore-size distribution from micropores to macropores resulting from the exchange of the different types of solvents and the migration of the amphiphilic block copolymer during the phase inversion process.30 The hierarchical porous structure may provide an excellent pathway and large surface area for enhanced electrode/electrolyte interfacial contact during the electrochemical process. As a comparation, the N2 adsorption–desorption measurements of FME-NP and FME-NC were also carried out (Fig. S6 in the ESI†). From the N2 adsorption–desorption isotherms, it can be observed that the two samples exhibited a same type of isotherm (type-IV) with FME-Ni(OH)2. The pore size distributions calculated using the BJH method showed a wide pore-size distribution from micropores to macropores, which were similar to that of FME-Ni(OH)2.
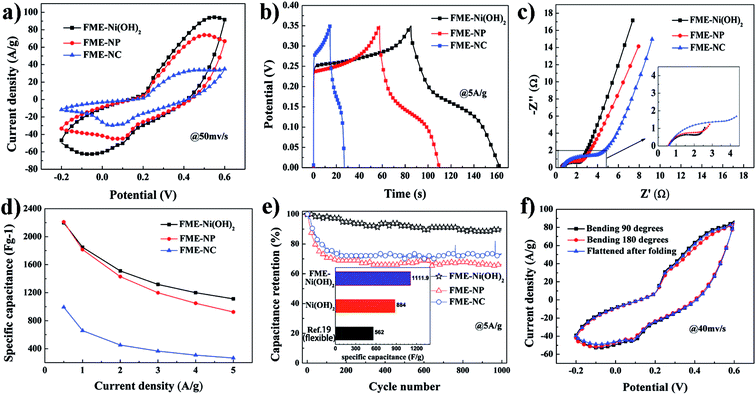
The fabricated FME-Ni(OH)2 exhibits a hierarchical porous structure, high electric conductivity, and super-high electrolyte affinity that will benefit the electrochemical process; moreover, it provides an excellent pathway for the electrolyte ions and enhances the electrode/electrolyte interfacial contact efficiency, in a fast electronic transmission, which is essential for achieving a high rate capability based on the rational design of the electrode membrane. The electrochemical performance measured in a 6 mol L−1 KOH aqueous solution is shown in Fig. 3. The shapes of the cyclic voltammogram (CV) curves (Fig. 3a) for the FME-Ni(OH)2, FME-NP, and FME-NC electrodes tested at the potential range from −0.2 to 0.6 V at the scan rate of 50 mV s−1 were very close. A pair of strong redox peaks was found and was attributed to the reversible redox reaction of Ni(II) ↔ Ni(III), which can be described as: Ni(OH)2 + OH− ↔ NiOOH + H2O + e−.31 These electrochemical behaviors resulted from the active material of Ni(OH)2 embedded in the electrode membrane. The electrochemical performance of the flower-like Ni(OH)2 nanoparticles is also given in Fig. S7 in the ESI.† Obviously, the current of the FME-Ni(OH)2 electrode was much higher than that of FME-NP and FME-NC. Since the specific capacitance was proportional to the area under the CV curves, the FME-Ni(OH)2 electrode showed a much larger charge storage capability than FME-NP and FME-NC.32 All the CV curves for the FME-Ni(OH)2 electrode obtained at the different scan rates ranging from 5 to 50 mV s−1 showed very similar curve shapes with a pair of strong redox peaks, demonstrating the good rate capability of the FME-Ni(OH)2 electrode (Fig. S8a in the ESI†). The galvanostatic charge–discharge (GCD) curves (Fig. 3b) obtained within the potential window from 0 to 0.35 V at the current density of 5 A g−1 showed that the charge and discharge time of FME-Ni(OH)2 was much longer than that of FME-NP and FME-NC; this implies that the specific capacitance of FME-Ni(OH)2 was significantly higher than that of FME-NP and FME-NC. Via calculation, the specific capacitance of FME-Ni(OH)2, FME-NP, and FME-NC was found to be 1111.9, 924.0, and 267.3 F g−1 (389.2, 323.4, and 93.6 C g−1), respectively. All the nonlinear charge–discharge curves of the FME-Ni(OH)2 showed that the discharge curves were almost symmetrical to their corresponding charge curves, demonstrating a good electrochemical reversibility (Fig. S8b in the ESI†). As calculated from the discharge curves according to the equation C = I × t/(ΔV × m), Cm = I × t/ΔV,33 the specific capacitances were 2198.6, 1849.1, 1510.9, 1318.3, 1200.0, and 1111.9 F g−1 (769.5, 647.2, 528.8, 461.4, 420.0, and 389.2 C g−1), respectively. The electrochemical impedance spectroscopy curves of the electrodes, as shown in Fig. 