Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single operation palladium catalysed C(sp3)–H functionalisation of tertiary aldehydes: investigations into transient imine directing groups†
S.
St John-Campbell
 ,
A. J. P.
White
,
A. J. P.
White
 and
J. A.
Bull
and
J. A.
Bull
 *
*
Department of Chemistry, Imperial College London, South Kensington, London, SW7 2AZ, UK. E-mail: j.bull@imperial.ac.uk
First published on 4th May 2017
Abstract
Simple amine and diamine derivatives can promote the palladium catalysed direct β-C–H arylation of aliphatic aldehydes via transient imine formation. Trifluoroacetate was shown to be crucial in promoting the reaction. Sub-stoichiometric quantities of simple N-tosylethylenediamine was shown to form a bidentate directing group with an imine linkage. Isolation of an unsymmetrical palladacycle has shown different potential binding modes of the secondary NTs coordinating group by single crystal X-ray diffraction analysis, suggestive of a hemilabile ligand.
Introduction
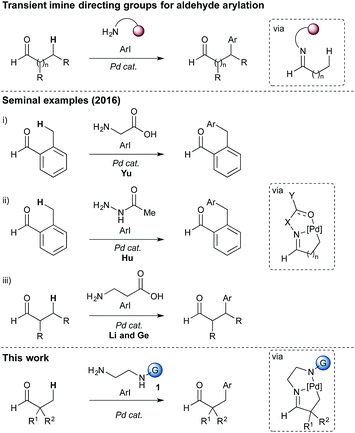
Catalytic C–H functionalisation promises powerful strategies to derivatise otherwise inert sites of organic molecules.1 Using transition metal catalysts, in particular using palladium, this approach is becoming increasingly viable to streamline complex molecule synthesis. For C(sp3)–H functionalisation issues of low reactivity and regioselectivity are paramount. Several strategies have been described to control these issues, invoking an adjacent functional group to position a catalyst in an appropriate spatial arrangement to enable C–H activation,2,3 commonly through a concerted metalation–deprotonation mechanism.4Daugulis,5 Yu,6 and others, made seminal advances in C(sp3)–H functionalisation through the use of amide linked bidentate and monodentate directing groups through Pd0/PdII and PdII/PdIV redox cycles.2 The potential to cleave these groups from the substrate provided clear synthetic advantages, which encouraged the development of alternative directing groups to allow more facile removal.7,8 Nonetheless, these strategies require additional steps to install and remove the directing groups, which can be problematic and reduces the overall efficiency.
Very recently, and during the course of our investigations, the concept of transient directing groups has emerged, whereby the directing group is installed, C(sp3)–H functionalisation occurs, then the directing group is removed, all in one reaction pot (Scheme 1).9 The first report in this field, by Yu, described a transient imine linkage with α-amino acids to promote β-arylation of aliphatic ketones and benzylic arylation of o-tolualdehydes.10 A bidentate coordination was proposed involving the imine nitrogen and the carboxylate. A sub-stoichiometric amount of glycine was shown to be an effective promoter using a palladium catalyst and AgTFA, in a HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH solvent mixture. The use of L-tert-leucine afforded enantioselective arylation. Hu showed that an acetylhydrazone could act as a directing group for the arylation of o-tolualdehyde.11 Very recently Li and Ge reported the arylation of aliphatic aldehydes, via a 5,6-palladacyclic intermediate, using a β-amino acid to form an imine linked directing group.12
AcOH solvent mixture. The use of L-tert-leucine afforded enantioselective arylation. Hu showed that an acetylhydrazone could act as a directing group for the arylation of o-tolualdehyde.11 Very recently Li and Ge reported the arylation of aliphatic aldehydes, via a 5,6-palladacyclic intermediate, using a β-amino acid to form an imine linked directing group.12
Dong,13 Ge,14 Murakami,15 and Yu16 also recently independently demonstrated the direct arylation of aliphatic amines, using aromatic or conjugated aldehydes to form directing groups incorporating the reverse imine. Yu has reported the ortho-C(sp2)–H functionalisation of benzaldehydes using transient directing groups to install various functional groups.17,18
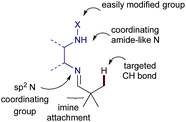
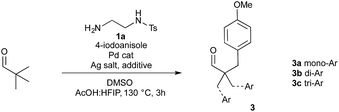
Here we report investigations into a new class of transient, sub-stoichiometric directing group for single step β-C(sp3)–H arylation of tertiary aldehydes using palladium catalysis and aryl iodides. Over 25 different mono- and bidentate directing groups are investigated either as preformed imines or through in situ reversible imine formation with simple amines (1), with many successful in mediating the reaction. Some mechanistic studies are also described, including isolation of a palladacycle as an unusual unsymmetrical dimer, characterized by single crystal X-ray diffraction.
