Doping of TiO2 as a tool to optimize the water splitting efficiencies of titania–hematite photoanodes†
Darinka
Primc
 *ab,
Mario
Bärtsch
a,
Davide
Barreca
c,
Giorgio
Carraro
d,
Chiara
Maccato
d,
Cinzia
Sada
e and
Markus
Niederberger
a
*ab,
Mario
Bärtsch
a,
Davide
Barreca
c,
Giorgio
Carraro
d,
Chiara
Maccato
d,
Cinzia
Sada
e and
Markus
Niederberger
a
aLaboratory for Multifunctional Materials, Department of Materials, ETH Zurich, Vladimir-Prelog-Weg 5, 8093 Zurich, Switzerland
bDepartment of Materials, Imperial College London, South Kensington Campus, London SW7 2AZ, UK. E-mail: d.primc@imperial.ac.uk
cCNR-ICMATE and INSTM, Department of Chemistry, Padova University, 35131 Padova, Italy
dDepartment of Chemistry, Padova University and INSTM, 35131 Padova, Italy
eDepartment of Physics and Astronomy, Padova University, 35131 Padova, Italy
First published on 7th February 2017
Abstract
Simple metal oxides such as hematite and titania draw tremendous interest as materials for photoelectrochemical (PEC) water splitting photoelectrodes to produce hydrogen as a clean and sustainable energy carrier. However, the high recombination rates of the photogenerated charges limit their application. Herein, we report on highly efficient and stable composite titania–hematite photoanodes prepared by combining doped TiO2 nanoparticles with amorphous iron oxide and subsequent annealing. Studying the effect of various TiO2 doping strategies, by in-depth structural and chemical characterization, carried out through a multiple technique approach, showed that doping of TiO2 allows subtle tuning of the phase composition, microstructure and surface topography of the photoanodes. When the photoanodes were prepared by combining Ta-doped TiO2 nanoparticles and amorphous iron oxide nanoparticles and subsequently annealed, remarkable photocurrents of up to 2.2 mA cm−2 at 1.23 V in 1 M NaOH under 1.5 AM simulated solar illumination were obtained. The high photocurrents, which were traced back to Ta-doping, were elucidated by rutile-hematite heterojunction energetics and the blocking layer formation. In addition to showing promise for a sustainable and cost-effective generation of an energy carrier, the presented strategies can also be expanded to other material combinations opening doors for new modified semiconductors or heterojunction photoanodes.
Introduction
Solar energy is the world's most abundant renewable energy source and its harvesting and subsequent storage in the form of chemical fuels hold promise for the current and future energy demands.1,2 In this regard, photoelectrochemical (PEC) water splitting over semiconductor electrodes using sunlight is an attractive and promising approach to supply hydrogen as a renewable and clean energy carrier.1–3 One of the key challenges in PEC water splitting is the choice of a suitable photoanode material, requiring the trade-off between band gap energy, determining its sensitivity to the vis part of the solar spectrum, and band edge positions, enabling water splitting to proceed with a minimum applied bias. Among the most studied candidates (Fe2O3,4,5 TiO2,6,7 WO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–10) hematite (α-Fe2O3) offers significant advantages due to its unique combination of visible light absorption (with the band gap Eg = 2.1 eV), stability in aqueous media, non-toxicity and abundance.4,5,11,12 Notwithstanding its appeals, hematite still presents a challenge, due to its intrinsic limitations, including high charge recombination, low hole and electron mobility, low conductivity and inappropriate band gap alignment for unassisted water splitting.2,4,5,11,13 As a result, the performance is greatly limited below its theoretical efficiency of 16.8% solar-to-hydrogen (STH) efficiency that corresponds to a photocurrent of 12.6 mA cm−2.4,5,14 Common approaches towards increasing the PEC efficiencies of hematite include its coupling with other materials, purposely designed towards improved photocatalytic activity or charge transport/transfer efficiencies. In this regard, elemental doping5,13,15 or coupling of hematite with oxygen evolution catalysts such as Ru-, Co- and Ir-oxides,13,16,17 resulted in enhanced PEC efficiencies. Alternatively, formation of a heterojunction2,6,7,18 or coupling with metal oxide underlayers for facilitating the charge transfer12,15 or overlayers to improve oxygen evolution kinetics5,19,20 brought notable improvements.
8–10) hematite (α-Fe2O3) offers significant advantages due to its unique combination of visible light absorption (with the band gap Eg = 2.1 eV), stability in aqueous media, non-toxicity and abundance.4,5,11,12 Notwithstanding its appeals, hematite still presents a challenge, due to its intrinsic limitations, including high charge recombination, low hole and electron mobility, low conductivity and inappropriate band gap alignment for unassisted water splitting.2,4,5,11,13 As a result, the performance is greatly limited below its theoretical efficiency of 16.8% solar-to-hydrogen (STH) efficiency that corresponds to a photocurrent of 12.6 mA cm−2.4,5,14 Common approaches towards increasing the PEC efficiencies of hematite include its coupling with other materials, purposely designed towards improved photocatalytic activity or charge transport/transfer efficiencies. In this regard, elemental doping5,13,15 or coupling of hematite with oxygen evolution catalysts such as Ru-, Co- and Ir-oxides,13,16,17 resulted in enhanced PEC efficiencies. Alternatively, formation of a heterojunction2,6,7,18 or coupling with metal oxide underlayers for facilitating the charge transfer12,15 or overlayers to improve oxygen evolution kinetics5,19,20 brought notable improvements.
