 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct structural identification of carbenium ions and investigation of host–guest interaction in the methanol to olefins reaction obtained by multinuclear NMR correlations†
Dong
Xiao
 abc,
Shutao
Xu
d,
Xiuwen
Han
a,
Xinhe
Bao
abc,
Shutao
Xu
d,
Xiuwen
Han
a,
Xinhe
Bao
 a,
Zhongmin
Liu
ad and
Frédéric
Blanc
a,
Zhongmin
Liu
ad and
Frédéric
Blanc
 *ce
*ce
aState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, China
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cDepartment of Chemistry, University of Liverpool, Crown Street, Liverpool, L69 7ZD, UK. E-mail: frederic.blanc@liverpool.ac.uk
dNational Engineering Laboratory for Methanol to Olefins, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics Chinese Academy of Sciences, Dalian 116023, China
eStephenson Institute for Renewable Energy, University of Liverpool, Crown Street, Liverpool, L69 7ZD, UK
First published on 10th October 2017
Abstract
Probing and determining the intermediates formed during catalytic reactions in heterogeneous catalysis are strong challenges. Using 13C labelling and two dimensional 13C–13C through-bond NMR correlations, we directly reveal the structures of a range of carbenium ion species formed during the conversion of methanol to olefins on acidic H-ZSM-5 zeolite by mapping the carbon–carbon bond connectivities. Additionally, we use 13C–27Al and 29Si–13C through-space NMR experiments to probe the interactions between the confined carbon species (including carbenium ions) and the framework of the zeolite, which quantitatively provide an estimate for the carbon–aluminium and carbon–silicon distances, respectively.
Introduction
The methanol-to-olefins (MTO) reaction is an important process for the production of light olefins (mainly ethylene and propene) from non-petrochemical resources such as coal and natural gas.1 The reaction is catalysed by microporous solid acids, in particular a wide range of zeolites (e.g. H-ZSM-5 and H-SAPO-34), and has been successfully commercialised since the 1990s.1–3 Nevertheless, there is a need for a deeper understanding of the catalytic active sites and reaction mechanism in order to identify catalyst deactivation pathways and further optimise the catalytic performance.4–11Solid-state NMR is a well-developed technique for structural determination and host–guest investigation studies, and has played an important role in increasing our understanding of heterogeneous catalytic processes.12–15 In particular, solid-state NMR has enabled the detection of the intermediates formed during the MTO reaction and their interactions with zeolite, which are both important in unravelling the mechanism of the reaction. The intermediates that have been observed previously by NMR are cyclic carbenium ions such as polymethylcyclopentenyl cations and polymethylbenzenium cations on H-SAPO-34,16 H-SSZ-13,16 DNL-6 (ref. 17) and β-zeolite,18 and polymethylcyclopentenyl cations and ethylated cyclopentenyl cations on H-ZSM-5 zeolite.19,20 These cyclic carbenium ions are crucial intermediates involved in the hydrocarbon pool mechanism21 in which the cyclic organic species in the zeolite pores act as co-catalysts for the conversion of methanol to olefins.5 More specifically, two reaction routes have been proposed namely the side-chain methylation route in which the olefins are produced through the methylation of polymethylbenzenium ions and the subsequent elimination of the side chain groups, and the paring route in which the olefins are released via the expansion of polymethylcyclopentenyl cations and the subsequent contraction of polymethylbenzenium ions.16,22–24 The structural identification of the carbenium ions is crucial for the determination of the dominant route and specific reaction path in different zeolites.
Previous work relied on computational methods for the assignments of the 13C solid-state NMR spectra of the carbenium ions.16,19 Although these computational methods are recognised as robust approaches for NMR spectral interpretation,25,26 there is still a direct lack of experimental data supporting these assignments and therefore this may lead to possible misinterpretation of the carbenium ions produced. Additionally, the identification of the carbenium ions was indirectly obtained by digesting the dienes (the deprotonated counterparts of the cyclic carbenium ions) with concentrated sulfuric acid and analysing the obtained solutions by liquid-state NMR.16,20 This procedure assumes that the states of the carbenium ions in the solutions are the same as those confined in the solid zeolite pores, and also requires independent synthesis of the dienes, and therefore entails prior knowledge of the possible carbenium ions’ structures.
