 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nickel-catalyzed transamidation of aliphatic amide derivatives†
Jacob E.
Dander‡
,
Emma L.
Baker‡
and
Neil K.
Garg
 *
*
Department of Chemistry and Biochemistry, University of California, 607 Charles Young Drive East, Box 951569, Los Angeles, CA 90095, USA. E-mail: neilgarg@chem.ucla.edu
First published on 10th July 2017
Abstract
Transamidation, or the conversion of one amide to another, is a long-standing challenge in organic synthesis. Although notable progress has been made in the transamidation of primary amides, the transamidation of secondary amides has remained underdeveloped, especially when considering aliphatic substrates. Herein, we report a two-step approach to achieve the transamidation of secondary aliphatic amides, which relies on non-precious metal catalysis. The method involves initial Boc-functionalization of secondary amide substrates to weaken the amide C–N bond. Subsequent treatment with a nickel catalyst, in the presence of an appropriate amine coupling partner, then delivers the net transamidated products. The transformation proceeds in synthetically useful yields across a range of substrates. A series of competition experiments delineate selectivity patterns that should influence future synthetic design. Moreover, the transamidation of Boc-activated secondary amide derivatives bearing epimerizable stereocenters underscores the mildness and synthetic utility of this methodology. This study provides the most general solution to the classic problem of secondary amide transamidation reported to date.
Introduction
The ability to convert one amide to another, known as the transamidation reaction, represents a long-standing synthetic challenge.1,2 Although significant progress has been made with regard to the transamidation of 1° amides,3 the corresponding reaction involving secondary amides (1 + 2 → 3 + 4) has remained largely underdeveloped (Fig. 1). Two factors are primarily responsible for the difficulty of this transformation. First, the kinetic barrier to break the amide C–N bond is considered high because of well-known resonance effects.4 The second complication stems from thermodynamics, as the energetics of starting materials and products in transamidation reactions are often comparable, resulting in thermoneutral rections.1a,5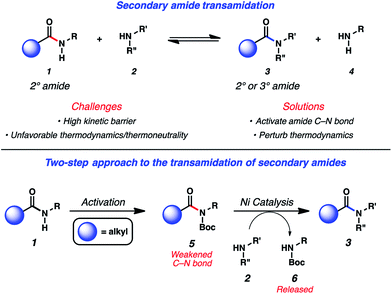 | ||
| Fig. 1 Challenges associated with secondary amide transamidation and the two step-approach to realize this challenging synthetic transformation. | ||
Despite these challenges, several breakthroughs have been reported with regard to secondary amide transamidation. Gellman and Stahl utilized a dimeric aluminum complex to affect secondary amide transamidation, albeit with equilibrium mixtures resulting, thus highlighting the difficulty regarding thermodynamics.6 Bertrand has reported a means to achieve secondary amide transamidation of simple substrates using excess AlCl3.7 In both of these cases, the transamidation is made possible by Lewis acid activation of the amide carbonyl. Most recently, Szostak reported two simple protocols for achieving the transamidation of secondary amide derivatives, each with a focus on benzamide-derived substrates. The first uses Lewis base catalysis,8 while the other utilizes Pd–NHC complexes.9 Despite these discoveries, a general solution to the transamidation of aliphatic 2° amides has remained elusive.