3c, exhibit a similar shape composed of one semicircle at high-frequency and a linear component at low frequency. The diameters of the semicircles in the high frequency region for the FME-Ni(OH)2 and FME-NP are similar, which are smaller than those of FME-NC. This indicated lower charge-transfer resistance (RCT) of FME-Ni(OH)2 and FME-NP than that of FME-NC, which was attributed to the addition of the conductive agent. Moreover, at lower frequencies, the slope of the straight line represented diffusive resistance (Warburg impedance, W0) of the electrode/electrolyte interfaces. The curves of FME-Ni(OH)2 and FME-NC showed a larger slope than that of FME-NP, indicating lower diffusion resistance of FME-Ni(OH)2 and FME-NC due to large number of –PAA chains as hydrophilic polymeric brushes formed on the surface layer of the membrane. In addition, the equivalent circuit of the samples is shown in Fig. S14 in the ESI;† the detailed values of RS, RCT, and W0 are summarized in Table S1 in the ESI.†Fig. 3d shows the specific capacitance of different composites measured at different current densities; the detailed data are summarized in Table S2 in the ESI.† The specific capacitance of FME-Ni(OH)2 at the current density of 0.5 A g−1 was as high as 2198.6 F g−1 (769.5 C g−1) and even retained 1111.9 F g−1 (389.2 C g−1) when the current density was increased to 10 times its initial value (5 A g−1). The capacitance retention from 0.5 A g−1 to 5 A g−1 for FME-Ni(OH)2 was 50.6%, indicating an excellent rate capability. In contrast, the capacitance retentions for FME-NP and FME-NC were 41.8% and 26.9%, respectively. The cyclic stability of the electrodes is another important property for their application in electrochemical supercapacitors. To evaluate the cycling stability of different composites, the charge–discharge cycling tests were performed at the high current density of 5 A g−1 for 1000 cycles. As shown in Fig. 3e, after 1000 cycles, the capacitance retention of FME-Ni(OH)2 was 89.7%, which was far higher than that of FME-NP (66.1%) and FME-NC (73.5%). Fig. S9 in the ESI† displays the coulombic efficiency of different membrane electrodes and all the values were near 100% during the tests, demonstrating excellent electrochemical reversibility. Interestingly, the specific capacitance of FME-Ni(OH)2 (1111.9 F g−1, 389.2 C g−1) was much higher than that of pristine flower-like Ni(OH)2 at the current density of 5 A g−1 (884 F g−1, 309.4 C g−1) (inset in Fig. 3e). This supercapacitance performance was also superior to that of the Ni(OH)2-based flexible electrode membrane previously reported in the literature (562 F g−1).19 The porous and electrolyte-affinity membrane structure was beneficial to the fast faradic reaction, which enabled a high specific capacitance. Based on the results, the excellent electrochemical behavior of FME-Ni(OH)2 was obtained, which was ascribed to the rational design of the membrane electrode and the unique membrane structure.
As a flexible electrode membrane, note that the electrochemical behavior of FME-Ni(OH)2 did not show any obvious changes in the CV curves after bending to 90° and 180°, and then flattening at the scan rate of 40 mV s−1, as shown in Fig. 3f. The electrochemical impedance spectroscopy curves of FME-Ni(OH)2 at different bending states were also tested (Fig. S10 in the ESI†). No obvious changes in the curves can be found, which indicate the good conductive properties of the hybrid membrane at all the bending states. The excellent stability of the electrochemical performance can be ascribed to the membrane substrate PES. Thus, this type of flexible membrane electrode can be used in constantly bending environments.