Results and discussion
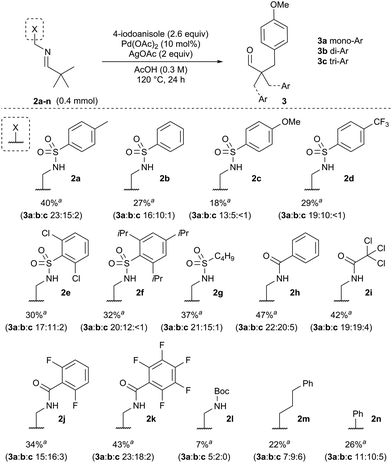
At the outset of our investigation we envisaged that a reversible imine formation could transiently introduce directing functionality to promote C–H arylation on aldehyde substrates using simple primary amines. To mimic many of the successful bidentate amide-linked directing groups with a PdII/PdIV catalytic cycle, we proposed moving the amide or related functional groups to a distal position, presenting a reversal of the N,N′-coordinating functionality (Fig. 1).19 Such groups would be easily prepared from ethylenediamine and we anticipated that simple modification of the exo-amide group would be suitable to tune the properties of the directing group.To establish the viability of the C–H arylation and to design the distal coordinating group, we initially preformed a series of imines from pivaldehyde and primary amines. These were designed to offer potential bidentate coordination through varied sulfonamides (2a–g), amides (2h–k) and a carbamate (2l) (Scheme 2). Simple alkyl and benzyl examples (2m,n) which could form only monodentate directing groups were also prepared. We initially subjected preformed imines to common arylation conditions using 4-iodoanisole.5,7,20 A low yield was obtained in the absence of solvent [e.g. 8% yield for 2a], and although various other solvents were unsuccessful an effective arylation was obtained in acetic acid.21
After preliminary optimisation, various imines were subjected to the arylation conditions, using Pd(OAc)2 and AgOAc in acetic acid at 0.3 M concentration, over 24 h (Scheme 2). The screen revealed that both the amide and the sulfonamide containing imines could successfully direct the C–H arylation. After a simple work up, involving a filtration through silica, only the hydrolysed aldehyde product 3 was observed, with no residual imine 2. A mixture of mono-, di- and tri-arylated products were obtained (3a–c).
Sulfonamide-containing imine 2a gave the best result, with a 40% combined yield of aldehydes 3a–c. Various other sulfonamides, with differing steric and electronic properties, gave similar yields. Generally, the amide-containing directing groups showed higher reactivity than the sulfonamides, with amide containing imine 2h giving the highest yield of aldehydes 3a–c. However, these gave both poorer selectivity of the monoarylated product, and also formed more highly functionalised aldehydes, giving overall a more complex mixture of products.20 Carbamate derivative 2l showed much reduced yield though was presumably unstable under the reaction conditions. Interestingly, amines which formed monodentate directing groups also promoted arylation, with benzylamine 2n affording a 26% yield. This highlighted that secondary binding was not crucial, but did enhance the yield. Indeed, the similarity across the different amines examined suggests that the secondary binding site may not be continuously bound to the metal centre throughout the catalytic cycle.
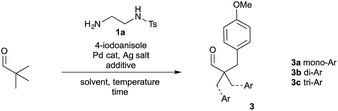
To realise a one-pot arylation of pivaldehyde, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of aldehyde and directing group was used in place of imine 2a (Table 1). Unfortunately, under the conditions used above, only trace product 3 was observed (entry 1). Therefore, we investigated the reaction parameters initially using a stoichiometric quantity of amine 1a and Pd(OAc)2 at 10 mol%. Importantly, on changing the silver source to AgTFA a 29% yield was achieved (entry 2). A wide range of solvents were investigated, along with solvent mixtures.20 The presence of acetic acid was critical, and various solvents were tolerated when used in combination with AcOH. The yield was improved when using hexafluoroisopropanol (HFIP) as a co-solvent.22 Similar yields were obtained with different AcOH
1 ratio of aldehyde and directing group was used in place of imine 2a (Table 1). Unfortunately, under the conditions used above, only trace product 3 was observed (entry 1). Therefore, we investigated the reaction parameters initially using a stoichiometric quantity of amine 1a and Pd(OAc)2 at 10 mol%. Importantly, on changing the silver source to AgTFA a 29% yield was achieved (entry 2). A wide range of solvents were investigated, along with solvent mixtures.20 The presence of acetic acid was critical, and various solvents were tolerated when used in combination with AcOH. The yield was improved when using hexafluoroisopropanol (HFIP) as a co-solvent.22 Similar yields were obtained with different AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HFIP ratios and we chose to progress with a 1
HFIP ratios and we chose to progress with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture for maximum reproducibility (entry 3). HFIP alone as a solvent was ineffective (entry 4). Using TFA as a co-solvent with acetic acid was also a slight improvement on acetic acid alone. Varying the palladium pre-catalyst gave a successful reaction with PdCl2 and an improvement with Pd(OPiv)2 to a 47% yield (entry 7).