In a previous study, we reported on nanostructured heterojunction photoanodes obtained by combining amorphous iron oxide with titania (TiO2), which were then fabricated into a photoanode following a colloidal route.18 A solid-state reaction during photoanode annealing led to the formation of pseudobrookite (Fe2TiO5) at the interfaces between iron oxide and titania nanoparticles. The observed 15-fold increase in photocurrent up to 1.3 mA cm2 (at 1.23 VRHE) compared to a pure hematite photoanode was attributed to the heterojunction formation between hematite and Fe2TiO5, among other effects like donor states for better conductivity and surface states acting as charge transfer mediators.18
Herein we report on the PEC efficiencies of titania–hematite photoanodes, which are improved by a remarkable 70% compared to hematite photoanodes and were prepared by combining doped TiO2 nanoparticles with amorphous iron oxide and subsequent annealing. We found that the selection of the dopant allowed tailoring the phase transformation during annealing. Depending on the type of dopant, coexistence of different TiO2 polymorphs or ternary Fe2TiO5 at the interface to hematite was observed. By means of in-depth chemical and structural analyses, we first compare the effect of Nb- and Ta-doping of TiO2 on the phase composition, microstructure and topography of the photoanodes. We then focus on the PEC characterization of the photoanodes delivering high photocurrent enhancements. High photocurrents are elucidated through the synergetic effects of phase coexistence, donor states and heterojunction energetics.
Results and discussion
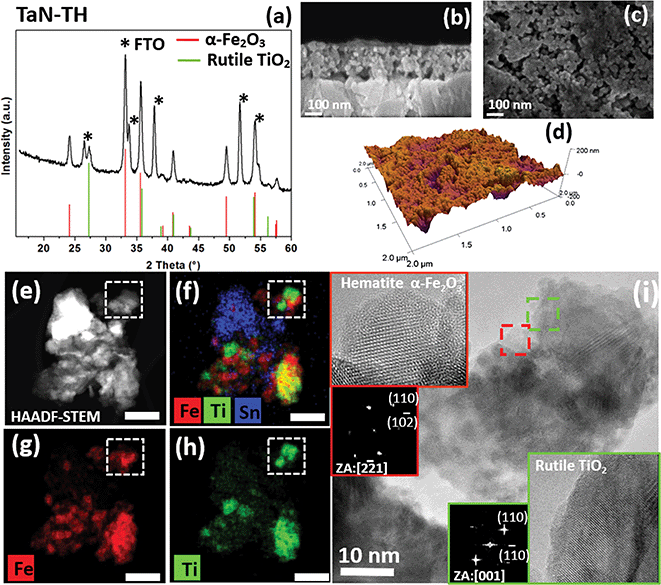
The photoanodes were prepared by spin coating a dispersion of amorphous iron oxide nanoparticles and well-faceted doped-TiO2 nanoparticles with the anatase structure in a Fe/Ti ratio of 8 (Fig. S1 in the ESI†). The final phase composition of the photoanode was varied by doping of TiO2 with either Nb or Ta. Additional co-doping of TiO2 with nitrogen was achieved by N-diffusion of TiO2 surface-bonded polydopamine during annealing (Fig. S1, ESI†).Fig. 1a shows the grazing incidence X-ray diffraction (GI-XRD) pattern of the photoanode, prepared by combining Ta-doped anatase TiO2 nanoparticles with amorphous iron oxide (sample TaN-TH) and annealed in N2 and subsequently in air. Apart from the diffraction peaks of orthorhombic SnO2 originating from the FTO substrate (JCPDS-077-0452) and rhombohedral hematite α-Fe2O3 (JCPDS: 033-0664), clear signals of rutile TiO2 (JCPDS-072-7374) as the only Ti-containing phase are detected. This indicates that Ta-doped TiO2 nanoparticles with the anatase structure, when combined with amorphous iron oxide in the photoanode, are completely converted into the rutile polymorph during the annealing step, whereas no ternary Fe–Ti–O phases were detected. Cross-sectional and top-view field-emission scanning electron microscopy (FE-SEM) analyses of TaN-TH (Fig. 1b and c) show an interconnected network of nanoparticles, with a noticeable high porosity. The uniform elemental distribution of Ti and Fe is confirmed by energy dispersive X-ray spectroscopy (EDXS) analysis (Fig. S2, ESI†). In order to get a deeper insight into the chemical composition of the photoanode, X-ray photoelectron spectroscopy (XPS) (Fig. S4 and S5, ESI†) was carried out. In addition to the signals attributed to Ti(IV) and Fe(III) oxides, also Ta(V) and N species can be detected, confirming the successful Ta and N doping (for further details regarding XPS analyses see the ESI, Section 2.2.†).
Secondary ion mass spectrometry (SIMS) analysis gives insight into the in-depth elemental distribution of the photoanodes (Fig. S6a, ESI†). An almost parallel profile is observed for Fe, Ti and Ta species, suggesting a homogeneous composition throughout the film thickness. On the other hand, N doping is mostly confined to the outermost layer region. In addition, the data indicate a Sn tail extending into the film, suggesting Sn diffusion from the FTO substrate into the photoanode, as also evidenced by XPS analyses (see the ESI, Section 2.2†). This interdiffusion was also observed in previous reports and typically results in improved PEC efficiency due to increased electron density.21,22 Regarding the surface topography, atomic force microscopy (AFM) images (Fig. 1d and S7, ESI†) show mean-square roughness of ∼27 nm, favorable for providing a high exposed area for the water photooxidation reaction.