In considering the challenges noted earlier, we sought to develop an alternative strategy to achieve the transamidation of secondary amides. As summarized in Fig. 1, it was envisioned that N-functionalization of secondary amide substrates 1 could lead to weakening of the acyl amide C–N bond,10 if the appropriate activating group was utilized. Electron-withdrawing groups, such as the Boc group, were viewed as ideal for this purpose,11 given the ease by which Boc groups can be introduced. From the resulting Boc-activated secondary amide 5, it was believed that oxidative addition with an appropriate nickel catalyst could occur through a reasonable kinetic process. In situ interception of this species with an amine nucleophile 2 would furnish transamidated product 3. The process would be driven thermodynamically by the favorable release of carbamate 6.12
Encouraged by the successful activation of amide C–N bonds using transition metal catalysis, as demonstrated by studies from Szostak, Shi, and our laboratory,13–17 we explored the sequence described above. In 2016, we validated this approach to achieve a two-step transamidation of N-Bn,Boc benzamide derivatives.18 However, the corresponding reaction sequence using substrates derived from aliphatic secondary amides was unsuccessful. In this manuscript, we describe our efforts to overcome this hurdle, which have led to the nickel-catalyzed transamidation of aliphatic amide derivatives. The methodology presented herein offers a robust solution to the classic problem of secondary aliphatic amide transamidation, and is expected to inform future efforts toward natural product synthesis and derivatization.
Results and discussion
Reaction discovery and optimization
To initiate our studies, we selected imide 7, obtained by Boc-activation of the corresponding aliphatic amide, and cyclohexylamine (8) as the reaction partners (Table 1). As both coupling partners possess α-branching and are sterically hindered, they were considered excellent challenges for methodology development. We first tested the amidation using 10 mol% Ni(cod)2 and 20 mol% SIPr (10), to parallel the conditions we employed in our original disclosure involving benzamide substrates (entry 1).18 As anticipated, only a low yield of amide 9 was obtained. We also tested terpyridine (11) as the ligand (entry 2), as this was shown to be effective in promoting the Ni-catalyzed esterification of aliphatic amide derivatives.14e To our surprise, no reaction occurred, which prompted us to evaluate additional NHC ligands. We were delighted to find that use of ligand precursor 12, in combination with NaOtBu for in situ free-basing, afforded the desired amide product 9 in quantitative yield (entry 3). We attribute the improved competency of 12 (compared to 10) to its more electron-rich nature, which ultimately renders oxidative addition more facile.19,20 Of note, the reaction also took place using lower amounts of catalyst, ligand, and base (entry 4). Finally, we found that by increasing the concentration, the equivalents of amine could be reduced from 2.0 to 1.5, while also allowing for shorter reaction times (entry 5). These conditions (entry 5) were found to be sufficiently general and were used to explore the reaction scope. Of note, in the absence of Ni(cod)2, no reaction occurs, thus demonstrating that the conversion of 7 to 9 is indeed catalyzed by nickel.21 Likewise, Lewis base-promoted transamidation conditions were also deemed ineffective.22| Entry | Mol% of Ni(cod)2 | Ligand (mol%) | Mol% of NaOtBu | Equiv. of 8 | Conc. | Time | Yield of 9a |
|---|---|---|---|---|---|---|---|
| a Yields determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. | |||||||
| 1 | 10 | 10 (20 mol%) | — | 2.0 | 1.0 M | 24 h | 15% |
| 2 | 10 | 11 (20 mol%) | — | 2.0 | 1.0 M | 24 h | 0% |
| 3 | 10 | 12 (20 mol%) | 22 | 2.0 | 1.0 M | 24 h | 100% |
| 4 | 5 | 12 (10 mol%) | 11 | 2.0 | 1.0 M | 24 h | 100% |
| 5 | 5 | 12 (10 mol%) | 11 | 1.5 | 1.5 M | 18 h | 90% |
|
|||||||
Scope of methodology