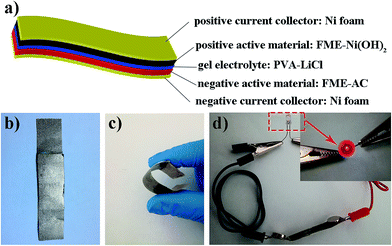
In all-solid-state SCs, the polymer gel electrolyte combines the functions of an electrolyte and a separator, reducing the thickness and weight of the SCs when compared with those of their liquid counterparts and simplifies the fabrication process of the devices.34,35 A schematic of the fabrication of an all-solid-state FASC is shown in Fig. 4a; the all-solid-state FASC was comprised of a current collector (Ni foam), positive electrode (FME-Ni(OH)2), negative electrode (FME-AC), and gel electrode (PVA-LiCl); an optical image of the prepared FASC is also shown in Fig. 4b. Fig. 4c shows a curved FASC, which can be bent to any angle, exhibiting excellent flexibility. Finally, to demonstrate the practical application of our FASC, a red LED light was powered by one FASC, as shown in Fig. 4d. All these results suggest that the FASC prepared herein can be expected to be an extremely promising candidate for wearable and flexible energy storage devices.
 | ||
| Fig. 4 (a) A schematic of the fabrication of the FME-Ni(OH)2//FME-AC FASC device, (b and c) optical images of the prepared FASC device, and (d) a powered red LED. | ||
For further assembling the FME-Ni(OH)2-based asymmetric supercapacitor (ASC), a flexible FME involving active carbon as the electroactive material (FME-AC) in the electrode membrane was also fabricated according to the same preparation procedure using phase-separation. FME-Ni(OH)2 was used as the positive electrode and the FME-AC as the negative electrode to assemble the asymmetric supercapacitor, i.e., FME-Ni(OH)2//FME-AC ASC. The electrochemical performance of FME-AC is shown in Fig. S11 in the ESI† and that of the FME-Ni(OH)2//FME-AC ASC device was tested in a 6 M KOH aqueous electrolyte, as shown in Fig. 5. For comparison, the symmetric supercapacitor based on FME-Ni(OH)2 was also assembled (Fig. S12 in ESI†) and the specific capacitance was very low. To construct a supercapacitor with high operating voltage and high energy density, it was important to balance the charges stored at the positive electrode (Q+) and the negative electrode (Q−), and the mass ratio of the positive and negative electrodes were calculated using the equation: m+/m− = (C− × ΔV−)/(C+ × ΔV+), where m is the mass, C is the specific capacitance, and ΔV is the potential drop of the positive or negative electrode during the discharge process. In this study, the calculated optimal mass ratio of FME-Ni(OH)2 and FME-AC was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
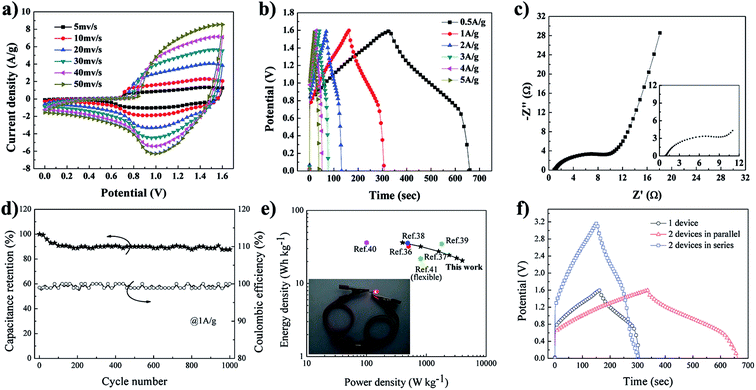
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The CV curves obtained for FME-Ni(OH)2//FME-AC ASC showed a large potential window up to 1.6 V at the various scan rates, as shown in Fig. 5a. Obviously, the broad redox peaks were observed, implying the pseudocapacitive nature of the device derived from the FME-Ni(OH)2 electrode. Fig. 5b depicts the GCD curves obtained for FME-Ni(OH)2//FME-AC ASC at different current densities ranging from 0.5 to 5 A g−1. All the discharge curves were nearly symmetrical to their corresponding charging counterparts, indicating its good electrochemical reversibility. In addition, no obvious voltage drop was observed in all the discharge curves, demonstrating the low internal resistance of the device. From the discharge time, the specific capacitance values of FME-Ni(OH)2//FME-AC ASC were calculated based on the total mass of the active materials of the two electrodes with a high capacitance of 102.2 F g−1 (163.5 C g−1) at the current density of 0.5 A g−1. The detailed capacitance performance is summarized in Table S2 in the ESI.