In-depth analysis of the morphology and phase of the nanoparticles was performed by high resolution transmission electron microscopy (HRTEM) and scanning transmission electron microscopy (STEM), employed in a high-angle annular dark-field (HAADF) imaging mode of STEM. These studies were complemented by energy dispersive X-ray spectroscopy (EDXS) analysis. Fig. 1e shows a representative HAADF-STEM of agglomerated particles and the corresponding EDXS elemental maps of Fe and Ti spatial distribution (Fig. 1g and h). A composite overlay map of Fe, Ti and Sn is also shown in Fig. 1f. Closer inspection of the elemental distribution maps revealed that the areas enriched with Fe or Ti are in contact, but never overlapped. This observation was confirmed by analysis of a larger area of the specimen (data not shown). These results are in line with GI-XRD, showing the absence of any Fe–Ti–O phases. The HRTEM analysis (Fig. 1i and S8, ESI†) over the area marked in Fig. 1e–h showed that Fe rich areas were composed of percolating nanoparticles of ≈5 nm in diameter. This size approximately matches the size of the amorphous iron oxide nanoparticles used for the preparation of the photoanode, which, upon annealing, crystallize into hematite α-Fe2O3 (Fig. 1i, inset). On the other hand, HRTEM of Ti-rich areas of an agglomerate shows the presence of elongated particles of 20 nm length and 8 nm width. Such morphology in fact corresponds well to the size of the colloidal anatase TiO2 nanoparticles used for the photoanode preparation (Fig. S1, ESI†). However, upon annealing these particles seem to form compact arrays of densely sintered particles. The HRTEM image of the highlighted area in green confirms the occurrence of rutile TiO2 (Fig. 1i, inset). The comparably small size of the hematite particles in TaN-TH might result from the annealing in an oxygen poor atmosphere, known to suppress the crystallization of amorphous species.23,24 In addition to providing a high surface area and enhanced contact with the electrolyte, such a small particle size could also greatly suppress charge recombination in hematite due to the intrinsically low hole diffusion length (in the range of only 2–3 nm).2,4,5 Thus the small size of hematite is expected to lead to an enhancement in PEC efficiencies.
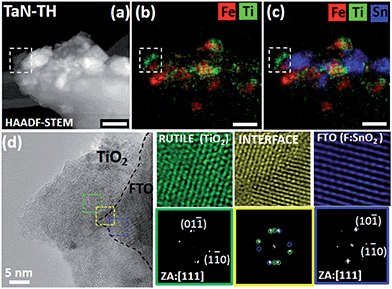
Since the rutile phase in TaN-TH, formed upon annealing, is structurally similar to F:SnO2 of the FTO substrate, we closely investigated the interfaces between the corresponding particles. A representative area at the interface to FTO is shown in the HAADF-STEM image (Fig. 2a), and EDXS maps of the same agglomerate are shown in Fig. 2b and c. The Fast Fourier Transform (FFT) analysis of the high resolution image allowed identifying the rutile (TiO2) and F:SnO2 in the examined area (Fig. 2a–d). Closer observation of the interface in the HRTEM image (Fig. 2d) reveals the coherent nature of the interface between TiO2 and F:SnO2 with the following crystallographic relationship: (111)Rutile ⊥ (111)FTO (Fig. 2, yellow square). The interface coherency suggests that the FTO substrate with its structure presumably acts as template for the rutile formed during the annealing. In any case, the crystallographic match with FTO is of significant importance to boost the PEC performance since it can facilitate efficient electron transport to the FTO substrate, yet to date it has only seldom been investigated in photoanodes prepared by colloidal routes.25
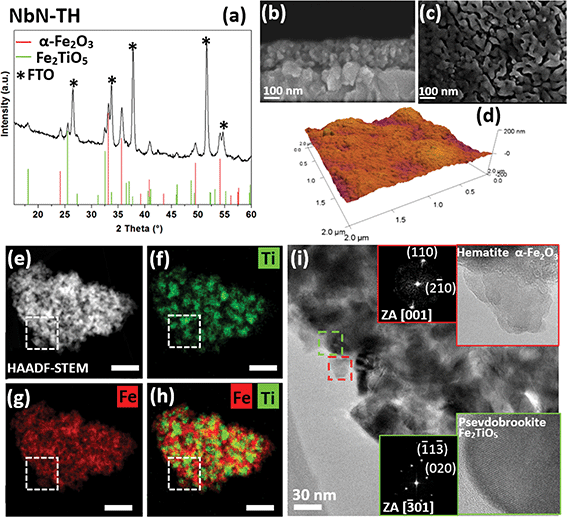
Remarkably, when Nb-doped TiO2 nanoparticles were used (sample NbN-TH), the phase, the particle sizes and the microstructure greatly differ compared to TaN-TH. The GI-XRD pattern of NbN-TH (Fig. 3a), apart from the reflections of SnO2 from FTO, reveals the presence of hematite and pseudobrookite (Fe2TiO5) (JCPDS: 01-076-1158), suggesting that the Nb-doped anatase TiO2 reacted with the amorphous iron oxide during annealing, thus forming a ternary phase. It is important to note that despite NbN-TH being annealed under the same conditions as TaN-TH, the particle sizes as revealed by in-plane and cross-sectional FE-SEM (Fig. 3b and c) and HRTEM (see below) greatly increased compared to TaN-TH. Since the particles are also much larger compared to the initial ones used for the photoanode preparation, this phenomenon indicates a remarkable growth parallel to the phase transformation during annealing. EDXS analysis shows uniform spatial distribution of Ti and Fe (Fig. S3, ESI†).
Chemical analyses by XPS show the presence of Ti(IV) and Fe(III) species, along with the occurrence of Nb(V) and N as dopants (see Fig. S4 and S5, ESI and Section S2.2.† for further details). Similar to TaN-TH, SIMS (Fig. S6b, ESI†) highlights a uniform in-depth distribution of Ti, Fe, and Nb, with intensity profiles decaying at the FTO interface, and the N content increases towards the photoanode surface. As for the TaN-TH, SIMS of NbN-TH also gives evidence for Sn diffusion from FTO. The investigation of the surface topography (Fig. 3d and S7, ESI†) of NbN-TH however shows considerable lower surface roughness (RMS ∼ 16) as compared to TaN-TH.
The increased particle sizes of NbN-TH upon annealing were confirmed by HRTEM and HAADF-STEM/EDXS analyses. Fig. 3e shows the HAADF-STEM micrograph of a representative agglomerate. The corresponding Ti and Fe mapping (Fig. 3f and g) and overlaid Fe/Ti map (Fig. 3h) reveal the presence of Fe throughout the agglomerate, while Ti areas are segregated and overlap with Fe, thus confirming the occurrence of a mixed Fe–Ti phase.