Having arrived at suitable reaction conditions to achieve the transamidation of aliphatic amide derivative 7, we explored the generality of our methodology by first varying the aliphatic amide partner (Fig. 2). Using cyclohexylamine (8), a variety of amide derivatives underwent the desired transamidation reaction. Beginning with the parent example, the desired amide product 9 was obtained in 82% isolated yield. Similarly, the corresponding cyclopentyl substrate could be utilized in the transamidation reaction to furnish 15 in excellent yield. As a further test of the methodology, an indane substrate was evaluated and found to undergo smooth coupling to give amide 16. Additionally, two substrates bearing sterically encumbered t-butyl groups were tested. When the t-butyl group was positioned on the alpha carbon, neopentylic amide 17 was obtained in 70% yield. Direct linkage of the t-butyl group to the amide carbonyl carbon also did not hinder the reaction, as judged by the formation of pivalamide 18. Lastly, we evaluated two piperidine-containing substrates, given the prevalence and importance of piperidines in medicinal chemistry.23,24 In both cases, the desired secondary amides were obtained in synthetically useful yields, as shown by the formation of 19 and 20.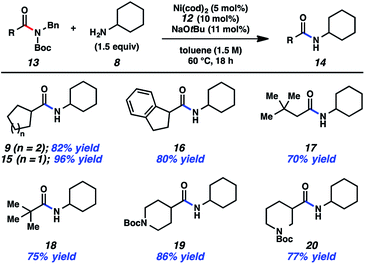 | ||
| Fig. 2 Variation of the amide substrate. Yields shown reflect the average of two isolation experiments. | ||
As shown in Fig. 3, the scope of this methodology was not limited to N-benzylamide derivatives. For example, N-n-Bu-containing substrate 22 could be employed, thus demonstrating that the aromatic benzyl substituent was not critical for success. Additionally, α-branching was tolerated, as judged by the successful coupling of the isopropylamine-derived substrate 23. The methodology also displayed notable tolerance to sterics, given that t-butylamine substrate 24 could be coupled with cyclohexylamine (8) to furnish 9 in 59% yield.
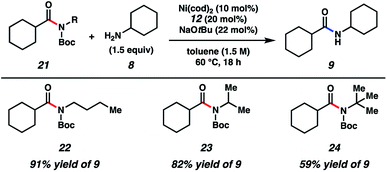 | ||
| Fig. 3 Scope of the amide substrate N-substituent. Yields shown reflect the average of two isolation experiments. | ||
The scope of this methodology with respect to the amine nucleophile was also evaluated (Fig. 4). Several α-branched primary amines were tested, such as cyclopentyl amine, iso-propylamine, and sec-phenethylamine. These experiments led to the desired amides, 26–28, respectively, in good to excellent yields. Even t-butylamine, which bears considerable steric hindrance, could be coupled as shown by the formation of 29, albeit in somewhat diminished yield. p-Trifluoromethylbenzylamine was also utilized and gave rise to amide 30 in 76% yield. This result showcases an unbranched primary amine nucleophile, while also incorporating the medicinally relevant –CF3 group.25 The formation of 31a and 31b demonstrate that aniline and aniline derivatives can be utilized in this methodology. It should also be emphasized that secondary amines can be employed in the transamidation reaction to give tertiary amide products. The formation of 32–34, which relied on the use of pyrrolidine, indoline, and morpholine, respectively, are representative of this notion.
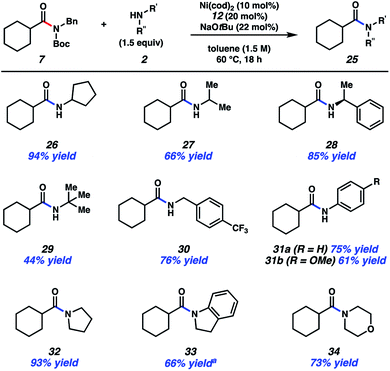 | ||
| Fig. 4 Scope of the amine nucleophile. Yields shown reflect the average of two isolation experiments. aYield determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. | ||
Amine competition studies
With the aim of identifying selectivity patterns that may aid in synthetic design, a series of competition experiments were performed using substrate 7 and various amine nucleophiles (Fig. 5). First, we compared p-trifluoromethylbenzylamine (35) and cyclohexylamine (8). The major product obtained was benzylamide 30 in 82% yield, with 9 being formed as the minor product. We attribute this selectivity to steric factors. Next, we compared p-trifluoromethylbenzylamine (35) and pyrrolidine (36). This reaction gave nearly a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of products 30 and 32, suggesting a fine balance between steric and electronic factors about the nucleophilic nitrogen in this case. In another comparison, pyrrolidine (36) and cyclohexylamine (8) were treated with amide 7. Pyrrolidine-derived tertiary amide 32 was formed as the major product, rather than secondary amide 9, consistent with the relative nucleophilicity of the amines being utilized.26 Lastly, we performed a competition experiment between cyclohexylamine (8) and t-butylamine (37), which led to the exclusive formation of cyclohexylamide 9, presumably as a result of steric factors.
