Open Access Article
Open Access ArticleMass spectrometric directed system for the continuous-flow synthesis and purification of diphenhydramine†
Bradley P.
Loren‡
a,
Michael
Wleklinski‡
 a,
Andy
Koswara‡
b,
Kathryn
Yammine
a,
Yanyang
Hu
a,
Zoltan K.
Nagy
*b,
David H.
Thompson
*a and
R. Graham
Cooks
a,
Andy
Koswara‡
b,
Kathryn
Yammine
a,
Yanyang
Hu
a,
Zoltan K.
Nagy
*b,
David H.
Thompson
*a and
R. Graham
Cooks
 *a
*a
aDepartment of Chemistry, Purdue University, West Lafayette, IN 47907, USA. E-mail: davethom@purdue.edu; cooks@purdue.edu
bDepartment of Chemical Engineering, Purdue University, West Lafayette, IN 47907, USA. E-mail: zknagy@purdue.edu
First published on 19th April 2017
Abstract
A highly integrated approach to the development of a process for the continuous synthesis and purification of diphenhydramine is reported. Mass spectrometry (MS) is utilized throughout the system for on-line reaction monitoring, off-line yield quantitation, and as a reaction screening module that exploits reaction acceleration in charged microdroplets for high throughput route screening. This effort has enabled the discovery and optimization of multiple routes to diphenhydramine in glass microreactors using MS as a process analytical tool (PAT). The ability to rapidly screen conditions in charged microdroplets was used to guide optimization of the process in a microfluidic reactor. A quantitative MS method was developed and used to measure the reaction kinetics. Integration of the continuous-flow reactor/on-line MS methodology with a miniaturized crystallization platform for continuous reaction monitoring and controlled crystallization of diphenhydramine was also achieved. Our findings suggest a robust approach for the continuous manufacture of pharmaceutical drug products, exemplified in the particular case of diphenhydramine, and optimized for efficiency and crystal size, and guided by real-time analytics to produce the agent in a form that is readily adapted to continuous synthesis.
Introduction
There is an extremely rich and rapidly growing knowledge base in the literature for organic transformations; however, the vast majority of this is based on batch processes. Continuous-flow reactor technology has generated significant interest due to the variety of advantages it offers over traditional batch processes such as much lower process costs and increased process efficiency.1 These advantages arise from the rapid mixing, superior heat transfer, safe operation at high temperatures and pressures, and control and automation that flow reactions can offer.2–4 These features provide the potential to execute organic reactions and purifications at rates and in yields not achievable in a batch system.5 Additionally, there is an inherent scalability with continuous-flow processes compared to batch processes, whereby process quality does not diminish as process scale increases.6,7These advantages have led to a growing interest in the recent literature in microfluidic technologies, as is exemplified by review articles,2–4,8,9 and the expanding scope of chemistry being employed in microreactors.5,10–14 In addition to the increasing diversity of chemistry reported for traditional solution-phase reactions in microreactors, this format also offers the flexibility for enabling technologies to be developed to cover other types of reactions, such as gas–liquid reactions,15,16 photochemical reactions,17 and electrochemical reactions,18 followed by different types of flow purifications, including extraction,19 crystallization20 and chromatography.21
All of the technologies described above have the potential for rapid optimization by means of on-line reaction monitoring.22 Real-time analysis provides the opportunity for rapid and automated process optimization.23–25 We are working to develop a continuous synthesis system that is driven by on-line analytics. Techniques that have previously been used to monitor microfluidics processes include UV-Vis,26 Raman,27 IR,28,29 NMR,25 and MS.30–32 The potential for rapid analysis times, as well as the detailed product and byproduct information that can be obtained from MS make it particularly attractive for the near real-time monitoring of reaction mixtures. Mass spectrometry has extensively been used to monitor both batch and continuous reactions, including, for example, Sonogashira and Negishi couplings.33–36 We report here the novel use of N2(g) segmented droplets and an inductive electrospray ionization method35,37 to provide MS feedback in under 30 seconds with minimal waste production.
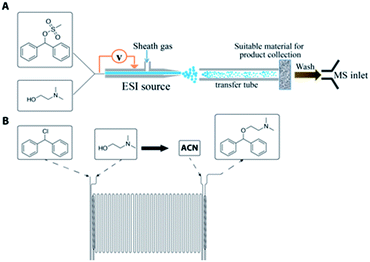
Another significant aspect of this work is the utilization of MS as a reaction screening module by accelerating reactions in charged microdroplets for high throughput reaction screening.38,39 The acceleration factors vary from 102 to 106 allowing accelerated access to slow reactions; reaction acceleration is due to increases in reagent concentration as solvent evaporates, pH effects, and interfacial effects.38,40–42 It is important to note that MS under the appropriate conditions can be used to either monitor or accelerate reactions. In this paper, both types of experiments are performed to help in the optimization of the synthesis of diphenhydramine. While microfluidics provides the opportunity to screen conditions more quickly than batch processes, the use of the droplet reactor as a screening tool prior to microfluidic optimization has the potential to significantly reduce the time required for route selection and, subsequently, reaction optimization. The droplet and microreactor systems are represented in Fig. 1. The continuous-flow reactors used for this system are glass microreactor chips from Chemtrix®.
