Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Asymmetric syntheses of 8-oxabicyclo[3,2,1]octane and 11-oxatricyclo[5.3.1.0]undecane from glycals†
Hongze
Liao
,
Wei-Lin
Leng
,
Kim
Le Mai Hoang
,
Hui
Yao
,
Jingxi
He
,
Amanda Ying Hui
Voo
and
Xue-Wei
Liu
 *
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371. E-mail: xuewei@ntu.edu.sg
First published on 7th August 2017
Abstract
Herein, we describe an efficient method to prepare enantiomerically pure 8-oxabicyclo[3.2.1]octanes via gold(I)-catalyzed tandem 1,3-acyloxy migration/Ferrier rearrangement of glycal derived 1,6-enyne bearing propargylic carboxylates. The resultant compounds could then undergo interrupted Nazarov cyclization to afford diastereomerically pure 11-oxatricyclo[5.3.1.0]undecanes.
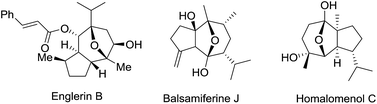
Chiral 8-oxabicyclo[3.2.1]octane and 11-oxatricyclo[5.3.1.0]-undecane are common structural motifs featured in many classes of natural products (Fig. 1), some of which show interesting biological activities. Englerin A, a potential anti-tumor reagent, is isolated from Phyllanthus engleri in Tanzania and shows selective activity to renal cancer cell lines at the nanomolar level.1 Balsamiferine J, isolated from Blumea balsamifera, represents a novel type of sesquiterpenoids with NO inhibitory activity against murine microglial cell lines.2 Homalomenol C, isolated from the roots of Homalomena aromatica, is reported as the bioactive component of the Vietnamese traditional medicine as an anti-inflammatory agent.3
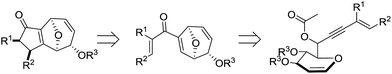
Driven by their important bioactivities, many chemists are interested in the synthesis of this type of natural products. Attempts to gain access to this unique 8-oxabicyclo[3.2.1]-octane ring asymmetrically include [4 + 3],4 [5 + 2],5 [3 + 2]6 cycloadditions and cascade reactions.7 Despite achieving moderate to good diastereoselectivities, these methods suffer from the drawbacks of requiring expensive chiral metal catalysts, requiring installation of non-atom economical auxiliaries and tedious chiral substrate synthesis. However, to our knowledge, there is still no report on the asymmetric synthesis of 11-oxatricyclo[5.3.1.0]undecane.
Tandem and sequential reactions are useful synthetic approaches as they offer the advantages of efficiency as well as reduction of cost and waste.8 Gold-catalyzed transformations of 1,n-enyne bearing propargylic carboxylates to form complex molecules have progressed rapidly in the past decade and an impressive series of transformations have been reported.9 It is well established that the propargylic esters could undergo 1,2- or 1,3-acyloxy migration to give the corresponding gold vinyl carbenoid or allene intermediates10 and these two intermediates could initiate further transformations depending on the reaction conditions and properties of substrates.11 Hence, we envisaged that the glycal linked 1,6-enyne bearing propargylic carboxylates 1 could undergo the 1,3-acyloxy migration and the resulting intermediate would be prone to subsequent Ferrier rearrangement, thus furnishing the enantiomerically pure disubstituted 8-oxabicyclo[3.2.1]octanes 2. If successful, this tandem 1,3-acyloxy migration/Ferrier rearrangement would set the stage for a subsequent Nazarov cyclization12 to form the 11-oxatricyclo[5.3.1.0]undecane derivatives 3 (Scheme 1). To the best of our knowledge, this mode of reactivity is unprecedented in reactions involving 1,n-enyne bearing propargylic carboxylates. Furthermore, this glycal derived 1,6-enyne could be synthesized from readily available glycals through simple steps, making this chirality source cheap and atom economical.