1 mixture for maximum reproducibility (entry 3). HFIP alone as a solvent was ineffective (entry 4). Using TFA as a co-solvent with acetic acid was also a slight improvement on acetic acid alone. Varying the palladium pre-catalyst gave a successful reaction with PdCl2 and an improvement with Pd(OPiv)2 to a 47% yield (entry 7).
| Entrya | Solvent | Pd cat. | Ag salt | Yieldb (%) 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b 3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c 3c |
Yield 3 combined (%) |
|---|---|---|---|---|---|
| a Reaction conditions: pivaldehyde (0.2 mmol), 1a (1 equiv.), 4-iodoanisole (2.6 equiv.), Pd catalyst (10 mol%), silver salt (2 equiv.), 0.3 M, 120 °C, 24 h, unless otherwise stated. b Yields determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. c Reaction performed using 0.5 equiv. 1a, 5 mol% Pd(OPiv)2. d 3 h reaction time. e Added DMSO (1 equiv.). f Reaction performed at 130 °C, and 0.5 M concentration. g 0.25 equiv. 1a, 5 mol% Pd(OPiv)2. h 0.10 equiv. 1a, 5 mol% Pd(OPiv)2. | |||||
| 1 | AcOH | Pd(OAc)2 | AgOAc | Trace | Trace |
| 2 | AcOH | Pd(OAc)2 | AgTFA | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
29 |
| 3 | HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Pd(OAc)2 | AgTFA | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
36 |
| 4 | HFIP | Pd(OAc)2 | AgTFA | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
9 |
| 5 | TFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Pd(OAc)2 | AgTFA | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
34 |
| 6 | HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
PdCl2 | AgTFA | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
36 |
| 7 | HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Pd(OPiv)2 | AgTFA | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
47 |
| 8c | HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Pd(OPiv)2 | AgTFA | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
45 |
| 9c,d | HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Pd(OPiv)2 | AgTFA | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
46 |
| 10c,d,e | HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Pd(OPiv)2 | AgTFA | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
56 |
| 11 , , , |
HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
Pd(OPiv) 2 | AgTFA |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 22 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 10 10
|
61 |
| 12d,e,f,g | HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Pd(OPiv)2 | AgTFA | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
57 |
| 13d,e,f,h | HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Pd(OPiv)2 | AgTFA | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
47 |
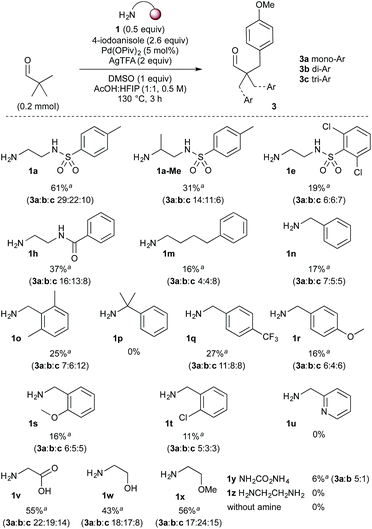
Pleasingly, the loadings of both amine 1a and Pd(OPiv)2 could be lowered to 0.5 equiv. and 5 mol% respectively without affecting the reaction yield (entry 8). Notably, an almost identical reaction profile over time was observed under these conditions (entries 7 and 8; and ESI†).20 The reaction was rapid, reaching maximum conversion within 3 h (entry 9). At this stage, numerous additives were investigated; while many Lewis basic additives were tolerated, the maximum increase in yield was on addition of DMSO, with 1 equiv. optimal (entry 10).20,23 A 61% overall yield of arylated products was obtained by performing the reaction at 130 °C and 0.5 M concentration. Finally, further reduction in the loading of amine 1a to 0.25 and 0.1 equiv. was well tolerated, resulting in only a marginal reduction in yield (57% and 47% respectively).
To highlight the crucial role of trifluoroacetate to the reaction conditions,24 we directly compared the effect of TFA additives (Table 2).
| Entrya | Pd cat. | Ag salt | Additive | Yieldb (%) 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b 3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c 3c |
Yield 3 combined (%) |
|---|---|---|---|---|---|
a Conditions: pivaldehyde (0.2 mmol), 1a (0.5 equiv.), 4-iodoanisole (2.6 equiv.), Pd cat (5 mol%), Ag salt (2 equiv.), DMSO (1 equiv.), HFIP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH (1 AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5 M), 130 °C, 3 h.
b Yields determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
c 2 equiv. 1, 0.5 M), 130 °C, 3 h.
b Yields determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
c 2 equiv.