1 ratio of products 30 and 32, suggesting a fine balance between steric and electronic factors about the nucleophilic nitrogen in this case. In another comparison, pyrrolidine (36) and cyclohexylamine (8) were treated with amide 7. Pyrrolidine-derived tertiary amide 32 was formed as the major product, rather than secondary amide 9, consistent with the relative nucleophilicity of the amines being utilized.26 Lastly, we performed a competition experiment between cyclohexylamine (8) and t-butylamine (37), which led to the exclusive formation of cyclohexylamide 9, presumably as a result of steric factors.
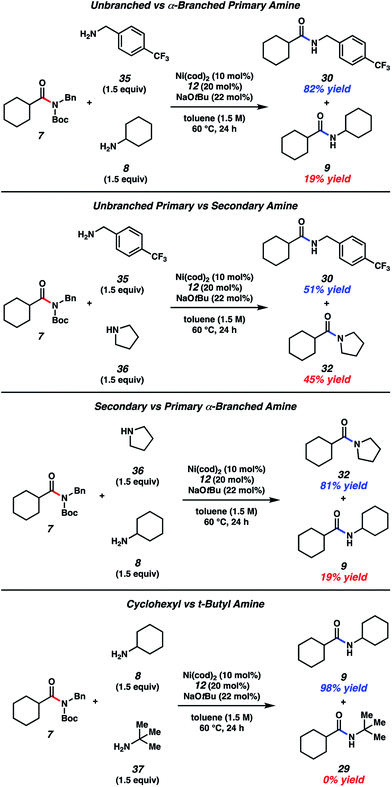 | ||
| Fig. 5 A series of amine competition experiments. Yields determined by 1H NMR analysis using hexamethylbenzene as an internal standard. | ||
As a final test of our methodology, we evaluated two substrates that each bear an epimerizable stereocenter (Fig. 6). Treatment of cyclohexenamide 38 with cyclohexylamine (8) under typical reaction conditions, notably using only 3 mol% Ni(cod)2, delivered amide 39 in 78% yield on gram-scale. Of note, product 39 was obtained in 90% ee, indicative of minimal racemization occurring. Attempts to couple proline-derived amide 40 using our standard reaction protocol, on the other hand, led to substantial epimerization. We attribute this to the increased acidity of the substrate's α-proton relative to 38. As a workaround, we developed a modified protocol that involves free-basing of ligand 12 in the presence of Ni(cod)2 in toluene to access the active catalyst in solution. Addition of the catalyst solution and amine 8 to substrate 40 afforded amide 41 after 18 h at 60 °C. Amide 41 was obtained in 60% yield and high optical purity. The mild and scalable nature of the reaction conditions bodes well for future synthetic applications.
