Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The lubricating role of water in the shuttling of rotaxanes†
Haohao
Fu
a,
Xueguang
Shao
abc,
Christophe
Chipot
 def and
Wensheng
Cai
def and
Wensheng
Cai
 *ab
*ab
aResearch Center for Analytical Sciences, College of Chemistry, Tianjin Key Laboratory of Biosensing and Molecular Recognition, Nankai University, Tianjin 300071, China. E-mail: wscai@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300071, China
cState Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin 300071, China
dLaboratoire International Associé Centre National de la Recherche Scientifique et University of Illinois at Urbana-Champaign, Unité Mixte de Recherche No. 7565, Université de Lorraine, B.P. 70239, 54506 Vandœuvre-lès-Nancy cedex, France
eTheoretical and Computational Biophysics Group, Beckman Institute, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, USA
fDepartment of Physics, University of Illinois at Urbana-Champaign, 1110 West Green Street, Urbana, Illinois 61801, USA
First published on 16th May 2017
Abstract
We have investigated at the atomic level amide-based rotaxanes set in motion in four different solvents, namely, ethyl ether, acetonitrile, ethanol and water. In three non-aqueous solvents, shuttling of the macrocycle between two binding sites separated by a free-energy barrier is coupled with a conformational change and rotation, driven primarily by hydrogen-bonding interactions. The mechanism that underlies the shuttling is completely altered when the non-aqueous solvent is replaced by water. In aqueous solution, hydrophobic interactions chiefly control shuttling of the rotaxane, leading to a sharp decrease of the free-energy barrier, thereby speeding up the process. The binding sites and the reaction pathway describing shuttling vary significantly in water compared with in the other three solvents. We found that the high polarity, the hydrogen-bond donor and acceptor ability, and the minimal steric hindrance of water conspire to modify the mechanism. These three physicochemical properties are also responsible for the lubrication by water. That water completely changes the mechanism underlying the shuttling of rotaxanes, is addressed for the first time in this study, and provides valuable guidelines for the de novo design of molecular machines.
Introduction
Over the past decades, artificial molecular machines, or nanomachines, have proven extremely useful as devices that mimic macroscopic instruments.1–6 Rotaxanes,7 which consist of a dumbbell-shaped (or chain-like) molecule threaded onto a macrocycle, form the primary component of many types of nanomachines, like nanomuscles,8–10 nanovalves,11–13 nanorecorders,14,15 drug carriers,16–18 product lines,19 or locks.20 In these molecular architectures, the macrocyclic rings usually shuttle between different binding sites in response to an external stimulus,21,22 such as light,23–26 redox,27,28 a change of pH,29,30 temperature31,32 or solvent.31,33 The polarity of the solvent is generally considered to be crucial in controlling the motion of a rotaxane. However, in some hydrogen-bonded rotaxanes, like the amide-based rotaxane investigated by Panman et al.,34 only water can accelerate the shuttling rate of the macrocycle, whereas other polar solvents appear to have a limited effect. Why water alone possesses this unique property remains in large measure poorly understood. The main thrust of the present contribution is to shed light onto the distinctive role played by water in amide-based rotaxanes from the perspective of the thermodynamics of shuttling and the structural changes that accompany it.Our previous theoretical investigations have revealed that contrary to chemical intuition, shuttling is usually coupled to other different movements. Mapping the free-energy landscape that describes shuttling, therefore, requires careful modeling of the underlying reaction coordinate.35,36 Toward this end, in the present study, we have decoupled the overall shuttling process of the amide-based rotaxane34 into several possible movements. We have determined the free-energy landscape characterizing the shuttling process in four different solvents, i.e. diethyl ether (Et2O), acetonitrile (ACN), ethanol (EtOH) and water. Key differences between the reaction pathways describing the shuttling of the macrocycle in water and in non-aqueous solvents rationalize the distinctive shuttling mechanism observed in water.