|
|||||
| 1 | Pd(OAc)2 | AgOAc | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
2 |
| 2 | Pd(OAc)2 | AgTFA | — | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
54 |
| 3 | Pd(OAc)2 | AgOAc | TFAc | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
47 |
| 4 | Pd(TFA)2 | AgOAc | — | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trace trace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
9 |
Using the optimised conditions, but with palladium acetate and AgOAc, only a trace amount of aldehyde 3 was formed (entry 1). Using AgTFA (2 equiv.) in place of AgOAc returned the yield to 54%. Interestingly, when using AgOAc with trifluoroacetic acid (2 equiv.) as an additive a 47% yield could still be achieved, despite the large excess of acetate from the solvent.25 Using Pd(TFA)2 (5 mol%; comparable to 0.1 equiv. of TFA) gave a 9% yield of monoarylated aldehyde 3a. In contrast, in the arylation of the preformed imines the presence of TFA in the reaction mixture was not a requirement. Indeed, running the arylation on imine 2a with AgTFA gave the same yield as using AgOAc. Given the lower concentration of imine in the one-pot process,21 a more electrophilic Pd(TFA)X species may improve coordination to the imine and overall facilitate the CMD process.
With the highest yielding conditions (Table 1, entry 11), various mono- and bidentate directing groups were investigated, to further explore potential directing group design. Remarkably, the vast majority promoted the arylation to some extent (Scheme 3). N-Tosylethylenediamine 1a continued to provide the best directing group.26 Adding a methyl group adjacent to the primary amine gave a decreased yield, presumably due to reduced imine formation. Sulfonamide 1e and amide 1h, which gave similar yields in the preformed imines, were less successful than 1a. The monodentate directing groups gave generally lower yields. Several benzylamine derivatives were investigated (1n–u), including those with secondary coordinating groups (1s–u), for which the best yield of 27% was with 4-trifluoromethylbenzylamine 1q. No arylation occurred with hindered derivative 1p. 2-Picolylamine appeared to directly complex with palladium and was unsuccessful in the reaction. Other simple bidentate directing groups were also examined. Under these conditions glycine 1v, as used by Yu,10 formed the arylated product in 55% yield with an increased proportion of the tri and diarylated products. Amino alcohol 1w and 2-methoxyethan-1-amine 1x gave good yields, also with a greater tendency to form di- and triarylated aldehydes. A simple NH imine formed from ammonium carbamate 1y also gave the arylated product, though in very low yield. Using ethylenediamine itself (1z) also gave no product formation, most likely due to coordination to the Pd catalyst. Crucially with no amine added, there was no evidence of product formation.
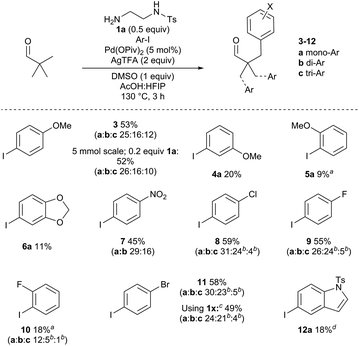
Next, the steric and electronic requirements of the aryl iodide coupling partner were explored using pivaldehyde and amine 1a (Scheme 4). Under the optimised conditions, 4-iodoanisole afforded a 53% combined isolated yield of the separated mono-, di- and triarylated aldehydes 3a–c. While arylation occurred at each CH3 group, there was no evidence of arylation at the benzylic methylene position under these conditions. The reaction was performed on a larger scale with a reduced amine loading (5 mmol scale, 0.2 equiv. 1a). The reaction efficiency was maintained, providing arylated aldehyde 3 in 52% yield. The meta- and importantly the ortho-iodoanisole examples were viable, albeit with sequentially lower yields of 4 and 5 respectively due to increased steric demands. Using 5-iodo-1,3-benzodioxole gave a low yield of arylated product 6a. The electron poor 1-iodo-4-nitrobenzene gave 45% of 7, along with small amounts of the homo-dimer of the aryl iodide. para-Chloro- and fluoro-iodobenzenes gave good yields of aldehydes 8 and 9, and the ortho-fluoro example gave 18% yield aldehyde 10. para-Bromoiodobenzene was run with both 1a and 1x as the directing group to form 11. Although the yields for both directing groups were similar (within 10%), 1x gave a higher proportion of diarylation. It is likely that for pivaldehyde multiple arylations may occur without exiting the catalytic cycle after the monoarylation, the extent of which is affected by the structure the directing groups.27 Pyridyl and thienyl iodides were not well tolerated under the reaction conditions (<10% yield). However, a 5-iodoindole derivative was successful to afford 12a in 18% yield.