Recently, the interactions between the carbon species and the Al sites of the H-ZSM-5 zeolite were qualitatively investigated using spatially encoded 13C–27Al dipolar coupling NMR experiments (employing a S-RESPDOR, Symmetry-based Resonance-Echo Saturation-Pulse DOuble-Resonance sequence27). The work demonstrated the formation of supramolecular reaction centres composed of confined carbon species and the inorganic framework of zeolite which possesses higher reactivity toward methanol in the H-ZSM-5 zeolite.7
Here, we unambiguously experimentally identified several cyclic carbenium ions on MTO activated H-ZSM-5 zeolite, including a previously undetected 1,5-dimethyl-3-sec-butyl cyclopentenyl cation, using a refocused INADEQUATE (Incredible Natural Abundance DoublE QUAntum Transfer Experiment)28 NMR sequence. This experiment relies on scalar J couplings and yields through-bond correlations, providing a straightforward pathway for 13C spectral assignments. Moreover, the interactions between the confined carbon species and the H-ZSM-5 zeolite framework are quantitatively probed via through-space 13C{27Al} S-RESPDOR27,29 and 29Si{13C} REDOR (Rotational Echo DOuble Resonance) experiments (see Fig. S1 in the ESI†).30
Results and discussion
Structural identification of the confined carbon species in MTO activated H-ZSM-5
In this work, the MTO activated H-ZSM-5 materials were prepared by passing 13C enriched CH3OH over H-ZSM-5 at 285 °C for 20 minutes followed by quenching the reaction mixture with liquid N2 (see the Experimental section in the ESI†). The 13C CP (Cross-Polarisation) MAS (Magic Angle Spinning) NMR spectra of the 13C enriched MTO activated H-ZSM-5 are given in Fig. 1a at 9.4 T (and in Fig. S2† at 20 T) and show multiple signals ranging from 0 to 260 ppm, highlighting the complexity of the confined carbon species. It is worth pointing out that the unusual downfield 13C resonances (235–260 ppm) are characteristic signals of protonated carbenium ions and these have previously been assigned to several methylated and ethylated cyclopentenyl cations19,20 whose overlapping resonances prevent their unequivocal assignments.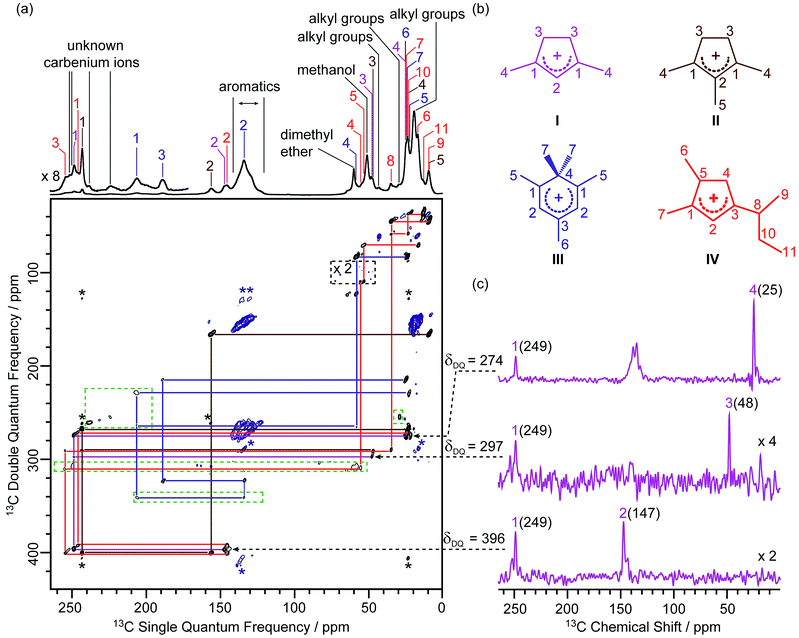 | ||
| Fig. 1 (a) 2D 13C–13C refocused INADEQUATE spectrum of 13C enriched MTO activated H-ZSM-5 at B0 = 9.4 T and at a MAS frequency of vr = 14 kHz. The signals in the black dashed box have been magnified by a factor of 2, while signals in the green dashed boxes have been processed with a smaller number of t1 points and larger line broadening to account for the shorter T′2 values (see Table S4† for details) and weak intensities of these 13C signals to make them more easily visible. Signals corresponding to carbenium ions (black) and to other neutral carbon species (blue) are highlighted to distinguish them. The assignments of the different carbenium species are given in different colours. Asterisks (*) denote spinning sidebands. (b) Molecular structures of the carbenium ions are identified, colour-coded according to their assignments. (c) Extracted horizontal traces of carbenium ion I with arrows in dashed lines indicating their positions in the 2D map. The corresponding double quantum frequency δDQ of each slice is also given in the figure. The chemical shifts of different 13C sites are given in parenthesis. Unlabelled peaks are from other carbenium ions or aromatic species. Traces obtained for other identified carbeniums ions are shown in Fig. S3–S5.† | ||