Results and discussion
Movements and reaction coordinate model
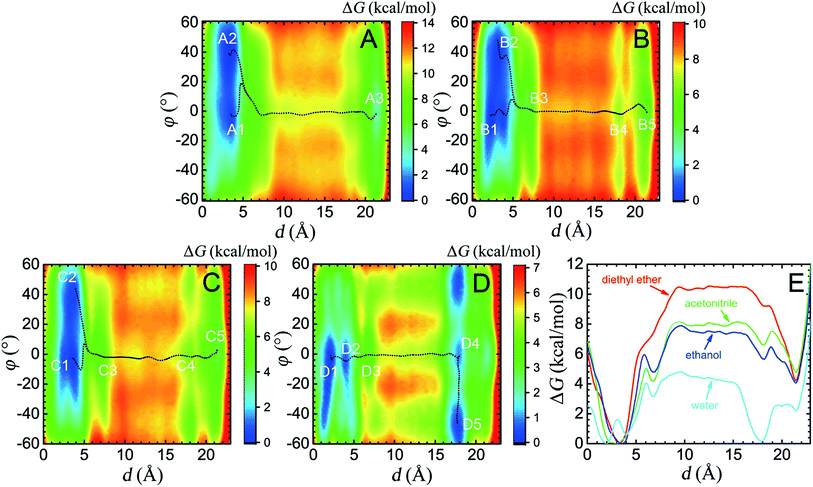
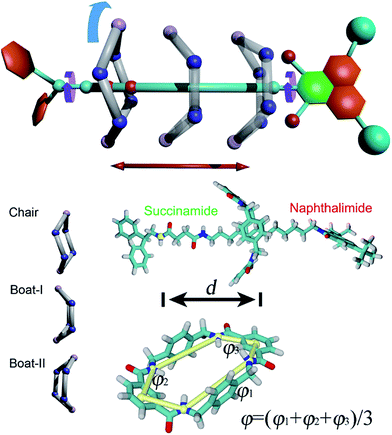
The structure of the rotaxane investigated herein is depicted in Fig. 1A and S1 of the ESI.† The dumbbell-like molecule contains a succinamide moiety forming the left-hand binding site of the chain, and a naphthalimide group forming its right-hand binding site, capped with a stopper. The macrocycle is a benzylic amide ring including four amide moieties. In light of a preliminary simulation, the nature of the shuttling of this rotaxane was found to be particularly intricate. At least four possible movements were found to be coupled to each other, namely the translational movement, the conformational change and the rotation of the ring-like molecule, as well as the spin of the terminal groups of the dumbbell-shaped molecule (Scheme 1). Due to the inherent complexity of the intercoupled movements and the limitation of brute-force molecular dynamics simulations, importance-sampling-based free-energy calculations37 were employed here. Exhaustive scanning of the free-energy hyperplane is, however, computationally expensive when the number of dimension exceeds 3. We, therefore, dissected the shuttling process of the rotaxane carefully, and selected two primary coarse variables to form the transition coordinate, namely the distance, d, and the average dihedral angle, φ, defined in Scheme 1, to describe the translation and the conformational change of the ring-like molecule, respectively. To validate the choice of the coarse variable φ, the free-energy profile (or potential of mean force, PMF) for the isomerization of the macrocycle was determined, as shown in Fig. S2 of the ESI.† The resulting PMF clearly suggests that φ represents an appropriate variable. In the following two-dimensional free-energy calculations, the recently developed extended adaptive biasing force method38–40 was used. Alternate methodologies are detailed in the Methods section of the ESI.†To support our choice of the reaction coordinate model, the PMF delineating the translation coupled with the conformational change of the ring in the rotaxane in a vacuum was calculated as depicted in Fig. S3.† Two binding sites can be clearly identified in the one dimensional free-energy profile of Fig. S3B,† namely, one on the succinamide group (left-hand binding site) and the other on the two carbonyl moieties of the naphthalimide moiety (right-hand binding site). The four types of intercoupled movements depicted in the Scheme 1 are thoroughly analyzed in Fig. S4–S6.† To further investigate whether the chosen transition coordinate is germane to characterize the movements of the rotaxane, or, in other words, whether the translation and the conformational change are the principal components of the rotaxane shuttling, a committor analysis41,42 was performed. The results shown in Fig. S7† clearly suggest that the applied coarse variables are suitable for modeling the rotaxane in motion.