†Fig. 5c presents the electrochemical impedance spectroscopy curve obtained for FME-Ni(OH)2//FME-AC ASC over the frequency range from 10.0 kHz to 10.0 mHz. The small semicircle indicates low charge transfer resistance (RCT = 4.569 Ω) and the vertical line demonstrates low diffusive resistance (W0 = 0.146 Ω) of the device. The equivalent circuit and the resistance values are shown in Fig. S14 and Table S1 in the ESI,† respectively. The long-term cycle life and coulombic efficiency of FME-Ni(OH)2//FME-AC ASC were investigated by charge–discharge cycling at the current density of 1 A g−1. As shown in Fig. 5d, after 1000 charge–discharge cycles, the capacitance retention of FME-Ni(OH)2//FME-AC ASC remained 88.8% (82.7% after 5000 cycles, as shown in Fig. S13a in the ESI†), indicating the good cycling stability of the device. Moreover, the flexibility of the device was further tested by repeated bending to 180° for 500 cycles, and only a small decrease in the capacitance was observed (Fig. S13b†). The coulombic efficiency remained about 98% during the test, demonstrating its excellent electrochemical reversibility. The Ragone plot is shown in Fig. 5e, in which the energy density was plotted versus the power density. Note that the energy of FME-Ni(OH)2//FME-AC ASC can reach 36.3 W h kg−1 at the power density of 400 W kg−1, and still remained 20.6 W h kg−1 at the high power density of 4000 W kg−1. This value surpassed those of many previously reported ASCs (Table S3 in the ESI†) such as Ni(OH)2/AC/CNT//AC (32.3 W h kg−1 at 504.8 W kg−1),36 Ni(OH)2 nanosheets//AC (22 W h kg−1 at 800 W kg−1),37 Ni(OH)2//AC (35.7 W h kg−1 at 490 W kg−1),38 CNT/Ni(OH)2//rGO (35 W h kg−1 at 1800 W kg−1),39 and β-Ni(OH)2/Ni-foam//AC (36.2 W h kg−1 at 100.6 W kg−1),40 which were not flexible devices. This result was also superior to that obtained for the flexible ASCs based on Ni(OH)2 (18 W h kg−1 at 850 W kg−1)41 and some based on nanocarbon materials.12,15,42 Due to the limited working potential and work current of one single ASC, using serial or multipled assemblies will be a facile way to extend their applications. Fig. 5f presents the GCD curves for a single ASC device and two devices connected in series and parallel, obtained at the same current. The two ASC devices connected in parallel showed the same voltage with a double charge/discharge time when compared with that of a single ASC; moreover, the two ASC devices connected in series showed a 3.2 V charge/discharge voltage with a similar charge/discharge time when compared with that of the single ASC, following the theorem of series connections of capacitors. As a practical application of the FME-Ni(OH)2//FME-AC ASC, one red LED (at the working voltage of 3 V) was powered by two ASCs in series after charging to 3.2 V (the inset in Fig. 5e).