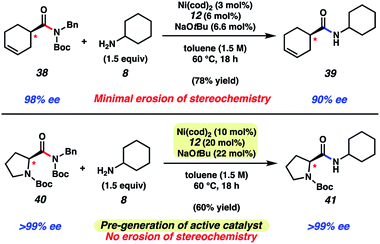 | ||
| Fig. 6 Transamidation of enantioenriched amide substrate 38 on gram-scale and transamidation of enantioenriched N-Boc proline substrate 40 using a modified protocol. | ||
Conclusions
We have developed a facile approach to achieve the transamidation of secondary aliphatic amides, an unmet challenge in organic synthesis. Our strategy involves first preparing Boc-activated secondary amide derivatives and subsequently treating them with appropriate amine coupling partners under Ni-mediated reaction conditions. The methodology delivers secondary and tertiary amide products in synthetically useful yields across a range of substrates and amine nucleophiles. A variety of competition experiments were undertaken to reveal selectivity patterns, the results of which are expected to influence future synthetic design. Moreover, the transamidation of N-functionalized secondary amide derivatives bearing epimerizable stereocenters highlights the mildness and synthetic utility of this transformation. This study addresses the long-standing problem of secondary amide transamidation through the use of a general and mild nickel catalysis platform.Acknowledgements
The authors are grateful to the NIH (T32-GM067555 and R01-GM117016), the NSF (DGE-1144087 for E. L. B.), the Foote Family (J. E. D. and E. L. B.), and the University of California, Los Angeles, for financial support. These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the NIH NCRR (S10RR025631).Notes and references
- (a) M. E. González-Rosende, E. Castillo, J. Lasri and J. Sepúlveda-Arques, Prog. React. Kinet. Mech., 2004, 29, 311–332 CrossRef; (b) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed; (c) A. Ojeda-Porras and D. J. Gamba-Sanchez, J. Org. Chem., 2016, 81, 11548–11555 CrossRef CAS PubMed.
- For a recent review on amide bond formation, see: R. M. Figueiredo, J.-S. Suppo and J.-M. Campagne, Chem. Rev., 2016, 116, 12029–12122 CrossRef PubMed.
- For primary amide transamidations, see: (a) T. A. Dineen, M. A. Zajac and A. G. Myers, J. Am. Chem. Soc., 2006, 128, 1419–1422 CrossRef PubMed; (b) C. L. Allen, B. N. Atkinson and J. M. J. Williams, Angew. Chem., Int. Ed., 2012, 51, 1383–1386 CrossRef CAS PubMed; (c) L. Becerra-Figueroa, A. Ojeda-Porras and D. Gamba-Sánchez, J. Org. Chem., 2014, 79, 4544–4552 CrossRef CAS PubMed; (d) S. N. Rao, D. C. Mohan and S. Adimurthy, Green Chem., 2014, 16, 4122–4126 RSC; (e) S. N. Rao, D. C. Mohan and S. Adimurthy, Org. Lett., 2013, 15, 1496–1499 CrossRef CAS PubMed; (f) S. N. Rao, D. C. Mohan and S. Adimurthy, RSC Adv., 2015, 5, 95313–95317 RSC.
- (a) The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science, ed. A. Greenberg, C. M. Breneman, and J. F. Liebman, John Wiley & Sons, Inc., Hoboken, N.J., 2003 Search PubMed; (b) L. Pauling, R. B. Corey and H. R. Branson, Proc. Natl. Acad. Sci. U. S. A., 1951, 37, 205–211 CrossRef CAS PubMed.
- Z. Mucsi, G. A. Chass and I. G. Csizmadia, J. Phys. Chem. B, 2008, 112, 7885–7893 CrossRef CAS PubMed.
- S. E. Eldred, D. A. Stone, S. H. Gellman and S. S. Stahl, J. Am. Chem. Soc., 2003, 125, 3422–4036 CrossRef CAS PubMed.
- E. Bon, D. C. H. Bigg and G. Bertrand, J. Org. Chem., 1994, 59, 4035–4036 CrossRef CAS.
- Y. Liu, S. Shi, M. Achtenhagen, R. Liu and M. Szostak, Org. Lett., 2017, 19, 1614–1617 CrossRef CAS PubMed.
- G. Meng, P. Lei and M. Szostak, Org. Lett., 2017, 19, 2158–2161 CrossRef CAS PubMed.