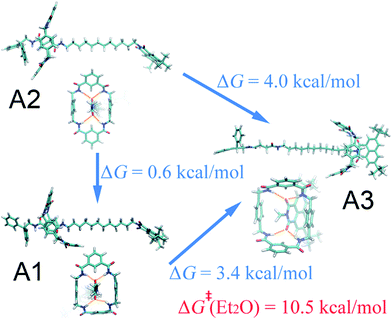
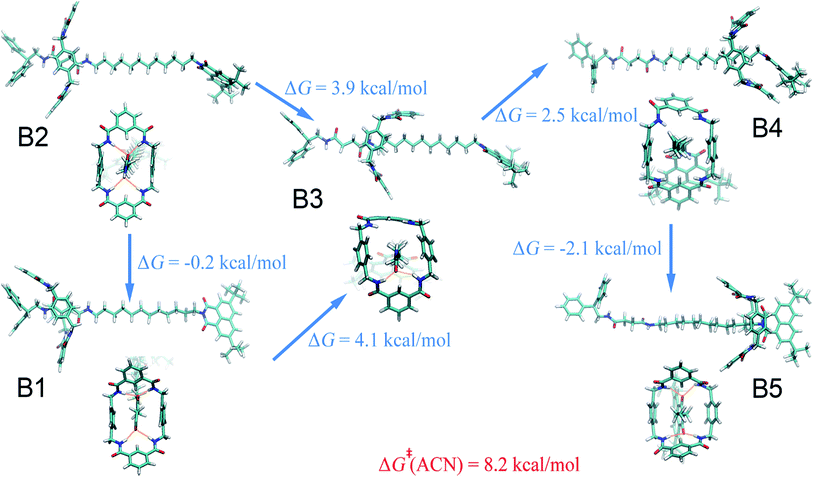
Effects of different solvents on the mechanism underlying shuttling
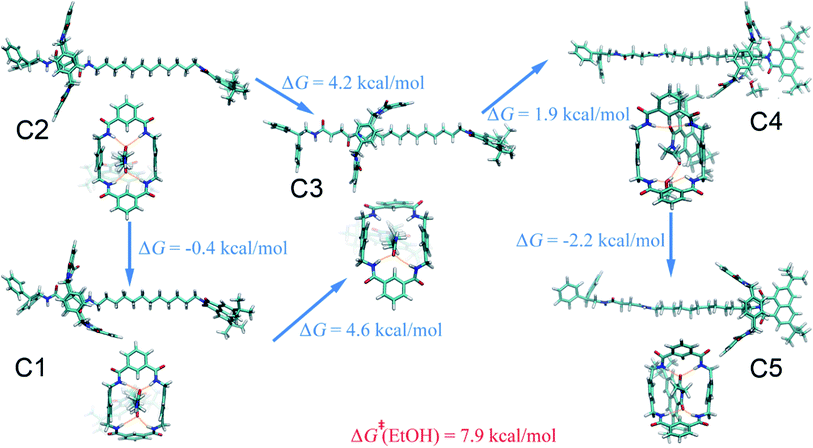
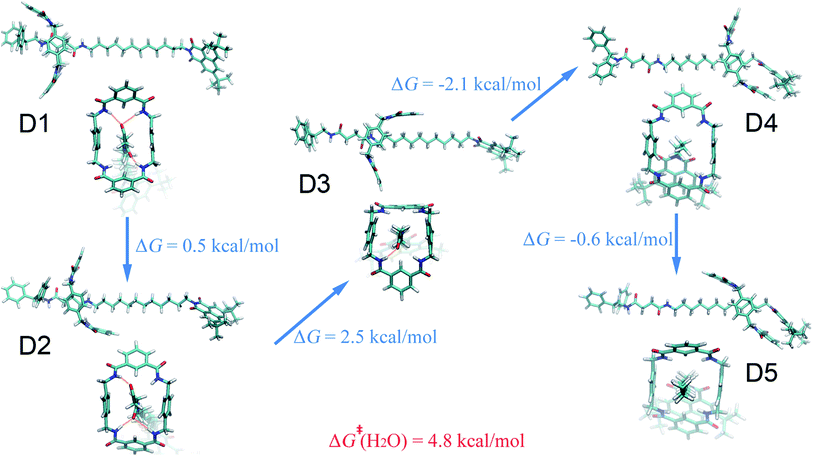
Four solvents with different properties were adopted in this study, i.e. diethyl ether, acetonitrile, ethanol and water. The properties of these solvents are highlighted in Table S1.† Detail of the molecular assemblies studied in this work is provided in Table S2.† The free-energy landscapes characterizing the translation and conformational change of the ring in different solvents are shown in Fig. 1A–D. The least free-energy pathways connecting the local minima on the hyperplane were determined using the LFEP algorithm.43The special property of the rotaxane in water can be intuitively explained by the representative structures depicted in Fig. 5. Hydrogen-bonding interactions of the macrocycle with the chain are significantly weakened in water, as shown in Fig. S9.† In motifs D1 and D2, the phenylene groups of the ring interact with the stopper and/or the aliphatic chain connecting with the succinamide moiety. The macrocycle does not interconvert spontaneously in a chair conformation at the left-hand binding site (Fig. S10†) to minimize the surface area of the hydrophobic part of the chain exposed to the aqueous environment (Fig. S11†). No ring-chain hydrogen bond is formed in structures D4 and D5 (Fig. 5 and S9†). A favorable π–π stacking interaction greatly stabilizes structure D5 instead, making the latter almost as energetically favorable as structure D1. It can be inferred that hydrogen bonding is no longer the driving force responsible for the translocation of the macrocycle from the left-hand binding site to the right-hand one. On the contrary, hydrophobic interactions contribute the most to the shuttling process. This result is also supported by the free-energy decomposition of Fig. S8D.†
Shuttling rate and mechanism of lubrication by water
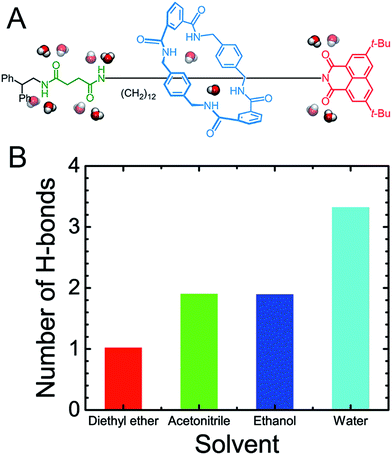
As may be seen in Fig. 1E, the free-energy barrier to be overcome for translocating the ring from the left-hand binding site to the right-hand one drops as the polarity of the solvent increases. In particular, the free-energy barrier reduced sharply in water, compared with non-aqueous solvents, thereby, accelerating shuttling significantly. The inherent properties of water can rationalize its unique lubricant role. First, when the macrocycle interacts with the succinamide moiety, the contribution to the free energy of the hydrogen bonds formed between the