3. The CV curves obtained for FME-Ni(OH)2//FME-AC ASC showed a large potential window up to 1.6 V at the various scan rates, as shown in Fig. 5a. Obviously, the broad redox peaks were observed, implying the pseudocapacitive nature of the device derived from the FME-Ni(OH)2 electrode. Fig. 5b depicts the GCD curves obtained for FME-Ni(OH)2//FME-AC ASC at different current densities ranging from 0.5 to 5 A g−1. All the discharge curves were nearly symmetrical to their corresponding charging counterparts, indicating its good electrochemical reversibility. In addition, no obvious voltage drop was observed in all the discharge curves, demonstrating the low internal resistance of the device. From the discharge time, the specific capacitance values of FME-Ni(OH)2//FME-AC ASC were calculated based on the total mass of the active materials of the two electrodes with a high capacitance of 102.2 F g−1 (163.5 C g−1) at the current density of 0.5 A g−1. The detailed capacitance performance is summarized in Table S2 in the ESI.†Fig. 5c presents the electrochemical impedance spectroscopy curve obtained for FME-Ni(OH)2//FME-AC ASC over the frequency range from 10.0 kHz to 10.0 mHz. The small semicircle indicates low charge transfer resistance (RCT = 4.569 Ω) and the vertical line demonstrates low diffusive resistance (W0 = 0.146 Ω) of the device. The equivalent circuit and the resistance values are shown in Fig. S14 and Table S1 in the ESI,† respectively. The long-term cycle life and coulombic efficiency of FME-Ni(OH)2//FME-AC ASC were investigated by charge–discharge cycling at the current density of 1 A g−1. As shown in Fig. 5d, after 1000 charge–discharge cycles, the capacitance retention of FME-Ni(OH)2//FME-AC ASC remained 88.8% (82.7% after 5000 cycles, as shown in Fig. S13a in the ESI†), indicating the good cycling stability of the device. Moreover, the flexibility of the device was further tested by repeated bending to 180° for 500 cycles, and only a small decrease in the capacitance was observed (Fig. S13b†). The coulombic efficiency remained about 98% during the test, demonstrating its excellent electrochemical reversibility. The Ragone plot is shown in Fig. 5e, in which the energy density was plotted versus the power density. Note that the energy of FME-Ni(OH)2//FME-AC ASC can reach 36.3 W h kg−1 at the power density of 400 W kg−1, and still remained 20.6 W h kg−1 at the high power density of 4000 W kg−1. This value surpassed those of many previously reported ASCs (Table S3 in the ESI†) such as Ni(OH)2/AC/CNT//AC (32.3 W h kg−1 at 504.8 W kg−1),36 Ni(OH)2 nanosheets//AC (22 W h kg−1 at 800 W kg−1),37 Ni(OH)2//AC (35.7 W h kg−1 at 490 W kg−1),38 CNT/Ni(OH)2//rGO (35 W h kg−1 at 1800 W kg−1),39 and β-Ni(OH)2/Ni-foam//AC (36.2 W h kg−1 at 100.6 W kg−1),40 which were not flexible devices. This result was also superior to that obtained for the flexible ASCs based on Ni(OH)2 (18 W h kg−1 at 850 W kg−1)41 and some based on nanocarbon materials.12,15,42 Due to the limited working potential and work current of one single ASC, using serial or multipled assemblies will be a facile way to extend their applications. Fig. 5f presents the GCD curves for a single ASC device and two devices connected in series and parallel, obtained at the same current. The two ASC devices connected in parallel showed the same voltage with a double charge/discharge time when compared with that of a single ASC; moreover, the two ASC devices connected in series showed a 3.2 V charge/discharge voltage with a similar charge/discharge time when compared with that of the single ASC, following the theorem of series connections of capacitors. As a practical application of the FME-Ni(OH)2//FME-AC ASC, one red LED (at the working voltage of 3 V) was powered by two ASCs in series after charging to 3.2 V (the inset in Fig. 5e).
In summary, a super-high performance asymmetric supercapacitor device consisting of FME-Ni(OH)2 as the positive electrode and FME-AC as the negative electrode has been developed. Due to the well-designed individual electrode of FME, the FME-Ni(OH)2//FME-AC ASC presented a maximum energy density of 36.3 W h kg−1 at the power density of 400 W kg−1. After 1000 cycles, the capacitance retention can reach up to 88.8% of the initial capacitance value. These outstanding electrochemical properties of the ASC can be attributed to the porous structure, good conductivity, and excellent electrolyte affinity of the electrode membrane prepared based on the liquid–liquid phase separation of hydrophobic components and the migration and self-assembly of an amphiphilic block copolymer. The flexible electrode membrane prepared via the convenient, fast, and low-cost method exhibited comparable electrochemical performance, providing the possibility for practical applications in wearable and flexible energy storage devices.