A 2D 13C–13C refocused INADEQUATE spectrum is displayed in Fig. 1a and gives correlations mapping out the carbon skeleton of each of the carbenium ions. In this J-based experiment, two directly bonded 13C nuclei share a common frequency in the double quantum (vertical) dimension at the sum of their 13C individual frequencies in the single quantum (horizontal) dimension.28 The peaks observed in the INADEQUATE spectrum notably allowed us to explicitly identify the three methylated carbenium ions, namely the dimethylcyclopentenyl cation I, trimethylcyclopentenyl cation II and pentamethylbenzenium cation III (see Fig. 1b) which have been previously proposed but were identified based on a combination of 1D 13C CP NMR spectra, GC-MS (Gas Chromatography-Mass Spectrometry) and DFT (Density Functional Theory) calculations.19 More explicitly, the dimethylcyclopentenyl cation I can be identified through correlations C1(I) (249 ppm) – C2(I) (147 ppm), C1(I) (249 ppm) – C3(I) (48 ppm) and C1(I) (249 ppm) – C4(I) (25 ppm) as identified in purple in Fig. 1a (and in Fig. 1c for all horizontal traces). A similar approach is used to directly establish the carbon connectivities in cations II (Fig. S3†) and III (Fig. S4†).
A previously unprecedented observed 1,5-dimethyl-3-sec-butyl cyclopentenyl cation IV, is also identified on H-ZSM-5 as revealed by the C3(IV) (255 ppm) – C8(IV) (35 ppm), C8(IV) (35 ppm) – C9(IV) (9 ppm), C8(IV) (35 ppm) – C10(IV) (23 ppm), C10(IV) (23 ppm) – C11(IV) (13 ppm) correlations and correlations amongst C1(IV) to C8(IV) observed in the 2D 13C–13C refocused INADEQUATE spectra (red lines in Fig. 1a and S5†). A cyclopentenyl cation with a tert-butyl group has previously been reported to be involved as a key intermediate in the aromatic-based paring route proposed by theoretical modelling for the formation of isobutene in the MTO reaction, however, it was not experimentally observed.24 In contrast, some work proposed that butenes are formed through an alkene-based cycle involving the methylation/cracking of alkenes.31,32 Hence, the experimental identification of cation IV provides direct support for the aromatic-based paring route for butene formation in H-ZSM-5. The elimination of the sec-butyl group of cation IV is likely to produce but-1-ene and but-2-ene which are also the products of the MTO reaction.33
Several correlations relating to 13C signals in the 235 ppm to 260 ppm region (black lines in Fig. S6†) are also obtained and can be assigned to some additional carbenium ions, most likely cyclopentenyl cations.19,20 The correlation involving the weak signal at 225 ppm may arise from the polymethylcyclohexenyl cations.34 However, the lack of correlations relating these signals with the aliphatic region of the 13C NMR spectrum limits the complete assignments of these signals and is probably due to the low concentration of these carbenium ions as evidenced by their weak signal intensities in the 1D 13C CP MAS spectrum. The low concentration may also account for the apparent absence of ethylated cyclopentenyl cations20 in our MTO activated H-ZSM-5.