HRTEM analysis shows particles of around 20–30 nm in size, with particles enriched with Fe corresponding to the hematite structure (Fig. 3i, inset, red square), while particles enriched with Fe–Ti match the orthogonal phase of pseudobrookite (Fig. 3i, inset, green square). In fact, the particle size of pseudobrookite roughly matches the dimensions of the mixed Ti–Fe areas that are, as revealed by a combined map (Fig. 3h), uniformly distributed in the hematite matrix. These observations point to a possibility of heterojunction formation at the hematite–pseudobrookite interface, which was previously reported to improve the charge transfer efficiencies, yielding enhancement in photocurrents.5,6,18
Differences in the phase composition of both photoanodes can be traced back to the variations in the doping of the TiO2 nanoparticles. In fact, studies on the doping effect of TiO2 for anatase to rutile (A → R) conversion upon annealing26 have shown that Nb-doping (up to 10 at%) suppresses the A → R transformation. On the other hand, Ta-doping of a similar doping level accelerates it, even at temperatures lower than those required for undoped A → R transformation.26 To some extent, this behavior can be correlated with the reactivity of Nb- or Ta-doped anatase TiO2 toward amorphous iron oxide as in the present case. In line with this, the Nb-doping in NbN-TH, suppresses the A → R conversion during annealing, resulting in anatase reacting with iron oxide nanoparticles. This is also supported by our previous study,18 in which annealing of undoped TiO2 with amorphous iron oxide particles of similar size also leads to the formation of hematite and pseudobrookite. On the other hand, the Ta-doping in TaN-TH accelerates the A → R conversion, and the rutile, present as sintered particle arrays, is apparently less reactive toward iron oxide and retains its phase integrity. Additionally, the size and morphology of the initial Ta- and Nb-doped TiO2 nanoparticles are similar, confirming that doping is mainly responsible for the triggered phase transformation (Fig. S1, ESI†).
Considering that the structural and chemical investigation showed large variations between TaN-TH and NbN-TH, it is interesting to investigate and compare the PEC behaviors of the two materials as photoanodes. In the absence of irradiation, TaN-TH and NbN-TH showed currents below 0.5 mA cm−2 until 1.7 V vs. RHE (Fig. S9a, ESI†). Under solar irradiation a photocurrent density of 1.6 mA cm−2 (at 1.23 V) was observed for NbN-TH, while for TaN-TH a remarkable photocurrent of 2.2 mA cm−2 (at 1.23 V) was obtained, without the addition of any co-catalysts (Fig. 4a). The measured photocurrents of both photoanodes are much higher compared to those consisting of only hematite (Fig. S9b, ESI†) or only Ta- or Nb-doped TiO2 nanoparticles (Fig. S10, ESI†). The measured photocurrents are also higher compared to photoanodes of similar thickness, where undoped TiO2 nanoparticles were combined with hematite.18 In fact, the efficiency of TaN-TH is comparable to the best photocurrents ever reported for hematite and hematite–TiO2 based photoanodes prepared by colloidal routes and without addition of any co-catalysts.11,21 For example, photocurrents comparable to ours were reported by Wang et al., who reported on high-efficient Ti-doped hematite based photoanodes by using simple deposition of Fe–metal salt solutions with subsequent annealing.11 High PEC efficiencies were also observed for hematite photoanodes, where high photocurrents were mainly attributed to Sn-doping of hematite obtained through annealing at temperatures of 800 °C,27 which is much higher than the temperatures used herein. The efficiency of our TaN-TH is also comparable to the benchmark values obtained with gas phase deposition routes such as chemical vapor16,28 or atomic layer deposition.20,29 However, compared to a simple colloidal based approach, as employed in our study, these routes are characterized by higher complexity, being less promising for up-scale. The photocurrent stability of the TaN-TH and NbN-TH photoanodes was measured during prolonged irradiation of six hours (Fig. 4b). Since the photocurrent measurements are done at the side of the film/solution interface the oxygen bubbles formed as a result of the water oxidation scatter the illuminated light, resulting in periodic decays of the photocurrent. Nevertheless, for TaN-TH the removal of the bubbles results in instant increase of the photocurrent to the original value, demonstrating its photostability. On the other hand, a gradual decay of photocurrent was observed for NbN-TH.
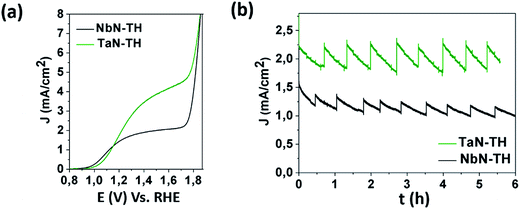 | ||
| Fig. 4 Photocurrent density for TaN-TH and NbN-TH photoanodes as a function of (a) applied potential and (b) time. | ||
The high PEC efficiencies of NbN-TH and TaN-TH can be ascribed to several factors all induced through different types of TiO2 doping: particle size, phase coexistence and electronic states. At first, higher photocurrents of TaN-TH compared to NbN-TH might result from smaller hematite particle sizes, which are beneficial for hole transport and for providing a higher exposed surface area for the water oxidation reaction.4,6,18,29 Another effect of the particle size is reflected by the onset potential, which is found to be shifted to the cathodic direction in the case of NbN-TH. In line with literature studies, this observation could be attributed to the passivation of the surface states as a result of particle growth during annealing of NbN-TH.3,20
Furthermore, TEM analyses of NbN-TH point to the presence of a hematite–pseudobrookite heterojunction. Due to the favorable band edge positions of both materials, electron injection into the hematite conduction band is facilitated, while holes transfer to the electrolyte due to the valence band alignment between both materials, in accordance with the observations in other studies.6,18,19 The high photocurrents of NbN-TH can also be rationalized on the basis of doping, where Nb-doping is expected to result in increased carrier density, leading to better conductivity.30
On the other hand, the high PEC efficiency of TaN-TH cannot be simply traced back to the heterojunction energetics. Due to the more negative conduction band (CB) potential of TiO2 compared to hematite a higher recombination rate would be expected due to the band offset.6,31 However, by applying electrochemical impedance spectroscopy, PEC and electron paramagnetic resonance studies, Luan et al. evidently confirmed the occurrence of an uncommon transfer of visible-excited high-energy electrons from hematite to rutile TiO2.32 It was suggested that in this process, the excited electrons in hematite populate different energy levels and while low energy electrons recombine with VB holes, high-energy electrons transfer to the CB of rutile.6,32 Such an uncommon electron transfer, leading to improved charge separation and enhanced PEC properties, was also observed in BiVO4–ZnO heterojunction photoanodes33 and recently also for BiVO4–TiO2 heterojunction photoanodes.6,34,35 In addition, the high efficiency could as well be explained on the basis of the formation of a rutile blocking layer. Previous studies demonstrated that thin oxide blocking underlayers at the hematite–FTO interface drastically enhance the PEC efficiencies by facilitating electron transfer into FTO.12,15 Consistently with this observation, compact arrays of rutile nanoparticles in TaN-TH likely act as an electron collector. The efficiency of electron transfer is expected to be enhanced, because these compact rutile arrays are not limited only to the FTO interface, but appeared to be present throughout a larger volume of the photoanodes. Additionally, the structural coherency of F:SnO2 and rutile is expected to further enhance the electron transfer efficiency.25 Finally, the high photocurrents of TaN-TH can be correlated with higher carrier density due to Ta-doping,36 however, more detailed studies like impedance spectroscopy and incident photon-to-current analysis are needed to fully disclose this effect.