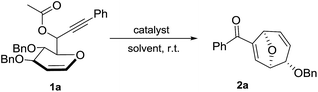
D-Glucal derived 1,6-enyne bearing propargylic carboxylates 1a was selected as the starting material to commence the investigation of this gold-catalyzed tandem reaction.13 To our delight, the reaction could go smoothly with compound 1a as the starting material in the presence of AuCl3 to give the desired product 2a in 69% yield within 5 min (Table 1, entry 1). Other commercially available gold catalysts could also promote this reaction, further proving the feasibility of our strategy (Table 1, entries 2 and 3). Activated Ph3PAuSbF6 generated in situ from Ph3PAuCl/AgSbF6 was found to be most suitable for this reaction (Table 1, entry 4). Subsequently, the counter-ion effect was investigated through examining various silver salts and the AgSbF6 presented the best performance (Table 1, entries 4–6). Attempt to employ the Brønsted acid catalyst, p-TsOH resulted in decomposition of the starting material (Table 1, entry 7). Notably, Ph3PAuCl and AgSbF6 were found to be inactive when they were employed individually (Table 1, entries 8 and 9). Solvent screening showed that CH2Cl2 was superior to other commonly used solvents (Table 1, entries 10–13). To demonstrate the utility of this method, a reaction on a larger scale (0.5 mmol) was carried out and 2a was afforded in 81% yield (Table 1, entry 14). Interestingly, when the propargylic ester group was switched from acetyl group to pivaloyl or benzoyl groups, no significant change was observed (Table 1, entries 15 and 16).
| Entrya | Catalyst | Solvent | Time | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: propargylic ester 1 (0.1 M in CH2Cl2), 5 mol% gold catalyst, 10 mol% silver catalyst. b Isolated yield. c Reaction was carried out on scale of 0.5 M of 1a. d Pivaloyl ester substrate. e Benzoyl ester substrate. DCE = ClCH2CH2Cl, n.r. = no reaction. | ||||
| 1 | AuCl3 | CH2Cl2 | 5 min | 69% |
| 2 | AuCl | CH2Cl2 | 5 min | 42% |
| 3 | Ph3PAuNTf2 | CH2Cl2 | 5 min | 72% |
| 4 | Ph3PAuCl/AgSbF6 | CH2Cl2 | 5 min | 80% |
| 5 | Ph3PAuCl/AgClO4 | CH2Cl2 | 5 min | 62% |
| 6 | Ph3PAuCl/AgOTf | CH2Cl2 | 5 min | 68% |
| 7 | p-TsOH | CH2Cl2 | — | Trace |
| 8 | Ph3PAuCl | CH2Cl2 | — | n.r. |
| 9 | AgSbF6 | CH2Cl2 | — | n.r. |
| 10 | Ph3PAuCl/AgSbF6 | DCE | 5 min | 76% |
| 11 | Ph3PAuCl/AgSbF6 | Toluene | 2 h | 52% |
| 12 | Ph3PAuCl/AgSbF6 | CH3NO2 | 5 min | 66% |
| 13 | Ph3PAuCl/AgSbF6 | CH3CN | 1 h | 48% |
| 14c | Ph3PAuCl/AgSbF6 | CH2Cl2 | 5 min | 81% |
| 15d | Ph3PAuCl/AgSbF6 | CH2Cl2 | 5 min | 79% |
| 16e | Ph3PAuCl/AgSbF6 | CH2Cl2 | 5 min | 72% |
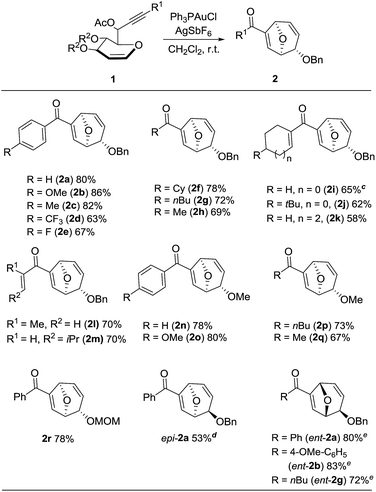
Using the optimal conditions, a range of the glycal derived 1,6-enyne bearing propargylic carboxylates were investigated to demonstrate the wide application of this transformation. In general, the desired products were rapidly obtained in good to excellent yields. The stereochemistry was unambiguously confirmed by X-ray analysis of compound 2b.14 A comparison of the para-substituents of aryl substrates showed that those with electron donating aryl substituents (2b, c) afforded higher yields than those with electron withdrawing substituents (2d, e). Replacing the aryl substituent at the alkyne position with less bulky alkyl substituents, such as cyclohexyl, n-butyl and methyl substituent (2f–h) furnished the corresponding products in good yields. Alkene substituents were also tolerated (2i–m), but the yield decreased when the reaction was carried out on a larger scale (0.5 M). It should be noted that the reaction proceeded readily to give the rearranged products when the benzyl group was changed to methyl or methoxymethyl groups (2n–r). To the best of our knowledge, methoxy group has never been used as the leaving group for Ferrier rearrangement. When L-glycal derived propargylic acetates were tested for this reaction, similar results were obtained (ent-2a, 2b and 2g). As expected, D-galactal derived substrate led to a diminished yield due to the steric effect between benzyl group and propargylic ester (epi-2a). Notably, complete diastereoselectivity was detected in all the examples (Table 2).