The increase in resolution offered in the vertical dimension of the 2D spectrum enables a more accurate determination of the 13C chemical shift values of the different carbon sites from these carbenium ions (Table S4†). Those signals are usually poorly resolved in the 1D CP MAS NMR spectrum (Fig. 1a and Fig. S2† for data at 9.4 T) even at a high magnetic field (see Fig. S2†).
In the 2D 13C–13C refocused INADEQUATE spectrum, signals ranging from 120 to 140 ppm show strong correlations with each other and with signals in the 13–22 ppm region (maroon lines in Fig. S6†). These correlations are attributed to correlations amongst carbons of the benzene rings and between benzene ring carbons and alkyl group carbons of neutral aromatic species, respectively. Aromatics with various types and numbers of substituted alkyl groups have very close chemical shifts,35 which makes individual assignments of signals from these aromatics challenging due to a lack of resolution.
Signals at 60 and 51 ppm have strong intensities in the 1D CP MAS spectrum and show no correlations with any other signals in the 2D spectrum (Fig. 1a). This observation is consistent with their assignments to dimethyl ether and residual adsorbed methanol, respectively.20 Correlations among peaks between 10 and 45 ppm (orange lines in Fig. S6†) can be assigned to alkyl groups of aromatics or carbenium ions.20
Quantitative investigation of the interactions between the confined carbon species and the H-ZSM-5 framework
The interactions between the confined carbon species and zeolite are initially investigated by recording 13C{27Al} S-RESPDOR data, in which the 13C–27Al dipolar couplings are reintroduced by the SR421 recoupling sequences36 on the 13C spins, and provide access to the intramolecular distance. With 27Al irradiation and at a recoupling time of 15 ms, the S-RESPDOR dephasing ΔS/S0 obtained at 9.4 T can be clearly observed, indicating spatial proximities between the 13C and 27Al spins. More specifically, signals from 0 to 40 ppm, corresponding to the alkyl groups of both carbenium ions and aromatics, all have similar signal reduction ΔS/S0 due to being coupled to the 27Al spins (Fig. 2a) and are integrated against the recoupling time in Fig. 2b. Due to the small concentration of 27Al atoms in ZSM-5 (SiO2/Al2O3 = 50), the 13C spins are unlikely to be coupled with multiple 27Al spins and a single spin pair model is used to fit the S-RESPDOR data27 (see ESI† for further details). A 13C–27Al dipolar coupling constant D of 74 ± 12 Hz is extracted and corresponds to an average 13C–27Al distance of 4.7 ± 0.3 Å between the alkyl groups of the confined carbon species and the 27Al sites, and the 27Al NMR spectrum in Fig. S7† shows mainly the framework tetrahedral Al.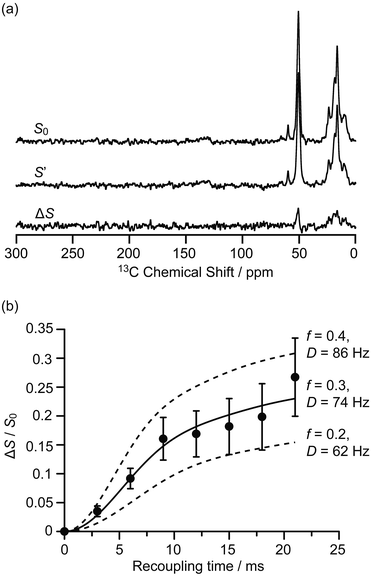 | ||
| Fig. 2 (a) 13C{27Al} S-RESPDOR signals with (S′) and without (S0) 27Al irradiation at an external magnetic field B0 = 9.4 T and with a recoupling time of 15 ms. ΔS is the difference spectrum between S0 and S′. (b) 13C{27Al} S-RESPDOR fraction ΔS/S0 as a function of the recoupling time with the corresponding best-fit curve (black line) and fit boundaries (dashed lines). f is the pre-factor and D is the dipolar coupling constant (see ESI†). The error bars are determined from the signal to noise ratios of the S0 and S′ spectra as measured by the TopSpin3.2 NMR software. | ||
The 9.4 T 29Si CP MAS NMR spectra of the MTO activated H-ZSM-5 (Fig. 3a and S8†) show multiple broad signals at −102, −106, −112 and −117 ppm which are characteristic of the (SiO)3SiOH (Q3), Si(OSi)3(OAl), Si(OSi)4 (Q4) and the crystallographically inequivalent Si(OSi)4 (Q4′) sites, respectively, of which the Si(OSi)3(OAl) sites contribute to the Brønsted acid sites.37 Note that no 29Si signal for the Tn sites of the type R–Si(OSi)n(OH)3−n (typically observed around −60 ppm (ref. 38)) could be detected on H-ZSM-5, indicating that the confined carbon species are not directly covalently bonded to the 29Si nuclei.