- For studies on amide bond destabilization/activation, see: (a) A. Greenberg and C. A. Venanzi, J. Am. Chem. Soc., 1993, 115, 6951–6957 CrossRef CAS; (b) A. Greenberg, D. T. Moore and T. D. DuBois, J. Am. Chem. Soc., 1996, 118, 8658–8668 CrossRef CAS; (c) K. Tani and B. M. Stoltz, Nature, 2006, 441, 731–734 CrossRef CAS PubMed; (d) A. J. Kirby, I. V. Komarov, P. D. Wothers and N. Feeder, Angew. Chem., Int. Ed., 1998, 37, 785–786 CrossRef CAS; (e) R. Szostak, S. Shi, G. Meng, R. Lalancette and M. Szostak, J. Org. Chem., 2016, 81, 8091–8094 CrossRef CAS PubMed; (f) V. Pace, W. Holzer, G. Meng, S. Shi, R. Lalancette, R. Szostak and M. Szostak, Chem.–Eur. J., 2016, 22, 14494–14498 CrossRef CAS PubMed; (g) R. Szostak, J. Aubé and M. Szostak, Chem. Commun., 2015, 51, 6395–6398 RSC; (h) R. Szostak, G. Meng and M. Szostak, J. Org. Chem., 2017, 82, 6373–6378 CrossRef CAS PubMed.
- S. K. Davidsen, P. D. May and J. B. Summers, J. Org. Chem., 1991, 56, 5482–5485 CrossRef CAS.
- Z. Mucsi, G. A. Chass and I. G. Csizmadia, J. Phys. Chem. B, 2008, 112, 7885–7893 CrossRef CAS PubMed.
- For reviews on amide cross-couplings, see: (a) J. E. Dander and N. K. Garg, ACS Catal., 2017, 7, 1413–1423 CrossRef CAS PubMed; (b) G. Meng, S. Shi and M. Szostak, Synlett, 2016, 27, 2530–2540 CrossRef CAS; (c) C. Liu and M. Szostak, Chem.–Eur. J., 2017, 23, 7157–7173 CrossRef CAS PubMed.
- For nickel-catalyzed amide C–N bond activations from our laboratory, see: (a) L. Hie, N. F. Fine Nathel, T. K. Shah, E. L. Baker, X. Hong, Y.-F. Yang, P. Liu, K. N. Houk and N. K. Garg, Nature, 2015, 524, 79–83 CrossRef CAS PubMed; (b) N. A. Weires, E. L. Baker and N. K. Garg, Nat. Chem., 2016, 8, 75–79 CrossRef CAS PubMed; (c) B. J. Simmons, N. A. Weires, J. E. Dander and N. K. Garg, ACS Catal., 2016, 6, 3176–3179 CrossRef CAS; (d) J. E. Dander, N. A. Weires and N. K. Garg, Org. Lett., 2016, 18, 3934–3936 CrossRef CAS PubMed; (e) L. Hie, E. L. Baker, S. M. Anthony, J.-N. Desrosiers, C. Senanayake and N. K. Garg, Angew. Chem., Int. Ed., 2016, 55, 15129–15132 CrossRef CAS PubMed.
- For nickel-catalyzed amide C–N bond activations from other laboratories, see: (a) J. Hu, Y. Zhao, Y. Zhang and Z. Shi, Angew. Chem., Int. Ed., 2016, 55, 8718–8722 CrossRef CAS PubMed; (b) S. Shi, G. Meng and M. Szostak, Angew. Chem., Int. Ed., 2016, 55, 6959–6963 CrossRef CAS PubMed; (c) S. Shi and M. Szostak, Chem.–Eur. J., 2016, 22, 10420–10424 CrossRef CAS PubMed; (d) S. Shi and M. Szostak, Org. Lett., 2016, 18, 5872–5875 CrossRef CAS PubMed; (e) A. Dey, S. Sasmal, K. Seth, G. K. Lahiri and D. Maiti, ACS Catal., 2017, 7, 433–437 CrossRef CAS; (f) S. Shi and M. Szostak, Synthesis, 2017, 49 DOI:10.105/s-0036-1588845.