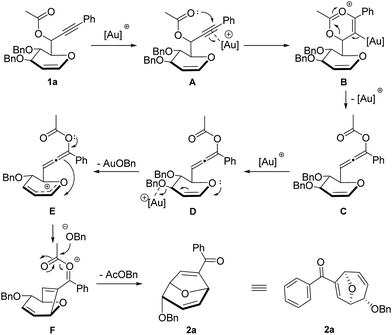
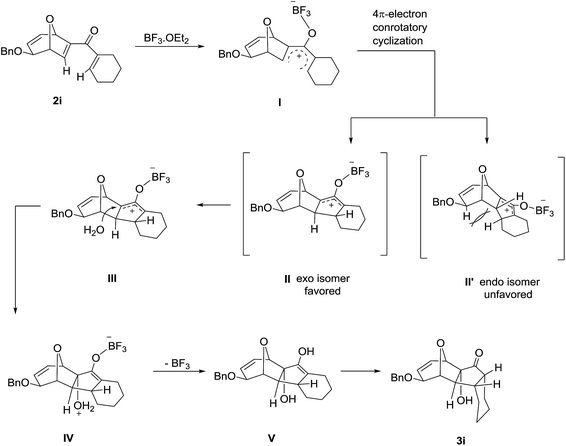
Based on the experimental results, a plausible mechanism for the formation of disubstituted 8-oxabicyclo[3.2.1]octane product is proposed with compound 1a (Scheme 2). The gold catalyst is proposed to have dual role in this transformation. Firstly, it helps to transform the propargylic ester motif of compound 1a into the nucleophilic allenic intermediate C through gold-catalyzed 1,3-acyloxy migration. Secondly, it serves as Lewis acid to facilitate the intramolecular Ferrier reaction by promoting the departure of benzyloxy group and leading to the formation of allylic oxocarbenium ion E.15 Subsequently, electrophilic attack of the allylic oxocarbenium motif to the allene generated oxa-bridged 7-membered ring intermediate F. Finally, the leaving group of Ferrier rearrangement attacks the oxonium species to generate the desired disubstituted 8-oxabicyclo[3.2.1]octane product 2a with benzyl acetate as the byproduct. The complete diastereoselectivity is attributed to cis face attack of allenic ester at C-5 position of glycal derived propargylic esters.
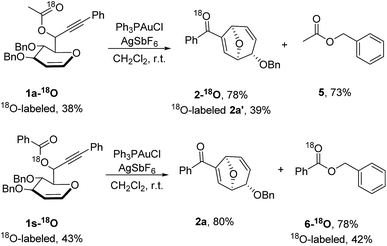
To confirm the mechanism, isotopic labeling experiments were conducted (Scheme 3). When the propargylic acetate 1a-18O with an 18O-enriched carbonyl oxygen atom was synthesized and subjected to the optimized reaction conditions, 18O-labeled 8-oxabicyclo[3.2.1]octane 2a-18O was isolated in 78% yield and 18O containing carbonyl fragment was detected (PhC18O+ with m/z 107.0393). Conversely, 18O label was present in benzyl benzoate 6-18O instead of 8-oxabicyclo[3.2.1]octane 2a-18O when propargylic benzoate 1s-18O with an 18O-enriched ester oxygen was used as the starting material. The following conclusions could be drawn from the isotopic labeling results detected by high-resolution mass spectrometry: firstly, 8-oxabicyclo[3.2.1]octane was generated exclusively from allene intermediate via gold-catalyzed 1,3-acyloxy migration rather than two sequential 1,2-acyloxy migration since scrambling of 18O-label was not detected in 5 or 2a. Secondly, Ferrier rearrangement was initiated by gold-catalyzed 1,3-acyloxy migration and its byproduct benzyl oxoanion quenched the reaction, accounting for the high efficiency and rapid rate for this transformation.