Conclusions
Following a simple colloidal route we successfully fabricated highly efficient and stable composite photoanodes. The photoanodes were prepared by combining amorphous iron oxide and crystalline doped TiO2 nanoparticles. The TiO2 doping strongly influenced the solid state reactions during annealing, allowing a subtle tuning of the final phase composition, which influenced the microstructure and surface topography of the photoanodes. When photoanodes were prepared by combining Ta-doped TiO2 with amorphous iron oxide, a remarkable increase in photocurrents was obtained, which was attributed to the rutile–hematite heterojunction, oxide blocking layer formation and doping. The resulting highly efficient and stable photoanodes, prepared by employing simple nanostructuring of abundant metal oxides, show great promise for sustainable and cost-effective generation of an energy carrier. It is expected that the presented strategy of using preformed nanoparticles could easily be expanded to other material combinations, opening doors for new modified semiconductors or heterojunction photoanodes.Experimental
Synthesis of doped-TiO2 nanoparticles
For the synthesis of doped-TiO2 nanoparticles typically 0.15 mmol Ta(OEt)5 (Sigma Aldrich, 99.98%) or Nb(OEt)5 (Sigma Aldrich, 99.95%) was dissolved in a solution of 1 mmol of Ti(OPr)4 (Sigma Aldrich, 98%) in a mixture of benzyl alcohol (Sigma Aldrich, anhydrous, 99.8%) and acetic acid (Sigma Aldrich >99.7%) (Vtot = 5 mL, vol. ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The reaction mixture, prepared in an Ar atmosphere, was sealed in a 10 mL microwave tube and heated in a microwave oven (CEM Discovery reactor operating at 2.45 GHz) at 200 °C for 15 min. After cooling to room temperature, the product was precipitated with diethyl ether (Sigma Aldrich, >99.8%), centrifuged off and washed twice with chloroform (Alfa Aesar, 99.5%). Subsequently, doped TiO2 nanoparticles were coated with polydopamine following a procedure from the literature, with slight modifications.1 Doped TiO2 nanoparticles were redispersed in a 10 mL solution containing 15 μL HCl (Sigma Aldrich, 37%) and 10 mg 2-amino-2-(hydroxymethyl)-1,3-propanediol (Trizma, Fluka, ≥99.7%). Subsequently, 20 mg dopamine hydrochloride (Sigma Aldrich, 98%) was added, followed by vigorous stirring overnight, initiating the self-polymerization of dopamine.37 Subsequently, the products were precipitated with acetone (Sigma Aldrich, ≥99.5%) and polydopamine coated TiO2 nanoparticles were colloidally stabilized in 2 mL of MeOH (Merck, EMSURE®) with addition of 150 μL of acetylacetone (Sigma Aldrich, ≥99.9%).
1). The reaction mixture, prepared in an Ar atmosphere, was sealed in a 10 mL microwave tube and heated in a microwave oven (CEM Discovery reactor operating at 2.45 GHz) at 200 °C for 15 min. After cooling to room temperature, the product was precipitated with diethyl ether (Sigma Aldrich, >99.8%), centrifuged off and washed twice with chloroform (Alfa Aesar, 99.5%). Subsequently, doped TiO2 nanoparticles were coated with polydopamine following a procedure from the literature, with slight modifications.1 Doped TiO2 nanoparticles were redispersed in a 10 mL solution containing 15 μL HCl (Sigma Aldrich, 37%) and 10 mg 2-amino-2-(hydroxymethyl)-1,3-propanediol (Trizma, Fluka, ≥99.7%). Subsequently, 20 mg dopamine hydrochloride (Sigma Aldrich, 98%) was added, followed by vigorous stirring overnight, initiating the self-polymerization of dopamine.37 Subsequently, the products were precipitated with acetone (Sigma Aldrich, ≥99.5%) and polydopamine coated TiO2 nanoparticles were colloidally stabilized in 2 mL of MeOH (Merck, EMSURE®) with addition of 150 μL of acetylacetone (Sigma Aldrich, ≥99.9%).