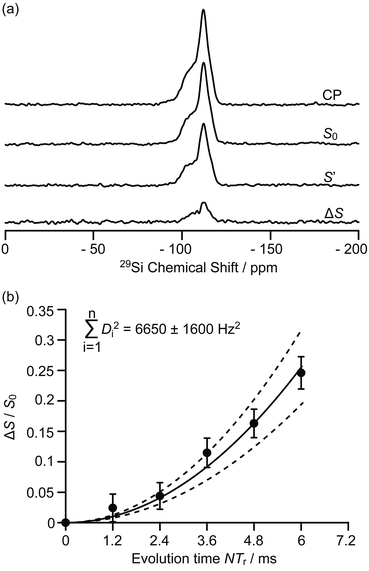 | ||
| Fig. 3 (a) 29Si CP, 29Si CP spin echo signal (S0) and 29Si{13C} REDOR signal with the reintroduction of dipolar couplings (S′) at an external magnetic field B0 = 9.4 T and with an evolution time NTr of 6 ms (Tr is the rotor period, N is the number of rotor periods, see Fig. S1†). ΔS = S0 − S′. (b) Plot of the REDOR fraction ΔS/S0 as a function of the evolution time NTr with the corresponding best-fit curve (black line) and fit boundaries (dashed lines). Vertical error bars are estimated as for the 13C{27Al} S-RESPDOR data. | ||
Spatial interactions between the confined carbon species and the H-ZSM-5 zeolite framework are further probed by 29Si detected 29Si{13C} REDOR experiments (Fig. 3a) which reintroduce the 29Si–13C dipolar couplings under MAS.30 At an evolution time of 6 ms, the intensity of the dephased 29Si NMR signal S′ is significantly reduced vs. the spin echo signal S0, indicating spatial proximities between 29Si and 13C spins. Fig. S8† shows that the different 29Si signals have a similar degree of intensity reduction and these signals cannot be well resolved even at a high field of 20 T (Fig. S9†). Therefore, integration of the whole 29Si NMR signals from −90 to −125 ppm was used to determine the REDOR fraction ΔS/S0 as a function of the evolution time (Fig. 3b). The number of retained carbon species and their unknown geometries with respect to the zeolite impose the use of a geometrically-independent REDOR curve model in which only data for a short dipolar evolution time (ΔS/S0 < 0.3) are needed.39
Fitting the 29Si{13C} REDOR data (see Fig. 3b and ESI†) yields ∑Di2 of 6650 ± 1600 Hz2 which gives an estimated 29Si–13C dipolar coupling constant D of 82 ± 10 Hz (assuming a simplified single spin pair model) and a 29Si to 13C internuclear distance of 4.2 ± 0.2 Å. This distance is comparable to the one obtained above for 13C to 27Al from the 13C{27Al} S-RESPDOR experiments, showing strong interactions between the confined hydrocarbon species and zeolite framework and providing quantitative information for the proposed supramolecular reaction centres in H-ZSM-5.7,40
The interactions between the neutral aromatics, carbenium ions and H-ZSM-5 have previously been investigated computationally.41–45 It was found that it is the confinement of pores via long-range van der Waals interactions between the neutral aromatics and zeolite framework that contributes considerably more to the aromatics’ adsorption in H-ZSM-5 than the short-range interactions between the acid OH group of the zeolite and the electrons of the aromatic ring. These previous works proposed that the aromatics prefer to adsorb in the intersection region between the straight and sinusoidal channels in which polycyclic aromatics grow and block the channels, leading to the catalysts’ deactivation.41–43 The 29Si{13C} REDOR spectra (Fig. S8†) show that different 29Si sites, including the Si(OSi)3(OAl) sites corresponding to the Brønsted acid sites, have apparent similar interactions with 13C nuclei, which indicates that the confinement effects dominate the adsorption of the main hydrocarbon species (neutral aromatics), and that the short-range interactions between the main hydrocarbon species and the Brønsted acid sites may not be strong enough to make a significant difference between these acid sites (and others). These observations suggest that the deactivation of zeolite may not result from the direct poisoning of acid sites, but from blockage of the channels due to the accumulation of aromatics, which is consistent with previous calculations.41 The adsorption model in previous studies showed that the acid O–H bond axis faces the aromatic ring in a nearly perpendicular orientation with the distance between the acidic H and the aromatic ring falling in the 2.2–2.9 Å range.41 Considering an Al–H distance of about 2.4 Å (ref. 43) and the size of aromatics, both 27Al–13C and 29Si–13C distances around 4–5 Å can be expected, matching the values measured above.