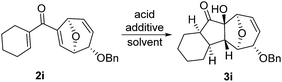
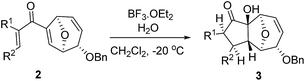
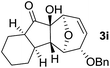
In order to investigate whether Nazarov cyclization could proceed sequentially after tandem 1,3-acyloxy migration/Ferrier rearrangement, a variety of Lewis and Brønsted acids were examined with 2a but gave either intractable mixtures or incomplete consumption of starting material. To our delight, when the divinyl ketone derivative 2i was used as the substrate instead, the interrupted Nazarov cyclization product 3i was furnished. The stereochemistry of compound 3i was confirmed by X-ray structure analysis of its derivative.16 Use of 2 equivalent BF3·OEt2 with 1 equivalent H2O as additive produced the optimal yield of 11-oxatricyclo[5.3.1.0]undecane 3i (Table 3, entry 3), while using CF3SO3H or in absence of H2O also effected conversion to 3i, albeit in lower yield (Table 3, entries 2, 4 and 5). H2SO4 and SnCl4 were examined but an intractable mixture was observed even when the reaction was conducted at a lower temperature (Table 3, entries 1 and 6). Reaction on larger scale (0.2 M) also proceeded smoothly and afforded 3i in 78% yield (Table 3, entry 7).
| Entry | Acid | Additive | Solvent | T/t (h) | Yieldb |
|---|---|---|---|---|---|
| a Reaction conditions: divinyl ketone 2i (0.1 M in CH2Cl2), acid (2 equiv.), additive (1 equiv.). b Isolated yield. c Reaction was carried out on scale of 0.2 M of 2i. | |||||
| 1 | H2SO4 | — | MeOH | −40 °C/1 | Mixture |
| 2 | BF3·OEt2 | — | CH2Cl2 | −20 °C/2 | Trace |
| 3 | BF3·OEt2 | H2O | CH2Cl2 | −20 °C/2 | 77% |
| 4 | CF3SO3H | — | CH2Cl2 | r.t./2 | 18% |
| 5 | CF3SO3H | H2O | CH2Cl2 | r.t./2 | 32% |
| 6 | SnCl4 | — | CH2Cl2 | −40 °C/1 | Mixture |
| 7c | BF3·OEt2 | H2O | CH2Cl2 | −20 °C/2 | 78% |
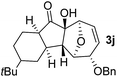
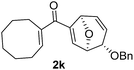
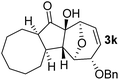
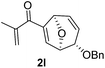
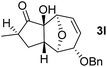
With the optimal interrupted Nazarov cyclization conditions in hand, five 8-oxabicyclo[3.2.1]octane derivatives 2 containing divinyl ketone motif were investigated. Similar with cyclohexenyl ketone 2i, substituted cyclohexenyl ketone 2j and cyclooctenyl ketone 2k could also undergo conversion to the corresponding polycyclic products 3j and 3k in good yields (Table 4, entry 3 and 4). In addition, β-substituted ketone 2m could be applied to form the 11-oxatricyclo[5.3.1.0]undecane 3l, which was the core structure of homalomenol C (Table 4, entry 5). However, α-substituted ketone 2l decomposed under the same condition (Table 4, entry 2).