- For Pd-catalyzed and Rh-catalyzed amide C–N bond activation studies, see: (a) G. Meng and M. Szostak, Org. Lett., 2015, 17, 4364–4367 CrossRef CAS PubMed; (b) G. Meng and M. Szostak, Org. Biomol. Chem., 2016, 14, 5690–5705 RSC; (c) C. Liu, G. Meng, Y. Liu, R. Liu, R. Lalancette, R. Szostak and M. Szostak, Org. Lett., 2016, 18, 4194–4197 CrossRef CAS PubMed; (d) G. Meng and M. Szostak, Angew. Chem., Int. Ed., 2015, 54, 14518–14522 CrossRef CAS PubMed; (e) G. Meng, S. Shi and M. Szostak, ACS Catal., 2016, 6, 7335–7339 CrossRef CAS; (f) X. Li and G. Zou, Chem. Commun., 2015, 51, 5089–5092 RSC; (g) G. Meng and M. Szostak, Org. Lett., 2016, 18, 796–799 CrossRef CAS PubMed; (h) C. Liu, G. Meng and M. Szostak, J. Org. Chem., 2016, 81, 12023–12030 CrossRef CAS PubMed; (i) P. Lei, G. Meng and M. Szostak, ACS Catal., 2017, 7, 1960–1965 CrossRef CAS; (j) M. Cui, H. Wu, J. Jian, H. Wang, C. Liu, S. Daniel and Z. Zeng, Chem. Commun., 2016, 52, 12076–12079 RSC; (k) H. Wu, Y. Li, M. Cui, J. Jian and Z. Zeng, Adv. Synth. Catal., 2016, 358, 3876–3880 CrossRef CAS.
- For reviews concerning Ni catalysis, see: (a) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299–309 CrossRef CAS PubMed; (b) T. Mesganaw and N. K. Garg, Org. Process Res. Dev., 2013, 17, 29–39 CrossRef CAS; (c) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346–1416 CrossRef CAS PubMed.
- E. L. Baker, M. M. Yamano, Y. Zhou, S. M. Anthony and N. K. Garg, Nat. Commun., 2016, 7, 11554 CrossRef PubMed.
- M. N. Hopkins, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef PubMed.
- The corresponding coupling of 7 and 8 under standard reaction conditions (e.g., entry 5, Table 1 conditions) using the related 1,3-dicyclohexylimidazolium (ICy) chloride ligand salt gave 9 in 82% yield via1H NMR analysis using hexamethylbenzene as an external standard.
- Control experiments using standard reaction conditions absent Ni(cod)2, as well as experiments performed without Ni(cod)2, 12, and NaOtBu led to minimal conversion to the desired transamidated product. See Section B of the ESI† for details.
- The Lewis base-promoted transamidation of an unbranched N-Ph,Boc aliphatic amide derivative using benzylamine as nucleophile was recently demonstrated (see ref. 7). Attempts to utilize these conditions for the transamidation of less activated N-Bn,Boc aliphatic amide derivatives have thus far been unsuccessful. See Section I of the ESI† for details.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- A. Gomtsyan, Chem. Heterocycl. Compd., 2012, 48, 7–10 CrossRef CAS.
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (b) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed; (c) K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305–321 CrossRef CAS; (d) C. Isanbor and D. O'Hagan, J. Fluorine Chem., 2006, 127, 303–319 CrossRef CAS.
- According to “Mayr's Database of Reactivity”, the nucleophilicity parameter (N) of pyrrolidine is 17.21 and 12.00 for comparable i-PrNH2 in H2O (a nucleophilicity parameter is not reported for cyclohexylamine (8)), http://www.cup.lmu.de/oc/mayr/reaktionsdatenbank/fe/showclass/41.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc01980g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |