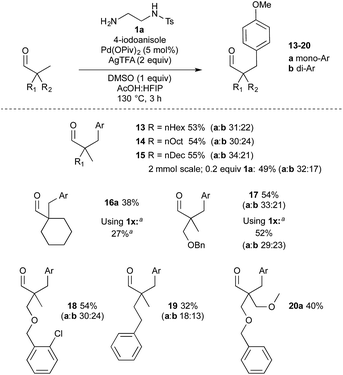
The aldehyde component was then investigated (Scheme 5). Higher molecular weight aldehydes bearing an alkyl chain, gave consistent yields around 55% of arylated products 13–15. The reaction to form aldehyde 15 was also performed on a larger scale using a lower loading of amine 1a (0.2 equiv.), which gave a comparable result. Cyclohexyl methyl example 16a was successful with both 1a and 1x directing groups affording 38% and 27% monoarylation respectively. Benzyl and 2-chlorobenzyl ether examples 17 and 18 were formed in good yield. Benzyl ether 17 was also formed using 2-methoxy-1-ethylamine 1x, which gave a comparable yield to 1a, again with an increased proportion of diarylation. Phenethyl example 19 was formed in a 32% total yield, and bis-ether-containing aldehyde 20 in 40% yield. Reapplying monoarylated aldehyde 3a and diarylated 3b to the reaction conditions afforded the corresponding further arylated aldehydes (Scheme 6) with some recovered substrate. Secondary centres, and aldehydes with acidic α-hydrogen atoms were not cleanly arylated under these conditions.
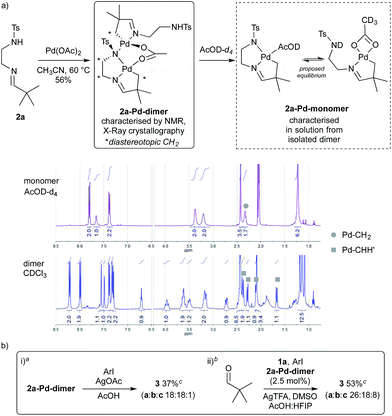
To gain some insight into the role of the sulfonamide as a secondary coordinating group, we formed a palladacycle from imine 2a by reaction with palladium acetate in MeCN (Scheme 6). The reaction was diluted with toluene and filtered through Celite to give a 56% yield of the palladacycle, a sample of which was further purified by recrystallisation. By 1H NMR this gave complex signals indicative of an unsymmetrical dimeric structure, containing different imine environments and several diastereotopic CH2 signals, as well as one NH signal with an integral of 1H (Scheme 7a).
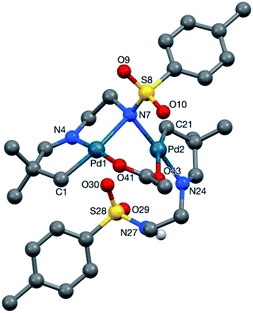
It was clear that the sulfonamide may display different coordination modes in its role as a secondary coordinating group.28 Pleasingly we obtained the crystal structure of 2a-Pd-dimer, proving the palladacycle structure, which indeed showed the two sulfonamides adopted very different binding modes (Fig. 2).29 The first directing group (C1-based ligand) binds to the palladium centre (Pd1) in a tridentate C,N,N′ fashion utilising C1, the imine nitrogen N4, and the sulfonamide nitrogen anion (N7), the latter of which also binds to Pd2.30,31 This binding mode is consistent with the imine and sulfonamide acting as a bidentate directing group. By contrast, the other imine (C21-based ligand), coordinates to Pd2 through C21 and the imine nitrogen (N24) only. The NH-sulfonamide was retained, and not coordinated to Pd.32 The coordination sphere at each metal centre was completed by a bridging acetate group. Each metal centre has a slightly distorted square planar coordination geometry, consistent with the PdII oxidation state.33 The two Pd-coordination planes are steeply inclined with respect to each other [82.63(7)°] such that the Pd1⋯Pd2 separation is 3.1098(3) Å.
When 2a-Pd-dimer was dissolved in AcOD a monomeric species 2a-Pd-monomer was formed, which was characterised in solution by 1H and 13C NMR. Due to the broadening of the directing group CH2 signals (3.38 and 3.20 ppm) we speculated that there may be an equilibrium between the free and coordinated sulfonamide group, complemented by changing acetate coordination (Scheme 7a). Furthermore, monitoring the reaction between 2a and palladium acetate in situ by 1H NMR in MeCN-d3 suggested a monomeric palladacycle was formed in solution.34 This suggests that a monomeric species would be dominant under the reaction conditions.
Reacting 2a-Pd-dimer with 4-iodoanisole in AcOH, afforded a 37% yield of arylated aldehyde 3 (Scheme 7b,i). Importantly, using 2a-Pd-dimer (2.5 mol%, i.e. 5% Pd) as the catalyst for the arylation of pivaldehyde under the optimised conditions, gave 53% yield of aldehyde 3, in the presence of additional 0.5 equiv. 1a (Scheme 7b,ii).35 These results support the palladacycle monomer being an intermediate in the reaction.
On the basis of these observations, and previous reports, we propose a PdII/PdIV mechanism with coordination of an electrophilic Pd(TFA)X species to a transiently formed imine, followed by palladacycle formation, likely through a CMD mechanism. Oxidative addition of the PdII-palladacycle into the aryl iodide followed by reductive elimination forms the C–C bond. The PdII species may decomplex and re-enter the catalytic cycle, or remain bound to the imine and undergo arylation again. The imine is then readily hydrolysed to the aldehyde to regenerate the directing group. The binding modes of the N–Ts groups in 2a-Pd-dimer, and the reactivity when using monodentate directing groups, suggests that the secondary binding site is not likely to be continuously bound to the catalyst while bound to the imine.