The high degree of torquoselectivity of 11-oxatricyclo-[5.3.1.0]undecane 3 is quite interesting in this study and we proposed the mechanism as follow (Scheme 4): activated by the BF3·OEt2, the pentadienyl cation I was generated and following by a 4π conrotatory electrocyclization to afford the cyclopentenyl cation intermediate II with complete exo selectivity. The similiar result about high exo selectivity for Nazarov cyclization of norbornene derivatives17b and bicyclo[3.2.1]octane17g,h were previously reported by West et al. and it was ascribed to the alkene predistortion such as transition state allylic bond staggering,17b combination of alkene pyramidalization,17c nonequivalent orbital extension,17d steric crowding17e and torsional strain.17f Houk and co-workers have shown that bicyclo[3.2.1]octane derivatives without through-space interaction would reach quite high exo selectivity in electrocyclization in DFT calculations.17a In our case, the nonconjugated alkene does not involve this reaction and the cycloalkene motifs create more steric repulsion and torsional strain in the transition state which further promote the exo stereoselectivity. Sequentially, the external H2O trapped the cyclopentenyl cation II from the endo face of the polycyclic system which is less bulky after electrocyclization. Finally, the intermediate IV was transformed into 11-oxatricyclo[5.3.1.0]undecane 3 through hydrogen shift and enol–keto tautomerism.
Conclusion
In summary, a novel homogeneous gold-catalyzed tandem 1,3-acyloxy migration/Ferrier rearrangement was developed successfully to access disubstituted 8-oxabicyclo[3.2.1]octane with high efficiency and complete diastereoselectivity using glycal-derived propargylic esters. The resultant products could then undergo an interrupted Nazarov cyclization serving as an efficient strategy for the facile synthesis of diastereomerically pure 11-oxatricyclo[5.3.1.0]undecanes which was applied to synthesize the core structure of homalomenol C. More studies on mechanism and natural product synthesis are currently in progress.Acknowledgements
We gratefully acknowledge Nanyang Technological University (RG6/13 and RG132/14) and the Ministry of Education, Singapore (MOE 2013-T3-1-002) for the financial support of this research. We thank Dr Ganguly Rakesh for X-ray analysis.Notes and references
- R. Ratnayake, D. Covell, T. T. Ransom, K. R. Gustafson and J. A. Beutler, Org. Lett., 2009, 11, 57–60 CrossRef CAS PubMed.
- J. Xu, D. Q. Jin, C. Liu, C. Xie, Y. Guo and L. Fang, J. Agric. Food Chem., 2012, 60, 8051–8058 CrossRef CAS PubMed.
- (a) L. R. Salgueiro, R. Vila, F. Tomi, X. Tomas, S. Cañigueral, J. Casanava, A. P. D. Cunha and T. Adzet, Phytochemistry, 1997, 45, 1177–1183 CrossRef CAS; (b) T. V. Sung, L. Kutschabsky, A. Porzel, W. Steglich and G. Adam, Phytochemistry, 1992, 31, 1659–1661 CrossRef CAS.
- (a) B. Lo, S. Lam, W.-T. Wong and P. Chiu, Angew. Chem., Int. Ed., 2012, 51, 12120–12123 CrossRef CAS PubMed; (b) J. Huang and R. P. Hsung, J. Am. Chem. Soc., 2005, 127, 50–51 CrossRef CAS PubMed; (c) M. Harmata, S. K. Ghosh, X. Hong, S. Wacharasindhu and P. Kirchhoefer, J. Am. Chem. Soc., 2003, 125, 2058–2059 CrossRef CAS PubMed; (d) C. B. W. Stark, U. Eggert and H. M. R. Hoffmann, Angew. Chem., Int. Ed., 1998, 37, 1266–1268 CrossRef CAS.
- (a) A. Orue, U. Uria, E. Reyes, L. Carrillo and J. L. Vicario, Angew. Chem., Int. Ed., 2015, 54, 3043–3046 CrossRef CAS PubMed; (b) M. R. Witten and E. N. Jacobsen, Angew. Chem., Int. Ed., 2014, 53, 5912–5916 CrossRef CAS PubMed; (c) N. Z. Burns, M. R. Witten and E. N. Jacobsen, J. Am. Chem. Soc., 2011, 133, 14578–14581 CrossRef CAS PubMed; (d) F. López, L. Castedo and J. L. Mascareñas, Org. Lett., 2002, 4, 3683–3685 CrossRef.