Synthesis of amorphous iron oxide nanoparticles
The synthesis of amorphous iron oxide nanoparticles followed the procedure from the literature with slight modifications.18 In brief, first 1 mmol Fe(acac)3 (Sigma-Aldrich, ≥99.9%) was dissolved in benzyl alcohol (V = 4 mL) with subsequent addition of 1,3-propanediol (Alfa Aesar, 99%) (Vtot = 5 mL, vol. ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by stirring for 20 min. The reaction mixture was sealed in a 10 mL microwave (MW) tube and treated in a microwave oven at 175 °C for 30 min with a high stirring rate. After cooling, the content of the two MW tubes was combined and centrifuged at 4000 rpm for 15 min. Only the supernatant was further used and mixed with 40 mL of diethyl ether. The precipitated products were washed twice with diethyl ether and immediately redispersed in 0.5 mL of methanol (Merck, EMSURE®) and 40 μL acetylacetone. A stable colloidal dispersion was obtained after 12 h without any stirring. The concentration, gravimetrically determined after annealing at 700 °C for 2 h was ≈60 mg mL−1.
1), followed by stirring for 20 min. The reaction mixture was sealed in a 10 mL microwave (MW) tube and treated in a microwave oven at 175 °C for 30 min with a high stirring rate. After cooling, the content of the two MW tubes was combined and centrifuged at 4000 rpm for 15 min. Only the supernatant was further used and mixed with 40 mL of diethyl ether. The precipitated products were washed twice with diethyl ether and immediately redispersed in 0.5 mL of methanol (Merck, EMSURE®) and 40 μL acetylacetone. A stable colloidal dispersion was obtained after 12 h without any stirring. The concentration, gravimetrically determined after annealing at 700 °C for 2 h was ≈60 mg mL−1.
Photoanode preparation
The photoanodes (TaN-TH and NbN-TH) were prepared by a procedure schematically shown in Fig. S1 (Section S1.6.†). Dispersions of polydopamine coated TiO2 nanoparticles doped with Nb or Ta were combined with amorphous iron oxide dispersion in the ratio M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti (mol%)/[M
Ti (mol%)/[M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti (mol%) + Fe (mol%)] of 20 mol% with respect to doped titania. The ratio M
Ti (mol%) + Fe (mol%)] of 20 mol% with respect to doped titania. The ratio M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti of 20% was selected as the optimal one, facilitating a complete transformation of anatase TiO2 nanoparticles into either the rutile polymorph or Fe2TiO5 when combined with iron oxide and annealed. The concentration of the mixed dispersion was ≈25 mg mL−1 with respect to M
Ti of 20% was selected as the optimal one, facilitating a complete transformation of anatase TiO2 nanoparticles into either the rutile polymorph or Fe2TiO5 when combined with iron oxide and annealed. The concentration of the mixed dispersion was ≈25 mg mL−1 with respect to M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 and Fe2O3 as determined gravimetrically after calcination at 700 °C. Prior to the preparation of thin films, the FTO substrates (Solaronix, TCO022-7, ∼7 Ω per square, 1.5 × 3.5 cm) were washed by sonicating in acetone followed by cleaning with a (1
TiO2 and Fe2O3 as determined gravimetrically after calcination at 700 °C. Prior to the preparation of thin films, the FTO substrates (Solaronix, TCO022-7, ∼7 Ω per square, 1.5 × 3.5 cm) were washed by sonicating in acetone followed by cleaning with a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of soap (Migros, Handy) and water. After rinsing the substrates thoroughly with distilled water, the substrates were washed with ethanol (Fluka, ≥99.8%) and dried in air at 200 °C for 2 h. A part of the substrate (approximately 1 × 1.5 cm) was coated with a polymer tape (Kapton® foil, VWR International) prior to coating. The uncoated part of FTO was later used as an electric contact for the electrodes in the photoelectrochemical (PEC) cell. The films were coated inside of a particle-free glovebox (GS inert gas glovebox®), which was constantly purged with synthetic air. Before each deposition cycle, the FTO substrate was spincoated (Laurell, WS-650MZ-23NPP) with isopropanol (Sigma-Aldrich, ≥99.8%). The mixed dispersion was coated twice on the FTO substrate at 4000 rpm for 25 s. After each deposition, the coated FTO was put on a hot plate and heated to 220 °C for 10 min with a ramp of 30 °C min−1. The final heat treatment was carried out in a tube furnace (Carbolite, MTF 12/38/250) at 680 °C (heat rate 20 °C min−1) for 1.5 h under a N2-flow, followed by 0.5 h in air and quenched in air within 5 min.
1) mixture of soap (Migros, Handy) and water. After rinsing the substrates thoroughly with distilled water, the substrates were washed with ethanol (Fluka, ≥99.8%) and dried in air at 200 °C for 2 h. A part of the substrate (approximately 1 × 1.5 cm) was coated with a polymer tape (Kapton® foil, VWR International) prior to coating. The uncoated part of FTO was later used as an electric contact for the electrodes in the photoelectrochemical (PEC) cell. The films were coated inside of a particle-free glovebox (GS inert gas glovebox®), which was constantly purged with synthetic air. Before each deposition cycle, the FTO substrate was spincoated (Laurell, WS-650MZ-23NPP) with isopropanol (Sigma-Aldrich, ≥99.8%). The mixed dispersion was coated twice on the FTO substrate at 4000 rpm for 25 s. After each deposition, the coated FTO was put on a hot plate and heated to 220 °C for 10 min with a ramp of 30 °C min−1. The final heat treatment was carried out in a tube furnace (Carbolite, MTF 12/38/250) at 680 °C (heat rate 20 °C min−1) for 1.5 h under a N2-flow, followed by 0.5 h in air and quenched in air within 5 min.
The photoanodes prepared from only doped TiO2 nanoparticles were fabricated following a procedure from the literature.38 In brief, 110 mg of the synthesized doped TiO2 nanoparticles were mixed in 25 mL of 10% acetyl acetone in hexyl alcohol and with 1.25 mL of 1% acetyl acetone in isopropanol. The mixture was sonicated for 1 h, and subsequently 20 mg hydroxypropyl cellulose was added and then stirred overnight. The (doped) TiO2 films were prepared by the doctor blade method, by depositing one layer of previously prepared slurry onto a cleaned FTO glass substrate. The films were annealed in air at 600°C for 1 hour.