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (51203071, 51363014, and 51463012), China Postdoctoral Science Foundation (2014M552509, and 2015T81064), Natural Science Funds of the Gansu Province (2015GS05123), and the Program for Hongliu Distinguished Young Scholars in Lanzhou University of Technology (J201402).References
- B. D. Gates, Materials science. Flexible electronics, Science, 2009, 323, 1566–1567 CrossRef CAS PubMed.
- S. Park, M. Vosguerichian and Z. A. Bao, A review of fabrication and applications of carbon nanotube film-based flexible electronics, Nanoscale, 2013, 5, 1727–1752 RSC.
- S. Kim, H. J. Kwon, S. Lee, H. Shim, Y. Chun, W. Choi, J. Kwack, D. Han, M. Song, S. Kim, S. Mohammadi, I. Kee and S. Y. Lee, Low-power flexible organic light-emitting diode display device, Adv. Mater., 2011, 23, 3511–3516 CrossRef CAS PubMed.
- M. Koo, K. I. Park, S. H. Lee, M. Suh, D. Y. Jeon, J. W. Choi, K. Kang and K. J. Lee, Bendable inorganic thin-film battery for fully flexible electronic systems, Nano Lett., 2012, 12, 4810–4816 CrossRef CAS PubMed.
- S. Shi, C. Xu, C. Yang, J. Li, H. D. Du, B. H. Li and F. Y. Kang, Flexible supercapacitors, Particuology, 2012, 11, 371–377 CrossRef.
- Y. Yang, H. Wang, R. Hao and L. Guo, Transition-metal-free biomolecule-based flexible asymmetric supercapacitors, Small, 2016, 12, 4683–4689 CrossRef CAS PubMed.
- G. K. Veerasubramani, K. Krishnamoorthy, P. Pazhamalai and S. J. Kim, Enhanced electrochemical performances of graphene based solid state flexible cable type supercapacitor using redox mediated polymer gel electrolyte, Carbon, 2016, 105, 638–648 CrossRef CAS.
- S. T. Senthilkumar, J. S. Kim, Y. Wang, H. T. Huang and Y. S. Kim, Flexible and wearable fiber shaped high voltage supercapacitors based on copper hexacyanoferrate and porous carbon coated carbon fiber electrodes, J. Mater. Chem. A, 2016, 4, 4934–4940 CAS.
- L. B. Dong, C. J. Xu, Y. Li, Z. H. Huang, F. Y. Kang, Q. H. Yang and X. Zhao, Flexible electrodes and supercapacitors for wearable energy storage: a review by category, J. Mater. Chem. A, 2016, 4, 4659–4685 CAS.
- K. Q. Qin, J. L. Kang, J. J. Li, C. S. Shi, Y. X. Li, Z. J. Qiao and N. Q. Zhao, Free-standing porous carbon nanofiber/ultra-thin graphite hybrid for flexible solid-state supercapacitors, ACS Nano, 2015, 1, 481–487 CrossRef PubMed.
- Z. Q. Niu, H. B. Dong, B. W. Zhu, J. Z. Li, H. H. Hng, W. Y. Zhou, X. D. Chen and S. S. Xie, Highly stretchable, integrated supercapacitors based on single-walled carbon nanotube films with continuous reticulate architecture, Adv. Mater., 2013, 7, 1058–1064 CrossRef PubMed.
- A. P. Yu, I. Roes, A. Davies and Z. W. Chen, Ultrathin, transparent, and flexible graphene films for supercapacitor application, Appl. Phys. Lett., 2010, 25, 253105 CrossRef.
- Y. M. He, W. J. Chen, X. D. Li, Z. X. Zhang, J. C. Fu, C. H. Zhao and E. Q. Xie, Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes, ACS Nano, 2012, 1, 174–182 Search PubMed.