- (a) F. Rodier, M. Rajzmann, J.-L. Parrain, G. Chouraqui and L. Commeiras, Chem.–Eur. J., 2013, 19, 2467–2477 CrossRef CAS PubMed; (b) K. Ishida, H. Kusama and N. Iwasawa, J. Am. Chem. Soc., 2010, 132, 8842–8843 CrossRef CAS PubMed; (c) N. Shimada, M. Anada, S. Nakamura, H. Nambu, H. Tsutsui and S. Hashimoto, Org. Lett., 2008, 10, 3603–3606 CrossRef CAS PubMed; (d) D. M. Hodgson, A. H. Labande, F. Y. T. M. Pierard and M. A. Expósito Castro, J. Org. Chem., 2003, 68, 6153–6159 CrossRef CAS PubMed; (e) S. Kitagaki, M. Anada, O. Kataoka, K. Matsuno, C. Umeda, N. Watanabe and S.-I. Hashimoto, J. Am. Chem. Soc., 1999, 121, 1417–1418 CrossRef CAS.
- (a) J. Fu, Y. Gu, H. Yuan, T. Luo, S. Liu, Y. Lan, J. Gong and Z. Yang, Nat. Commun., 2015, 6, 8617, DOI:10.1038/ncomms9617; (b) H. Faustino, I. Alonso, J. L. Mascareñas and F. Lõpez, Angew. Chem., Int. Ed., 2013, 52, 6526–6530 CrossRef CAS PubMed; (c) B. Li, Y.-J. Zhao, Y.-C. Lai and T. P. Loh, Angew. Chem., Int. Ed., 2012, 51, 8041–8045 CrossRef CAS PubMed.
- (a) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134–7186 CrossRef CAS PubMed; (b) J.-C. Wasilke, S. J. Obrey, R. T. Baker and G. C. B. Bazan, Chem. Rev., 2005, 105, 1001–1020 CrossRef CAS PubMed.
- (a) A. S. K. Hashmi, W. Yang, Y. Yu, M. M. Hansmann, M. Rudolph and F. Rominger, Angew. Chem., Int. Ed., 2013, 52, 1329–1332 CrossRef CAS PubMed; (b) J. W. Cran and M. E. Krafft, Angew. Chem., Int. Ed., 2012, 51, 9398–9402 CrossRef CAS PubMed; (c) W. Rao, D. Susanti and P. W. H. Chan, J. Am. Chem. Soc., 2011, 133, 15248–15251 CrossRef CAS PubMed; (d) S. Bhunia and R.-S. Liu, J. Am. Chem. Soc., 2008, 130, 16488–16489 CrossRef CAS PubMed; (e) N. Marion, P. de Frémont, G. Lemière, E. D. Stevens, L. Fensterbank, M. Malacria and S. P. Nolan, Chem. Commun., 2006, 2048–2050 RSC; (f) L. Zhang and S. Wang, J. Am. Chem. Soc., 2006, 128, 1442–1443 CrossRef CAS PubMed; (g) A. Buzas and F. Gagosz, J. Am. Chem. Soc., 2006, 128, 12614–12615 CrossRef CAS PubMed; (h) X. Shi, D. J. Gorin and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 5802–5803 CrossRef CAS PubMed; (i) V. Mamane, T. Gress, H. Krause and A. Fürstner, J. Am. Chem. Soc., 2004, 126, 8654–8655 CrossRef CAS PubMed.
- F. D. Toste, in Modern Gold Catalyzed Synthesis, ed. A. S. K. Hashmi and F. D. Toste, Wiley-VCH, Weinheim, 2012, pp. 75–134 Search PubMed.
- (a) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028–9072 CrossRef CAS PubMed; (b) L. Fensterbank and M. Malacria, Acc. Chem. Res., 2014, 47, 953–965 CrossRef CAS PubMed; (c) R. K. Shiroodi and V. Gevorgyan, Chem. Soc. Rev., 2013, 42, 4991–5001 RSC; (d) X.-Z. Shu, D. Shu, C. M. Schienebeck and W. Tang, Chem. Soc. Rev., 2012, 41, 7698–7711 RSC; (e) A. S. Dudnik, N. Chernyak and V. Gevorgyan, Aldrichimica Acta, 2010, 43, 37–46 CAS; (f) S. Wang, G. Zhang and L. Zhang, Synlett, 2010, 692–706 CAS; (g) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351–3378 CrossRef CAS PubMed; (h) E. Jiménez-Núñez and A. M. Echavarren, Chem. Commun., 2007, 333–346 RSC; (i) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed.