Carbenium ions were previously proposed to form ion-pair complexes with the Brønsted acid sites. In the DFT optimised geometry of this complex, the 13C nucleus directly involved in the ionic bonding interaction is around 3.1 Å away from the O of the Brønsted acid sites. Considering the Al–O and Si–O bond distances (1.7 and 1.6 Å respectively)44,45 and the local geometry of the complex, distances between this 13C nucleus and 27Al/29Si can be estimated to be around 4 Å. This 13C nucleus is on the carbenium ring in the optimised geometry and should be the closest one to the Al sites. Hence, we can expect a longer distance between the dangling alkyl groups of the carbenium ions and the Al sites, satisfying the experimental value of 4.7 ± 0.3 Å distance as measured by the 13C{27Al} S-RESPDOR experiments.
Conclusions
In conclusion, we show here that a 13C–13C refocused INADEQUATE experiment on a MTO activated H-ZSM-5 leads to the unambiguous assignment of the 13C NMR spectrum and the direct spectroscopic determination of the molecular structures of the retained carbon species inside the zeolite framework. The spatial proximities between these carbon species and the zeolite framework were probed by 13C{27Al} S-RESPDOR and 29Si{13C} REDOR experiments for which quantitative analysis reveals carbon–aluminium and carbon–silicon host–guest distances in the range of 4.2–4.7 Å, supporting pore confinement interactions (Fig. S10†).Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from BP, University of Liverpool and Dalian Institute of Chemical Physics for a studentship for D. X. (75139), from the National Natural Science Foundation of China (91545104 and 21473182) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2014165) to S. X. and the Engineering and Physical Science Research Councils (EP/M00869X/1) to F. B. are acknowledged. D. X. thanks Nick J. Brownbill and Kenneth K. Inglis (University of Liverpool) for fruitful discussions and assistance on sample preparation. The UK 850 MHz solid-state NMR Facility used in this research was funded by the EPSRC and BBSRC (contract reference PR140003), as well as the University of Warwick via part funding through Birmingham Science City Advanced Materials Projects 1 and 2 supported by Advantage West Midlands (AWM) and the European Regional Development Fund (ERDF). Collaborative assistance from the 850 MHz Facility Manager (Dr Dinu Iuga, University of Warwick) is acknowledged. The experimental data are provided as a supporting dataset from the University of Liverpool Data Catalogue portal at http://doi.org/10.17638/datacat.liverpool.ac.uk/403Notes and references
- P. Tian, Y. Wei, M. Ye and Z. Liu, ACS Catal., 2015, 5, 1922–1938 CrossRef CAS.
- B. V. Vora, T. L. Marker, P. T. Barger, H. R. Nilsen, S. Kvisle and T. Fuglerud, Stud. Surf. Sci. Catal., 1997, 107, 87–98 CrossRef CAS.
- H. Koempel and W. Liebner, Stud. Surf. Sci. Catal., 2007, 167, 261–267 CrossRef.
- M. Stöcker, Microporous Mesoporous Mater., 1999, 29, 3–48 CrossRef.