- B. Liang, L. Fang, Y. C. Hu, G. Yang, Q. Zhu and X. S. Ye, Bioinspired effective prevention of restacking in multilayered graphene films: towards the next generation of high-performance supercapacitors, Adv. Mater., 2011, 25, 2833–2838 Search PubMed.
- Y. M. Wang, J. C. Chen, J. Y. Cao, Y. Liu, Y. Zhou, J. H. Ouyang and D. C. Jia, Graphene/carbon black hybrid film for flexible and high rate performance supercapacitor, J. Power Sources, 2014, 271, 269–277 CrossRef CAS.
- K. Jost, G. Dion and Y. Gogotsi, Textile energy storage in perspective, J. Mater. Chem. A, 2014, 28, 10776–10787 Search PubMed.
- F. J. Miao, C. L. Shao, X. H. Li, K. X. Wang and Y. C. Liu, Flexible solid-state supercapacitors based on freestanding nitrogen-doped porous carbon nanofibers derived from electrospun polyacrylonitrile@polyaniline nanofibers, J. Mater. Chem. A, 2016, 4, 4180–4187 CAS.
- Q. F. Wang, X. F. Wang, B. Liu, G. Yu, X. J. Hou, D. Chen and G. Z. Shen, NiCo2O4 nanowire arrays supported on Ni foam for high-performance flexible all-solid-state supercapacitors, J. Mater. Chem. A, 2013, 7, 2468–2473 Search PubMed.
- D. Ghosh, M. Mandal and C. K. Das, Solid state flexible asymmetric supercapacitor based on carbon fiber supported hierarchical Co(OH)xCO3 and Ni(OH)2, Langmuir, 2015, 31, 7835–7843 CrossRef CAS PubMed.
- R. Shah, X. F. Zhang and S. K. Talapatra, Electrochemical double layer capacitor electrodes using aligned carbon nanotubes grown directly on metals, Nanotechnology, 2009, 39, 844–847 Search PubMed.
- W. Li, F. Zhang, Y. Dou, Z. Wu, H. Liu and X. Qian, A self-template strategy for the synthesis of mesoporous carbon nanofibers as advanced supercapacitor electrodes, Adv. Energy Mater., 2011, 1, 382–386 CrossRef CAS.
- J. Zhao, H. Lai and Z. Lyu, Hydrophilic Hierarchical Nitrogen–Doped Carbon Nanocages for Ultrahigh Supercapacitive Performance, Adv. Mater., 2015, 27, 3541 CrossRef CAS PubMed.
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie and G. O. Shitta-Bey, Energy storage in electrochemical capacitors: designing functional materials to improve performance, Energy Environ. Sci., 2010, 3, 1238–1251 CAS.
- M. P. Bichat, E. Raymundo-Pinero and F. Béguin, High voltage supercapacitor built with seaweed carbons in neutral aqueous electrolyte, Carbon, 2010, 48, 4351–4361 CrossRef CAS.
- H. M. Song, F. Ran, H. L. Fan, X. Q. Niu, L. Kang and C. S. Zhao, Hemocompatibility and ultrafiltration performance of surface-functionalized polyethersulfone membrane by blending comb-like amphiphilic block copolymer, J. Membr. Sci., 2014, 471, 319–327 CrossRef CAS.
- F. Ran, S. Q. Nie, W. F. Zhao, J. Li, B. H. Su, S. D. Sun and C. S. Zhao, Biocompatibility of modified polyethersulfone membranes by blending an amphiphilic triblock co-polymer of poly(vinyl pyrrolidone)-b-poly(methyl methacrylate)-b-poly(vinyl pyrrolidone), Acta Biomater., 2011, 7, 3370–3381 CrossRef CAS PubMed.
- J. W. Lang, L. B. Kong, W. J. Wu, M. Liu, Y. C. Luo and L. Kang, A facile approach to the preparation of loose-packed Ni(OH)2 nanoflake materials for electrochemical capacitors, J. Solid State Electrochem., 2009, 2, 333–340 CrossRef.