Characterization
The phase analysis was done by using a X'Pert Pro (PANalytical B.V., Netherlands) powder diffractometer in Bragg–Brentano mode and equipped with Cu Kα radiation (45 kV, 40 mA). Morphological and phase analyses of the photoanode materials were performed by transmission electron microscopy (TEM) on a FEI Talos F200X instrument operated at 200 kV and in TEM and scanning TEM (STEM) modes. An atomic number sensitive HAADF imaging was used for STEM studies with a probe size of ≈0.8 nm. The specimens for TEM were prepared from the photoanode material scratched off from the substrates in the form of powders. The powders were dispersed in isopropanol and then deposited as a drop onto a conventional, lacey carbon supported Cu-grid.The high resolution STEM micrographs and the corresponding FFTs patterns were used for the phase analyses of the materials. These analyses were complemented by EDXS spectroscopy employed in STEM mode. The SuperX EDX system, consisting of 4SDD segments and systematically positioned above a TEM specimen, was employed in a hypermap mode for spectral imaging of the elemental content profile.
FESEM micrographs were recorded by means of a Zeiss SUPRA 40VP and REM-LEO1530 apparatus. Plane-view and cross-sectional micrographs were obtained using primary beam voltages between 10 and 20 kV. XPS spectra were recorded on a Perkin Elmer Φ 5600ci spectrometer at a pressure lower than 10−8 mbar, using a non-monochromatized Al Kα excitation source (hν = 1486.6 eV). The Binding Energy (BE) shifts were corrected for charging by assigning the C1s line of adventitious carbon a value of 284.8 eV. The estimated standard deviation for BEs was ±0.2 eV. The atomic compositions were evaluated using sensitivity factors provided by Φ V5.4A software. The samples were introduced directly into the analysis chamber by a fast entry lock system.
SIMS investigation was performed by means of a IMS 4f mass spectrometer (Cameca) using a Cs+ primary beam (voltage = 14.5 keV; current = 12 nA, stability = 0.4%) and negative secondary ion detection, adopting an electron gun for charge compensation. Beam blanking mode and high mass resolution configuration were adopted. Signals were recorded by scanning over a 175 × 175 μm2 area and detecting secondary ions from a sub-region close to 10 × 10 μm2 in order to avoid crater effects.
Photoelectrochemical characterization
The photoanodes were tested for the PEC activity in a Teflon cell equipped with a quartz window by illuminating the photoanode from the side of a film/solution interface.8 Measurements were carried out by using a three-electrode setup with a Pt-counter electrode and a Hg/HgSO4 saturated K2SO4 reference electrode (MSE, Radiometer Analytical). Potential versus the reversible hydrogen electrode (RHE) was calculated by using the following equation:39| VRHE = VMSE + 0.640 + 0.059pH |
Measurements were conducted with a VMP3 BioLogic potentiostat. Simulated AM 1.5 G solar irradiation was provided by an Oriel solar simulator equipped with a 300 W Xe lamp and fitted with a 1.5 G filter. The light intensity was measured with a calibrated reference cell (Oriel, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 V) and adjusted to 100 mW cm−2 with a neutral density filter (Newport, FS-ND). The surface area illuminated on the photoanode was 0.28 cm2.
150 V) and adjusted to 100 mW cm−2 with a neutral density filter (Newport, FS-ND). The surface area illuminated on the photoanode was 0.28 cm2.
Acknowledgements
The authors gratefully thank ETH Zurich, the Polish-Swiss Research Programme PSPB-132/2010 (Hybrid Semiconducting Materials for Solar Energy Conversion) and the European Framework 7 program under the project SOLAROGENIX (FP7-NMP4-SL-2012-310333), as well as Padova University ex-60%, P-DiSC #SENSATIONAL BIRD2016-unipd projects and the post-doc fellowship ACTION, for financial support. We also acknowledge the Scientific Center for Optical and Electron Microscopy (ScopeM) of ETH Zurich for providing the facilities and Dr Alla Sologubenko for her help with HRTEM and HAADF-STEM studies. The authors also acknowledge Dr Derya Erdem and Dr Niklaus Kränzlin for their help with GI-XRD analysis and Mr Alain Reiser for AFM measurements.Notes and references
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- K. Zhang, M. Ma, P. Li, D. H. Wang and J. H. Park, Adv. Energy Mater., 2016, 6, 1600602–1600618 CrossRef.
- R. Liu, Z. Zheng, J. Spurgeon and X. Yang, Energy Environ. Sci., 2014, 7, 2504–2517 CAS.
- K. Sivula, F. Le Formal and M. Grätzel, ChemSusChem, 2011, 4, 432–449 CrossRef CAS PubMed.
- I. S. Cho, H. S. Han, M. Logar, J. Park and X. Zheng, Adv. Energy Mater., 2016, 6, 15001840–15001849 Search PubMed.
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z.-X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731–759 CAS.
- Y. Wang, Q. Wang, X. Zhan, F. Wang, M. Safdar and J. He, Nanoscale, 2013, 5, 8326–8339 RSC.
- S. Hilaire, M. J. Suess, N. Kranzlin, K. Bienkowski, R. Solarska, J. Augustynski and M. Niederberger, J. Mater. Chem. A, 2014, 2, 20530–20537 CAS.
- J. Brillet, J.-H. Yum, M. Cornuz, T. Hisatomi, R. Solarska, J. Augustynski, M. Grätzel and K. Sivula, Nat. Photonics, 2012, 6, 824–828 CrossRef CAS.
- M. Sarnowska, K. Bienkowski, P. J. Barczuk, R. Solarska and J. Augustynski, Adv. Energy Mater., 2016, 6, 1600526–1600532 CrossRef.
- G. Wang, Y. Ling, D. A. Wheeler, K. E. N. George, K. Horsley, C. Heske, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3503–3509 CrossRef CAS PubMed.
- T. Hisatomi, H. Dotan, M. Stefik, K. Sivula, A. Rothschild, M. Grätzel and N. Mathews, Adv. Mater., 2012, 24, 2699–2702 CrossRef CAS PubMed.