ring and the chain is greatly reduced due to the high polarity of water. The pronounced disrupting effect of water on the ring-chain hydrogen bonds can be seen in Fig. S9 (ESI†). Hydrophobic interactions, therefore, become dominant and drive the shuttling of the macrocycle ring from left to right. Second, the phenylene groups of the macrocycle can interact with the hydrophobic part of the supramolecular assembly, either the aliphatic chain or the aromatic stopper, during shuttling. This solvophobic interaction is significantly stronger in water, compared with other solvents. Last, when the ring moves away from the succinamide moiety to the alkly chain, water molecules can form hydrogen bonds with the carbonyl groups of the chain and the amino groups of the ring (Fig. 6), thus, compensating the energy loss due to breaking hydrogen bonds between the ring and the chain. As a result, the free-energy barrier against translocating the ring from the left-hand binding site to the right-hand one greatly reduced. Diethyl ether and acetonitrile, in stark contrast, are not hydrogen bond donors and, hence, cannot form hydrogen bonds with the carbonyl moieties of the chain. Moreover, diethyl ether, acetonitrile and ethanol are larger in volume, compared with the water molecule. It is, therefore, entropically difficult for the former molecules to insert into the cavity formed by the chain and the macrocycle to stabilize the amino moieties of the latter, as indicated in Fig. 6. To prove the proposed volumetric effect, the hydrogen bonds formed between the ring and the solvents when the coarse variable d = 12–16 Å, i.e., the ring is around the aliphatic part of the chain, were analyzed. Fig. 6 clearly indicates that the number of such hydrogen bonds in water is much greater than in any other solvent, thus, suggesting the importance of the small volume of water in acting as a lubricant.Conclusions
The amide ring of the rotaxane investigated in this study adopts different conformations along the reaction pathways connecting the left-hand and right-hand binding sites in the four solvents examined in this study on account of their distinct properties. The driving forces responsible for the shuttling of the macrocycle directly contribute to the distinct three-dimensional arrangements of the rotaxane. In diethyl ether, acetonitrile and ethanol, the translation coupled with conformational transition is driven by hydrogen bonding. In stark contrast, the left-to-right shuttling in an aqueous solution is controlled chiefly by hydrophobic interactions. The low free-energy barrier against