- J. F. Haw, W. Song, D. M. Marcus and J. B. Nicholas, Acc. Chem. Res., 2003, 36, 317–326 CrossRef CAS PubMed.
- U. Olsbye, S. Svelle, M. Bjørgen, P. Beato, T. V. W. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810–5831 CrossRef CAS PubMed.
- C. Wang, Q. Wang, J. Xu, G. Qi, P. Gao, W. Wang, Y. Zou, N. Feng, X. Liu and F. Deng, Angew. Chem., Int. Ed., 2016, 55, 2507–2511 CrossRef CAS PubMed.
- A. D. Chowdhury, K. Houben, G. T. Whiting, M. Mokhtar, A. M. Asiri, S. A. Al-Thabaiti, S. N. Basahel, M. Baldus and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2016, 55, 15840–15845 CrossRef CAS PubMed.
- Y. Liu, S. Müller, D. Berger, J. Jelic, K. Reuter, M. Tonigold, M. Sanchez-Sanchez and J. A. Lercher, Angew. Chem., Int. Ed., 2016, 55, 5723–5726 CrossRef CAS PubMed.
- X. Wu, S. Xu, W. Zhang, J. Huang, J. Li, B. Yu, Y. Wei and Z. Liu, Angew. Chem., Int. Ed., 2017, 56, 9039–9043 CrossRef CAS PubMed.
- J. D. A. Pelletier and J.-M. Basset, Acc. Chem. Res., 2016, 49, 664–677 CrossRef CAS PubMed.
- F. Blanc, R. Berthoud, C. Copéret, A. Lesage, L. Emsley, R. Singh, T. Kreickmann and R. R. Schrock, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12123–12127 CrossRef CAS PubMed.
- F. Blanc, C. Copéret, A. Lesage and L. Emsley, Chem. Soc. Rev., 2008, 37, 518–526 RSC.
- W. Zhang, S. Xu, X. Han and X. Bao, Chem. Soc. Rev., 2012, 41, 192–210 RSC.
- C. Copéret, A. Comas-Vives, M. P. Conley, D. P. Estes, A. Fedorov, V. Mougel, H. Nagae, F. Núñez-Zarur and P. A. Zhizhko, Chem. Rev., 2016, 116, 323–421 CrossRef PubMed.
- S. Xu, A. Zheng, Y. Wei, J. Chen, J. Li, Y. Chu, M. Zhang, Q. Wang, Y. Zhou, J. Wang, F. Deng and Z. Liu, Angew. Chem., Int. Ed., 2013, 52, 11564–11568 CrossRef CAS PubMed.
- J. Li, Y. Wei, J. Chen, P. Tian, X. Su, S. Xu, Y. Qi, Q. Wang, Y. Zhou, Y. He and Z. Liu, J. Am. Chem. Soc., 2012, 134, 836–839 CrossRef CAS PubMed.
- M. Zhang, S. Xu, J. Li, Y. Wei, Y. Gong, Y. Chu, A. Zheng, J. Wang, W. Zhang, X. Wu, F. Deng and Z. Liu, J. Catal., 2016, 335, 47–57 CrossRef CAS.
- C. Wang, Y. Chu, A. Zheng, J. Xu, Q. Wang, P. Gao, G. Qi, Y. Gong and F. Deng, Chem.–Eur. J., 2014, 20, 12432–12443 CrossRef CAS PubMed.
- C. Wang, X. Yi, J. Xu, G. Qi, P. Gao, W. Wang, Y. Chu, Q. Wang, N. Feng, X. Liu, A. Zheng and F. Deng, Chem.–Eur. J., 2015, 21, 12061–12068 CrossRef CAS PubMed.
- I. M. Dahl and S. Kolboe, J. Catal., 1994, 149, 458–464 CrossRef CAS.
- M. Bjørgen, U. Olsbye, D. Petersen and S. Kolboe, J. Catal., 2004, 221, 1–10 CrossRef.
- B. Arstad, J. B. Nicholas and J. F. Haw, J. Am. Chem. Soc., 2004, 126, 2991–3001 CrossRef CAS PubMed.