Conclusions
In summary, we have described a palladium catalysed β-C(sp3)–H functionalisation on tertiary aldehyde substrates with aryl iodides, assisted by simple bidentate and monodentate primary amine derivatives. We have shown a wide variety of simple amines and diamine derivatives, used in sub-stoichiometric quantities, form transient imine linkages as directing groups to promote aldehyde C–H arylation, in a single synthetic operation. Arylation protocols have been developed both for preformed imine species as well as through transient imine formation.Studies using a new directing group, derived from N-tosylethylenediamine 1a, have highlighted crucial factors in the success of the transient directing group. The reaction required small quantities of TFA, up to 2 equivalents, even in an acetic acid solvent, to promote the arylation of pivaldehyde. The presence of the second N–Ts coordination site, resulted in increased yields; a general observation with the potentially bidentate groups. However, it is likely that the second site is not coordinated to Pd throughout the catalytic cycle. The structure of a palladacycle formed from imine 2a was an unusual unsymmetrical dimer in the solid state, as confirmed by single crystal X-ray diffraction, where only one of the two N–Ts groups was coordinated to Pd. It is likely the monomeric form constitutes an intermediate in the reaction, presumed to be a PdII/PdIV cycle. The isolated palladacyclic dimer provided an effective catalyst for the reaction of pivaldehyde with 4-iodoanisole. The aldehyde arylation using N-tosylethylenediamine 1a was effective on a variety of aldehyde substrates and aryl iodide coupling partners with various steric and electronic properties. This study offers insight we expect to be valuable in the future development of directing groups to be formed transiently to promote C–H functionalisation. We are now examining the use of imine-linked directing groups for the functionalisation of unactivated C(sp3)–H bonds toward pharmaceutically relevant compounds.
Acknowledgements
For financial support, we gratefully acknowledge The Royal Society [University Research Fellowship (to J. A. B.) and URF appointed grant] the EPSRC [CAF to J. A. B. (EP/J001538/1), and DTP studentship to SSJC]. We acknowledge the EPSRC UK National Mass Spectrometry Facility at Swansea University.Notes and references
- (a) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369–375 CrossRef CAS PubMed; (b) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960–9009 CrossRef CAS PubMed; (c) K. Liao, S. Negretti, D. G. Musaev, J. Bacsa and H. M. L. Davies, Nature, 2016, 533, 230–234 CrossRef CAS PubMed; (d) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed; (e) K. Godula and D. Sames, Science, 2006, 312, 67–72 CrossRef CAS PubMed; (f) J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Q. Yu, Chem. Rev., 2017 DOI:10.1021/acs.chemrev.6b00622; (g) J. F. Hartwig and M. A. Larsen, ACS Cent. Sci., 2016, 2, 281–292 CrossRef CAS PubMed.
- (a) O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1053–1064 CrossRef CAS PubMed; (b) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS PubMed; (c) O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902–4911 RSC; (d) Z. Huang and G. Dong, Tetrahedron Lett., 2014, 55, 5869–5889 CrossRef CAS; (e) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726–11743 CrossRef CAS PubMed.
- (a) J. A. Garcia-Lopez and V. P. Mehta, ChemCatChem, 2017, 9, 1149–1156 CrossRef; (b) B. J. Knight, J. O. Rothbaum and E. M. Ferreira, Chem. Sci., 2016, 7, 1982–1987 RSC; (c) C. He and M. J. Gaunt, Angew. Chem., Int. Ed., 2015, 54, 15840–15844 CrossRef CAS PubMed; (d) J. J. Topczewski, P. J. Cabrera, N. I. Saper and M. S. Sanford, Nature, 2016, 531, 220–224 CrossRef CAS PubMed; (e) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem.–Eur. J., 2010, 16, 2654–2672 CrossRef CAS PubMed.
- (a) D. Lapointe and K. Fagnou, Chem. Lett., 2010, 39, 1118–1126 CrossRef; (b) L. Ackermann, Chem. Rev., 2011, 111, 1315–1345 CrossRef CAS PubMed; (c) M. Lafrance and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 16496–16497 CrossRef CAS PubMed.
- V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154–13155 CrossRef CAS PubMed.
- (a) R. Giri, N. Maugel, J.-J. Li, D.-H. Wang, S. P. Breazzano, L. B. Saunders and J.-Q. Yu, J. Am. Chem. Soc., 2007, 129, 3510–3511 CrossRef CAS PubMed; (b) D.-H. Wang, M. Wasa, R. Giri and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 7190–7191 CrossRef CAS PubMed; (c) M. Wasa, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 9886–9887 CrossRef CAS PubMed.