shuttling stems from lubrication effect by water. The special properties of water can be summarized as follows—a high polarity, the ability of being both a hydrogen donor and an acceptor, and a very small molecular volume, which can greatly weaken the hydrogen bonds between the ring and the dumbbell-like molecule and stabilize the transition states. These properties of water, hence, greatly reduce the friction between the chain and the ring of the rotaxane due to hydrogen bonding, making it act as a lubricant to enhance the shuttling in this supramolecular architecture. This conclusion obtained from amide-based rotaxanes can be used to rationalize the lubricating role of water in the motion of biological and abiological machines consisting of hydrogen-bonded components.44,45 For example, the rotation of the motor protein ATPase is generally believed to be controlled by electrostatic interactions. Water can, however, help hydrophobic residues induce local deformations prior to electrostatically driven rotation, thereby reducing the barriers of side-chain dissociation and association that drive stalk rotation.45Taking advantage of the carefully chosen transition coordinate and subsequent free-energy calculations, as well as other analyses, we bring to light the intertwined movements of the rotaxane, which lays the groundwork for exploring the features of more complex molecular machines. Moreover, we emphasize that translation coupled with conformational change of the rotaxane may be completely altered by solvation in a different environment, thereby providing a new perspective for the rational design of solvent-controlled nanomachines. Among the most common solvents ordinarily found at the bench, water is in many respects particularly unique, as has been shown in this study. Its special properties may modulate the driving force of molecular architectures, leading to unexpected phenomena.
Acknowledgements
This study is supported by National Natural Science Foundation of China (No. 21373117). Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase) of China and the GENCI and CINES, Montpellier, France, are gratefully acknowledged for provision of generous amounts of CPU time.Notes and references
- V. Balzani, A. Credi, F. M. Raymo and J. F. Stoddart, Angew. Chem., Int. Ed., 2000, 39, 3348–3391 CrossRef CAS PubMed.
- S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS PubMed.
- A. Coskun, M. Banaszak, R. D. Astumian, J. F. Stoddart and B. A. Grzybowski, Chem. Soc. Rev., 2012, 41, 19–30 RSC.
- B. Champin, P. Mobian and J.-P. Sauvage, Chem. Soc. Rev., 2007, 36, 358–366 RSC.