- A. G. Tamirat, J. Rick, A. A. Dubale, W.-N. Su and B.-J. Hwang, Nanoscale Horizons, 2016, 1, 243–267 RSC.
- A. B. Murphy, P. R. F. Barnes, L. K. Randeniya, I. C. Plumb, I. E. Grey, M. D. Horne and J. A. Glasscock, Int. J. Hydrogen Energy, 2006, 31, 1999–2017 CrossRef CAS.
- A. Annamalai, P. S. Shinde, A. Subramanian, J. Y. Kim, J. H. Kim, S. H. Choi, J. S. Lee and J. S. Jang, J. Mater. Chem. A, 2015, 3, 5007–5013 CAS.
- S. D. Tilley, M. Cornuz, K. Sivula and M. Grätzel, Angew. Chem., Int. Ed., 2010, 49, 6405–6408 CrossRef CAS PubMed.
- B. Zhang, X. Zheng, O. Voznyy, R. Comin, M. Bajdich, M. García-Melchor, L. Han, J. Xu, M. Liu, L. Zheng, F. P. García de Arquer, C. T. Dinh, F. Fan, M. Yuan, E. Yassitepe, N. Chen, T. Regier, P. Liu, Y. Li, P. De Luna, A. Janmohamed, H. L. Xin, H. Yang, A. Vojvodic and E. H. Sargent, Science, 2016, 352, 333–337 CrossRef CAS PubMed.
- D. Monllor-Satoca, M. Bärtsch, C. Fabrega, A. Genc, S. Reinhard, T. Andreu, J. Arbiol, M. Niederberger and J. R. Morante, Energy Environ. Sci., 2015, 8, 3242–3254 CAS.
- D. Barreca, G. Carraro, A. Gasparotto, C. Maccato, M. E. A. Warwick, K. Kaunisto, C. Sada, S. Turner, Y. Gönüllü, T.-P. Ruoko, L. Borgese, E. Bontempi, G. Van Tendeloo, H. Lemmetyinen and S. Mathur, Adv. Mater. Interfaces, 2015, 2, 1500313–1500324 CrossRef.
- F. Le Formal, N. Tetreault, M. Cornuz, T. Moehl, M. Gratzel and K. Sivula, Chem. Sci., 2011, 2, 737–743 RSC.
- R. H. Gonçalves, B. H. R. Lima and E. R. Leite, J. Am. Chem. Soc., 2011, 133, 6012–6019 CrossRef PubMed.
- Y. Ling, G. Wang, D. A. Wheeler, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 2119–2125 CrossRef CAS PubMed.
- M.-Y. Ho, H. Gong, G. D. Wilk, B. W. Busch, M. L. Green, W. H. Lin, A. See, S. K. Lahiri, M. E. Loomans, P. I. Räisänen and T. Gustafsson, Appl. Phys. Lett., 2002, 81, 4218–4220 CrossRef CAS.
- H. Takahashi, S. Toyoda, J. Okabayashi, H. Kumigashira, M. Oshima, K. Ikeda, G. L. Liu, Z. Liu and K. Usuda, Appl. Phys. Lett., 2007, 91, 012902 CrossRef.
- R. H. Goncalves and E. R. Leite, J. Mater. Res., 2014, 29, 47–54 CrossRef CAS.
- H. Pan, N. Chen, S. Shen and J. Huang, J. Sol-Gel Sci. Technol., 2005, 34, 63–69 CrossRef CAS.
- R. H. Goncalves and E. R. Leite, Energy Environ. Sci., 2014, 7, 2250–2254 CAS.
- M. Stefik, M. Cornuz, N. Mathews, T. Hisatomi, S. Mhaisalkar and M. Grätzel, Nano Lett., 2012, 12, 5431–5435 CrossRef CAS PubMed.
- J. Brillet, M. Grätzel and K. Sivula, Nano Lett., 2010, 10, 4155–4160 CrossRef CAS PubMed.
- C. Das, P. Roy, M. Yang, H. Jha and P. Schmuki, Nanoscale, 2011, 3, 3094–3096 RSC.
- S. Hoang, S. Guo, N. T. Hahn, A. J. Bard and C. B. Mullins, Nano Lett., 2012, 12, 26–32 CrossRef CAS PubMed.
- P. Luan, M. Xie, D. Liu, X. Fu and L. Jing, Sci. Rep., 2014, 4, 6180 CrossRef CAS PubMed.
- X. Fu, M. Xie, P. Luan and L. Jing, ACS Appl. Mater. Interfaces, 2014, 6, 18550–18557 CAS.
- M. Xie, X. Fu, L. Jing, P. Luan, Y. Feng and H. Fu, Adv. Energy Mater., 2014, 4, 1300995–1301001 CrossRef.
- X. An, T. Li, B. Wen, J. Tang, Z. Hu, L.-M. Liu, J. Qu, C. P. Huang and H. Liu, Adv. Energy Mater., 2016, 6, 1502268–1502278 CrossRef; X. An, T. Li, B. Wen, J. Tang, Z. Hu, L.-M. Liu, J. Qu, C. P. Huang and H. Liu, Adv. Energy Mater., 2016, 6, 1502268 CrossRef.
- M. Altomare, K. Lee, M. S. Killian, E. Selli and P. Schmuki, Chem.–Eur. J., 2013, 19, 5841–5844 CrossRef CAS PubMed; M. Altomare, K. Lee, M. S. Killian, E. Selli and P. Schmuki, Chem.–Eur. J., 2013, 19, 5841–5844 CrossRef PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- S. K. Biswas, J. O. Baeg, S.-J. Moon, K. J. Kong and W. W. So, J. Nanopart. Res., 2012, 14, 667–679 CrossRef.
- D. Pletcher and Royal Society of Chemistry (Great Britain), A First Course in Electrode Processes, Royal Society of Chemistry, Cambridge, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00005g |
| This journal is © The Royal Society of Chemistry 2017 |