- (a) M. Fan and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 12152–12155 CrossRef CAS PubMed; (b) N. Rodríguez, J. A. Romero-Revilla, M. Á. Fernández-Ibáñez and J. C. Carretero, Chem. Sci., 2013, 4, 175–179 RSC; (c) Q. Zhang, X.-S. Yin, S. Zhao, S.-L. Fang and B.-F. Shi, Chem. Commun., 2014, 50, 8353–8355 RSC; (d) G. He, S.-Y. Zhang, W. A. Nack, Q. Li and G. Chen, Angew. Chem., Int. Ed., 2013, 52, 11124–11128 CrossRef CAS PubMed; (e) D. Mu, F. Gao, G. Chen and G. He, ACS Catal., 2017, 1880–1885 CrossRef CAS; (f) D. P. Affron, O. A. Davis and J. A. Bull, Org. Lett., 2014, 16, 4956–4959 CrossRef CAS PubMed; (g) For AQ removal, see: M. Berger, R. Chauhan, C. A. B. Rodrigues and N. Maulide, Chem.–Eur. J., 2016, 22, 16805–16808 CrossRef CAS PubMed.
- For prior work using stoichiometrically preformed imines or oxime derivatives for C(sp3)–H arylation, see: (a) using stoichiometric Pd: B. D. Dangel, K. Godula, S. W. Youn, B. Sezen and D. Sames, J. Am. Chem. Soc., 2002, 124, 11856–11857 CrossRef CAS PubMed; (b) J. E. Baldwin, R. H. Jones, C. Najera and M. Yus, Tetrahedron, 1985, 41, 699–711 CrossRef CAS; (c) A. G. Constable, W. S. McDonald, L. C. Sawkins and B. L. Shaw, J. Chem. Soc., Chem. Commun., 1978, 1061–1062 RSC; (d) Using catalytic Pd, for oxidation see: S. R. Neufeldt and M. S. Sanford, Org. Lett., 2010, 12, 532–535 CrossRef CAS PubMed; (e) For arylation using iodonium salts, see: J. Peng, C. Chen and C. Xi, Chem. Sci., 2016, 7, 1383–1387 RSC; (f) For Ir catalysed arylation with iodonium salts, see: P. Gao, W. Guo, J. Xue, Y. Zhao, Y. Yuan, Y. Xia and Z. Shi, J. Am. Chem. Soc., 2015, 137, 12231–12240 CrossRef CAS PubMed; (g) Y. Dong and G. Liu, J. Org. Chem., 2017, 82, 3864–3872 CrossRef CAS PubMed.
- Reversibly bound directing groups had previously been reported with rhodium catalysts and on sp2 centres. For aldehydes, see: (a) C.-H. Jun, H. Lee and J.-B. Hong, J. Org. Chem., 1997, 62, 1200–1201 CrossRef CAS; (b) for arenes, see: R. B. Bedford, S. J. Coles, M. B. Hursthouse and M. E. Limmert, Angew. Chem., Int. Ed., 2003, 42, 112–114 CrossRef CAS; (c) for enamines: F. Mo and G. Dong, Science, 2014, 345, 68–72 CrossRef CAS PubMed.
- F.-L. Zhang, K. Hong, T.-J. Li, H. Park and J.-Q. Yu, Science, 2016, 351, 252–256 CrossRef CAS PubMed.
- F. Ma, M. Lei and L. Hu, Org. Lett., 2016, 18, 2708–2711 CrossRef CAS PubMed.
- K. Yang, Q. Li, Y. Liu, G. Li and H. Ge, J. Am. Chem. Soc., 2016, 138, 12775–12778 CrossRef CAS PubMed.
- Y. Xu, M. C. Young, C. Wang, D. M. Magness and G. Dong, Angew. Chem., Int. Ed., 2016, 55, 9084–9087 CrossRef CAS PubMed.
- Y. Liu and H. Ge, Nat. Chem., 2016, 9, 26–32 Search PubMed.
- A. Yada, W. Liao, Y. Sato and M. Murakami, Angew. Chem., Int. Ed., 2017, 56, 1073–1076 CrossRef CAS PubMed.
- Y. Wu, Y.-Q. Chen, T. Liu, M. D. Eastgate and J.-Q. Yu, J. Am. Chem. Soc., 2016, 138, 14554–14557 CrossRef CAS PubMed.
- X.-H. Liu, H. Park, J.-H. Hu, Y. Hu, Q.-L. Zhang, B.-L. Wang, B. Sun, K.-S. Yeung, F.-L. Zhang and J.-Q. Yu, J. Am. Chem. Soc., 2017, 139, 888–896 CrossRef CAS PubMed.
- Also see: (a) J. Xu, Y. Liu, Y. Wang, Y. Li, X. Xu and Z. Jin, Org. Lett., 2017, 19, 1562–1565 CrossRef CAS PubMed; (b) X.-Y. Chen, S. Ozturk and E. J. Sorensen, Org. Lett., 2017, 19, 1140–1143 CrossRef CAS PubMed.