- W. R. Browne and B. L. Feringa, Nat. Nanotechnol., 2006, 1, 25–35 CrossRef CAS PubMed.
- J. M. Abendroth, O. S. Bushuyev, P. S. Weiss and C. J. Barrett, ACS Nano, 2015, 9, 7746–7768 CrossRef CAS PubMed.
- V. Balzani, M. Gómez-López and J. F. Stoddart, Acc. Chem. Res., 1998, 31, 405–414 CrossRef CAS.
- J.-P. Collin, C. Dietrich-Buchecker, P. Gaviña, M. C. Jimenez-Molero and J.-P. Sauvage, Acc. Chem. Res., 2001, 34, 477–487 CrossRef CAS PubMed.
- M. C. Jimenez-Molero, C. Dietrich-Buchecker and J.-P. Sauvage, Chem. Commun., 2003, 1613–1616 RSC.
- C. J. Bruns and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 2186–2199 CrossRef CAS PubMed.
- S. Saha, K. C. F. Leung, T. D. Nguyen, J. F. Stoddart and J. I. Zink, Adv. Funct. Mater., 2007, 17, 685–693 CrossRef CAS.
- H. Meng, M. Xue, T. Xia, Y. Zhao, F. Tamanoi, J. F. Stoddart, J. I. Zink and A. E. Nel, J. Am. Chem. Soc., 2010, 132, 12690–12697 CrossRef CAS PubMed.
- J. Croissant and J. I. Zink, J. Am. Chem. Soc., 2012, 134, 7628–7631 CrossRef CAS PubMed.
- J. E. Green, J. W. Choi, A. Boukai, Y. Bunimovich, E. Johnston-Halperin, E. DeIonno, Y. Luo, B. A. Sheriff, K. Xu, Y. S. Shin, H. Tseng, J. F. Stoddart and J. R. Heath, Nature, 2007, 445, 414–417 CrossRef CAS PubMed.
- M. Feng, X. Guo, X. Lin, X. He, W. Ji, S. Du, D. Zhang, D. Zhu and H. Gao, J. Am. Chem. Soc., 2005, 127, 15338–15339 CrossRef CAS PubMed.
- X. Bao, I. Isaacsohn, A. F. Drew and D. B. Smithrud, J. Am. Chem. Soc., 2006, 128, 12229–12238 CrossRef CAS PubMed.
- X. Wang and D. B. Smithrud, Bioorg. Med. Chem. Lett., 2011, 21, 6880–6883 CrossRef CAS PubMed.
- G. Yu, D. Wu, Y. Li, Z. Zhang, L. Shao, J. Zhou, Q. Hu, G. Tang and F. Huang, Chem. Sci., 2016, 7, 3017–3024 RSC.
- B. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. Gramlich, D. Heckmann, S. M. Goldup, D. M. D'Souza, A. E. Fernandes and D. A. Leigh, Science, 2013, 339, 189–193 CrossRef CAS PubMed.
- D. H. Qu and B. L. Feringa, Angew. Chem., Int. Ed., 2010, 49, 1107–1110 CrossRef CAS PubMed.
- M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS PubMed.
- H. Tian and Q. Wang, Chem. Soc. Rev., 2006, 35, 361–374 RSC.
- A. M. Brouwer, C. Frochot, F. G. Gatti, D. A. Leigh, L. Mottier, F. Paolucci, S. Roffia and G. W. H. Wurpel, Science, 2001, 291, 2124–2128 CrossRef CAS PubMed.
- H. Murakami, A. Kawabuchi, K. Kotoo, M. Kunitake and N. Nakashima, J. Am. Chem. Soc., 1997, 119, 7605–7606 CrossRef CAS.
- C. A. Stanier, S. J. Alderman, T. D. W. Claridge and H. L. Anderson, Angew. Chem., 2002, 114, 1847–1850 CrossRef.