- (a) S. P. Simeonov, J. P. Nunes, K. Guerra, V. B. Kurteva and C. A. Afonso, Chem. Rev., 2016, 116, 5744–5893 CrossRef CAS PubMed; (b) M. J. Di Grandi, Org. Biomol. Chem., 2014, 12, 5331–5345 RSC; (c) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804–818 RSC; (d) W. T. Spencer III, T. Vaidya and A. J. Frontier, Eur. J. Org. Chem., 2013, 3621–3633 CrossRef PubMed; (e) N. Shimada, C. Stewart and M. A. Tius, Tetrahedron, 2011, 67, 5851–5870 CrossRef CAS PubMed; (f) T. N. Grant, C. J. Rieder and F. G. West, Chem. Commun., 2009, 5676–5688 RSC; (g) H. Pellissier, Tetrahedron, 2005, 61, 6479–6517 CrossRef CAS; (h) M. A. Tius, Eur. J. Org. Chem., 2005, 2193–2206 CrossRef CAS.
- Preparation of all the propargyl ester substrates 1 is included in Section B, general procedure A and B in the ESI.†.
- See Section E, Table 1 (2b, CCDC 1436794) in the ESI† for the crystal structure.
- (a) R. Roy, P. Rajasekaran, A. Mallick and Y. D. Vankar, Eur. J. Org. Chem., 2014, 5564–5573 CrossRef CAS; (b) A. M. Gómez, F. Lobo, C. Uriel and J. C. López, Eur. J. Org. Chem., 2013, 7221–7262 CrossRef; (c) E. Kumaran, M. Santhi, K. K. Balasubramanian and S. Bhagavathy, Carbohydr. Res., 2011, 346, 1654–1661 CrossRef CAS PubMed; (d) R. Balamurugan and S. R. Koppolu, Tetrahedron, 2009, 65, 8139–8142 CrossRef CAS; (e) P. Nagaraj and N. G. Ramesh, Eur. J. Org. Chem., 2008, 4607–4614 CrossRef CAS; (f) S. Kashyap, S. Rao Vidadala and S. Hotha, Tetrahedron Lett., 2007, 48, 8960–8962 CrossRef CAS.
- See Section E, Table 2 (9, CCDC 1520731) in the ESI† for the crystal structure.
- (a) A. Patel, F. G. West and K. N. Houk, J. Org. Chem., 2015, 80, 2790–2795 CrossRef CAS PubMed; (b) R. D. Mazzola Jr, T. D. White, H. R. Vollmer-Snarr and F. G. West, Org. Lett., 2005, 7, 2799–2801 CrossRef PubMed; (c) S. Giese, R. D. Mazola Jr, C. M. Amann, A. M. Arif and F. G. West, Angew. Chem., Int. Ed., 2005, 44, 6546–6549 CrossRef CAS PubMed; (d) N. G. Rondan, M. N. Paddon-Row, P. Caramella, J. Mareda, P. H. Muller and K. H. Houk, J. Am. Chem. Soc., 1982, 104, 4976–4978 CrossRef; (e) N. G. Rondan, M. N. Paddon-Row, P. Caramella and K. H. Houk, J. Am. Chem. Soc., 1981, 103, 2436–2438 CrossRef CAS; (f) S. Inagaki, H. Fujimoto and K. Fukui, J. Am. Chem. Soc., 1976, 98, 4054–4061 CrossRef CAS; (g) H. C. Brown, J. H. Kawakami and K.-T. Liu, J. Am. Chem. Soc., 1973, 95, 2209–2216 CrossRef CAS; (h) P. V. R. Schleyer, J. Am. Chem. Soc., 1967, 89, 701–703 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterisation data for all compounds are provided. CCDC 1436794 and 1520731. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc02625k |
| This journal is © The Royal Society of Chemistry 2017 |