- For computational studies on the potential of related directing groups, see: H. Tang, X.-R. Huang, J. Yao and H. Chen, J. Org. Chem., 2015, 80, 4672–4682 CrossRef CAS PubMed.
- D. P. Affron and J. A. Bull, Eur. J. Org. Chem., 2016, 139–149 CrossRef CAS PubMed.
- See ESI† for further details.
- To indicate the proportion of the imine present in solution, the pivaldehyde and N-tosylethylenediamine 1a were dissolved in AcOD-d4 at rt, which gave 8% formation of imine 2a. Using a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of AcOD-d4 and HFIP gave 20% formation of imine 2a. By contrast, dissolving the preformed imine in AcOD-d4 indicated 56% imine remaining at rt, and this proportion did not change on heating. See ESI† for further details.
1 mixture of AcOD-d4 and HFIP gave 20% formation of imine 2a. By contrast, dissolving the preformed imine in AcOD-d4 indicated 56% imine remaining at rt, and this proportion did not change on heating. See ESI† for further details. - For Pd-DMSO coordination, see: T. Diao, P. White, I. Guzei and S. S. Stahl, Inorg. Chem., 2012, 51, 11898–11909 CrossRef CAS PubMed.
- For an example of the importance of TFA in recent works see: (a) B. Wang, W. A. Nack, G. He, S.-Y. Zhang and G. Chen, Chem. Sci., 2014, 5, 3952 RSC; (b) See ref. 10 and 12 for use of AgTFA in C–H arylation with transient directing groups; (c) Y. Taniguchi, Y. Yamaoka, K. Nakata, K. Takaki and Y. Fujiwara, Chem. Lett., 1995, 24, 345–346 CrossRef.
- Varying the amount of TFA added from 0.1 to 3 equivalents gave a steady to rise in yield until 2 equiv. were used, with no further improvement at 3 equiv. (see ESI† for further details).
- N-Tosylethylenediamine 1a is commercially available or easily synthesised on a large scale.
- The reaction profile using 1a (see Fig. S1 and S2†) indicates that 3a, 3b and 3c form simultaneously and each increase steadily to a maximum at 3h. However, 3a and 3b are also viable substrates for the reaction in overall reduced yields (see Scheme 6).
- For example, Carretero and Fernández-Ibáñez previously observed a dimeric palladacycle involving coordination through the sulfonamide O atoms. See ref. 7b.
- CCDC 1534094 contains the supplementary crystallographic data for this paper.
- For the limited examples of palladacycles containing a bridging sulfonamide nitrogen, see: (a) L. Menabue and M. Saladini, Inorg. Chem., 1991, 30, 1651–1655 CrossRef CAS; (b) J. Sanmartín, F. Novio, A. M. García-Deibe, M. Fondo, N. Ocampo and M. R. Bermejo, Eur. J. Inorg. Chem., 2008, 1719–1726 CrossRef; (c) J. Sanmartín-Matalobos, C. Portela-García, M. Fondo, A. M. García-Deibe and A. L. Llamas-Saiz, J. Coord. Chem., 2016, 69, 1358–1370 CrossRef.
- The bridging-nitrogen (N7) is asymmetric, the bond to Pd1 [2.253(3) Å], trans to C1, being ca. 0.17 Å longer than that to Pd2 [2.085(3) Å], trans to N24. The bridging acetate group is similarly asymmetric with the Pd–O bond trans to carbon [Pd2–O43 2.244(2) Å], ca. 0.20 Å longer than that trans to the imine nitrogen N4 [Pd1–O41 2.046(2) Å].
- This N–H hydrogen atom is involved in an intermolecular hydrogen bond to the O41 acetate oxygen atom in an adjacent molecule with N⋯O and H⋯O separations of 2.882(3) and 1.988(5) Å, and an N–H⋯O angle of 172(2)°.
- The distortion of the square planar genometry is greater at Pd2 than at Pd1. For the Pd1 case, N7 lies 0.111(6) Å out of the {Pd1,C1,N4,O41} plane (the atoms of which are coplanar to within 0.0154(12) Å), whilst at Pd2 it is the C21 donor atom that lies 0.305(5) Å out of the {Pd2,N7,N24,O43} plane (the atoms of which are coplanar to within 0.0568(12) Å).
- In situ NMR experiments showed fast coordination, characterised by a shift in the peaks of 2a, then a gradual formation of a monomeric palladacycle species, reaching maximum conversion after approximately 80 min. See ESI† for further details.
- Without the inclusion of additional amine 1a, a 13% yield was obtained under the same conditions.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterisation data; full optimisation table for arylation of pivaldehyde; studies on imine formation/hydrolysis in AcOD-d4; reaction plots with time for different directing groups and catalyst loading and addition of DMSO; effect of TFA addition; 1H NMR spectra of sample aldehyde regions; 1H and 13C NMR spectra for characterised compounds and crystal structure data. CCDC 1534094. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc01218g |
| This journal is © The Royal Society of Chemistry 2017 |