- D. Qu, Q. Wang, Q. Zhang, X. Ma and H. Tian, Chem. Rev., 2015, 115, 7543–7588 CrossRef CAS PubMed.
- Y. Zhao, W. R. Dichtel, A. Trabolsi, S. Saha, I. Aprahamian and J. F. Stoddart, J. Am. Chem. Soc., 2008, 130, 11294–11296 CrossRef CAS PubMed.
- N. Kihara, M. Hashimoto and T. Takata, Org. Lett., 2004, 6, 1693–1696 CrossRef CAS PubMed.
- V. Blanco, A. Carlone, K. D. Hänni, D. A. Leigh and B. Lewandowski, Angew. Chem., 2012, 124, 5256–5259 CrossRef.
- D. Tuncel and M. Katterle, Chem.–Eur. J., 2008, 14, 4110–4116 CrossRef CAS PubMed.
- S. Dong, J. Yuan and F. Huang, Chem. Sci., 2014, 5, 247–252 RSC.
- Y. Inoue, P. Kuad, Y. Okumura, Y. Takashima, H. Yamaguchi and A. Harada, J. Am. Chem. Soc., 2007, 129, 6396–6397 CrossRef CAS PubMed.
- Z. Zhang, C. Han, G. Yu and F. Huang, Chem. Sci., 2012, 3, 3026–3031 RSC.
- M. R. Panman, B. H. Bakker, D. den Uyl, E. R. Kay, D. A. Leigh, W. J. Buma, A. M. Brouwer, J. A. J. Geenevasen and S. Woutersen, Nat. Chem., 2013, 5, 929–934 CrossRef CAS PubMed.
- P. Liu, X. Shao, C. Chipot and W. Cai, Chem. Sci., 2016, 7, 457–462 RSC.
- S. Wang, T. Zhao, X. Shao, C. Chipot and W. Cai, J. Phys. Chem. C, 2016, 120, 19479–19486 CAS.
- C. Chipot, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2014, 4, 71–89 CrossRef CAS.
- H. Fu, X. Shao, C. Chipot and W. Cai, J. Chem. Theory Comput., 2016, 12, 3506–3513 CrossRef CAS PubMed.
- L. Zheng and W. Yang, J. Chem. Theory Comput., 2012, 8, 810–823 CrossRef CAS PubMed.
- T. Lelièvre, M. Rousset and G. Stoltz, J. Chem. Phys., 2007, 126, 134111 CrossRef PubMed.
- P. G. Bolhuis, D. Chandler, C. Dellago and P. L. Geissler, Annu. Rev. Phys. Chem., 2002, 53, 291–318 CrossRef CAS PubMed.
- A. C. Pan, D. Sezer and B. Roux, J. Phys. Chem. B, 2008, 112, 3432–3440 CrossRef CAS PubMed.
- B. Ensing, A. Laio, M. Parrinello and M. L. Klein, J. Phys. Chem. B, 2005, 109, 6676–6687 CrossRef CAS PubMed.
- L. D. Barron, L. Hecht and G. Wilson, Biochemistry, 1997, 36, 13143–13147 CrossRef CAS PubMed.
- A. Singharoy, C. Chipot, M. Moradi and K. Schulten, J. Am. Chem. Soc., 2017, 139, 293–310 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Methods, structure of the rotaxane, free-energy calculation characterizing the isomerization of the ring-like molecule, free-energy landscape for the translation and conformational change of the ring in the rotaxane in vacuum, representative three-dimensional arrangements of the rotaxane in vacuum, rotation of the macrocycle along with translation, rotation of the terminal group of the dumbbell-like molecule accompanied with other movements in the rotaxane, one-dimensional free-energy decomposition, committor analysis, hydrogen bond analysis, boat–boat transformation of the macrocycle during the shuttling, solvent-accessible surface area (SASA) of the chain-like molecule along the transition coordinate in different solvents. Simulation parameters. See DOI: 10.1039/c7sc01593c |
| This journal is © The Royal Society of Chemistry 2017 |