- J. Y. Ji, L. L. Zhang, H. X. Ji, Y. Li, X. Zhao, X. Bai, X. B. Fan, F. B. Zhang and R. S. Ruoff, Nanoporous Ni(OH)2 thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
- Q. Q. Ke, M. R. Zheng, H. J. Liu, C. Guan, L. Mao and J. Wang, 3D TiO2@Ni(OH)2 core–shell arrays with tunable nanostructure for hybrid supercapacitor application, Sci. Rep., 2015, 5, 13940–13951 CrossRef PubMed.
- F. Ran, K. W. Shen, Y. T. Tan, B. W. Peng, S. H. Chen, W. J. Zhang, X. Q. Niu, L. B. Kong and L. Kang, Activated hierarchical porous carbon as electrode membrane accommodated with triblock copolymer for supercapacitors, J. Membr. Sci., 2016, 514, 366–375 CrossRef CAS.
- Z. Tang, C. H. Tang and H. Gong, A high energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/carbon nanotube electrodes, Adv. Funct. Mater., 2012, 6, 1272–1278 CrossRef.
- K. T. Cho, S. B. Lee and J. W. Lee, Facile synthesis of highly electrocapacitive nitrogen-doped graphitic porous carbons, J. Phys. Chem. C, 2014, 118, 9357–9367 CAS.
- Y. T. Tan, F. Ran and L. B. Kong, Polyaniline nanoparticles grown on the surface of carbon microspheres aggregations for electrochemical supercapacitors, Synth. Met., 2012, 162, 114–118 CrossRef CAS.
- K. Lu, J. T. Zhang, Y. Q. Wang, J. Z. Ma, B. Song and H. Y. Ma, Interfacial Deposition of Three-Dimensional Nickel Hydroxide Nanosheet-Graphene Aerogel on Ni Wire for Flexible Fiber Asymmetric Supercapacitors, ACS Sustainable Chem. Eng., 2017, 5, 821–827 CrossRef CAS.
- W. J. Liu, N. S. Liu, Y. L. Shi, Y. Chen and C. X. Yang, A wire-shaped flexible asymmetric supercapacitor based on carbon fiber coated with a metal oxide and a polymer, J. Mater. Chem. A, 2015, 3, 13461–13467 CAS.
- L. P. Sui, S. H. Tang, Y. D. Chen, Z. Dai, H. X. Huangfu, Z. T. Zhu, X. L. Qin, Y. X. Deng and G. M. Haarberg, An asymmetric supercapacitor with good electrochemical performances based on Ni(OH)2/AC/CNT and AC, Electrochim. Acta, 2015, 182, 1159–1165 CrossRef CAS.
- W. P. Sun, X. H. Rui, M. Ulaganathan, S. Madhavi and Q. Y. Yan, Few-layered Ni(OH)2 nanosheets for high-performance supercapacitors, J. Power Sources, 2015, 295, 323–328 CrossRef CAS.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials, Nat. Commun., 2013, 5, 54–56 CrossRef.
- R. R. Salunkhe, J. J. Lin, V. Malgras, S. X. Dou, J. H. Kim and Y. Yamauchi, Large-scale synthesis of coaxial carbon nanotube/Ni(OH)2 composites for asymmetric supercapacitor application, Nano Energy, 2015, 59, 211–218 CrossRef.
- J. C. Huang, P. P. Xu, D. X. Cao, X. B. Zhou, S. N. Yang, Y. J. Li and G. L. Wang, Asymmetric supercapacitors based on beta-Ni(OH)2 nanosheets and activated carbon with high energy density, J. Power Sources, 2014, 246, 371–376 CrossRef CAS.
- M. Li, Z. Tang, M. Leng and J. M. Xue, Flexible solid-state supercapacitor based on graphene-based hybrid Films, Adv. Funct. Mater., 2014, 24, 7495–7502 CrossRef CAS.
- L. F. Chen, Z. H. Huang, H. W. Liang, W. T. Yao, Z. Y. Yu and S. H. Yu, Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose, Energy Environ. Sci., 2013, 6, 3331–3338 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00076f |
| This journal is © The Royal Society of Chemistry 2017 |