- D. M. McCann, D. Lesthaeghe, P. W. Kletnieks, D. R. Guenther, M. J. Hayman, V. Van Speybroeck, M. Waroquier and J. F. Haw, Angew. Chem., Int. Ed., 2008, 47, 5179–5182 CrossRef CAS PubMed.
- C. Bonhomme, C. Gervais, F. Babonneau, C. Coelho, F. Pourpoint, T. Azaïs, S. E. Ashbrook, J. M. Griffin, J. R. Yates, F. Mauri and C. J. Pickard, Chem. Rev., 2012, 112, 5733–5779 CrossRef CAS PubMed.
- S. E. Ashbrook and D. M. Dawson, Acc. Chem. Res., 2013, 46, 1964–1974 CrossRef CAS PubMed.
- X. Lu, O. Lafon, J. Trébosc and J.-P. Amoureux, J. Magn. Reson., 2012, 215, 34–49 CrossRef CAS PubMed.
- A. Lesage, M. Bardet and L. Emsley, J. Am. Chem. Soc., 1999, 121, 10987–10993 CrossRef CAS.
- F. Pourpoint, J. Trébosc, R. M. Gauvin, Q. Wang, O. Lafon, F. Deng and J.-P. Amoureux, ChemPhysChem, 2012, 13, 3605–3615 CrossRef CAS PubMed.
- T. Gullion, Concepts Magn. Reson., 1998, 10, 277–289 CrossRef CAS.
- M. Bjørgen, S. Svelle, F. Joensen, J. Nerlov, S. Kolboe, F. Bonino, L. Palumbo, S. Bordiga and U. Olsbye, J. Catal., 2007, 249, 195–207 CrossRef.
- S. Ilias and A. Bhan, J. Catal., 2012, 290, 186–192 CrossRef CAS.
- P. Dejaifve, J. C. Védrine, V. Bolis and E. G. Derouane, J. Catal., 1980, 63, 331–345 CrossRef CAS.
- W. Dai, C. Wang, M. Dyballa, G. Wu, N. Guan, L. Li, Z. Xie and M. Hunger, ACS Catal., 2015, 5, 317–326 CrossRef CAS.
- Z. W. Qiu, D. M. Grant and R. J. Pugmire, J. Am. Chem. Soc., 1982, 104, 2747–2753 CrossRef CAS.
- A. Brinkmann and A. P. M. Kentgens, J. Am. Chem. Soc., 2006, 128, 14758–14759 CrossRef CAS PubMed.
- Q. Wang, S. Xu, J. Chen, Y. Wei, J. Li, D. Fan, Z. Yu, Y. Qi, Y. He, S. Xu, C. Yuan, Y. Zhou, J. Wang, M. Zhang, B. Su and Z. Liu, RSC Adv., 2014, 4, 21479–21491 RSC.
- S. de Monredon-Senani, C. Bonhomme, F. Ribot and F. Babonneau, J. Sol-Gel Sci. Technol., 2009, 50, 152–157 CrossRef.
- M. Bertmer and H. Eckert, Solid State Nucl. Magn. Reson., 1999, 15, 139–152 CrossRef CAS PubMed.
- J. F. Haw and D. M. Marcus, Top. Catal., 2005, 34, 41–48 CrossRef CAS.
- R. Y. Brogaard, B. M. Weckhuysen and J. K. Nørskov, J. Catal., 2013, 300, 235–241 CrossRef CAS.
- C. Raksakoon and J. Limtrakul, J. Mol. Struct.: THEOCHEM, 2003, 631, 147–156 CrossRef CAS.
- R. Rungsirisakun, B. Jansang, P. Pantu and J. Limtrakul, J. Mol. Struct., 2005, 733, 239–246 CrossRef CAS.
- J. F. Haw, J. B. Nicholas, W. Song, F. Deng, Z. Wang, T. Xu and C. S. Heneghan, J. Am. Chem. Soc., 2000, 122, 4763–4775 CrossRef CAS.
- H. Fang, A. Zheng, J. Xu, S. Li, Y. Chu, L. Chen and F. Deng, J. Phys. Chem. C, 2011, 115, 7429–7439 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc03657d |
| This journal is © The Royal Society of Chemistry 2017 |