In addition to conducting organic transformations in a continuous fashion, the potential advantages for executing continuous purifications are similarly important. This is particularly true for the production of active pharmaceutical ingredients (APIs), as exemplified by the recent on-demand continuous synthesis system developed at MIT.11 Liquid–liquid extraction19 and crystallization20 have both been demonstrated using continuous-flow technologies. We show here the integration of a miniaturized pharmaceutical purification platform (MiniPharm) with the microreactor and on-line inductive ESI-MS monitoring. This system consists of a mass-flow controller and temperature bath, equipped with in-line analytics for droplet and crystal detection, with control and process automation provided by a LabView based GUI.
Diphenhydramine (DPH) is an antihistamine found in several common medicines, including Benadryl®. A continuous-flow synthesis of DPH was published in 2013 by the Jamison group.10 We now demonstrate the use of both on-line and off-line MS to optimize the synthesis of diphenhydramine employing multiple routes using a Chemtrix Labtrix S1 microfluidic device, along with the rapid screening of reactions accelerated in microdroplets to guide reaction routes examined further in continuous-flow. The route screening in microdroplets expands upon earlier work done by Wleklinski et al.39 We also show the integration of one of the synthetic routes with a MiniPharm continuous purification system, which implements an air-segmented flow crystallizer to continuously produce solid products without clogging.
Results
Continuous-flow synthesis of DPH
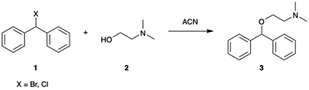
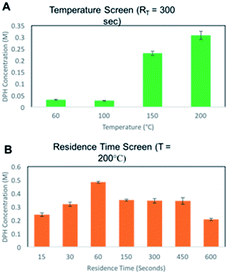
Microfluidic reactors have the potential to rapidly mix materials by diffusion, and the addition of micromixers can enhance the process by convection.43 A 19.5 μL Chemtrix reactor chip equipped with staggered oriented ridge (SOR) mixing sections was used for this. The SOR sections of the chip create turbulent flow immediately upon mixing two reagents to provide rapid and efficient mixing. The outlet of the reactor chip is fed into a pressure sensor and back pressure regulator (BPR) that was used to run reactions at pressures ranging from 10–20 bar. The first routes explored with this reactor system used either bromo- or chlorodiphenylmethane reacting with dimethylaminoethanol (DMAE) in ACN, a route previously reported in flow (Scheme 1).10 Reaction screening using accelerated reactions in microdroplets was attempted but proved unsuccessful due to the formation of a gas phase hetero-dimer between dimethylaminoethanol and the dibenzylic carbocation which has the same nominal mass as diphenhydramine. This represents a case in which accelerated reaction screening failed; however, due to the immense number of synthetic routes it is still a powerful technique as will be shown later in this paper.A 2.0 M solution of the halo-diphenylmethane was mixed with neat DMAE in the first SOR section of the chip and acetonitrile dilution was performed prior to the second SOR section as a reaction quench. We first explored the temperature dependence of this reaction by screening temperatures from 60–200 °C at a residence time of 5.0 min. A strong dependence on temperature was observed with no significant product being formed below 150 °C for both bromo- and chlorodiphenylmethane. While conducting reactions at temperatures higher than 200 °C would be advantageous to potentially optimize the process further, this was not possible due to a hardware limitation of the commercial system. These results are summarized in Table 1 and Fig. 2 with 1HNMR found in ESI (Fig. S1–S3†).
| Entry | X | Temp. (°C) | RT (min) | 3:(1 + 3)a | 3:(1 + 3)b |
|---|---|---|---|---|---|
| a 3:(1 + 3) calculated using 1H NMR. b 3:(1 + 3) calculated using MS. | |||||
| 1 | Br | 60 | 5 | 3.70 | 42.2 |
| 2 | Br | 100 | 5 | 0.65 | 50.2 |
| 3 | Br | 150 | 5 | 20.3 | 77.8 |
| 4 | Br | 200 | 5 | 60.6 | 96.9 |
| 5 | Cl | 60 | 5 | 6.9 | 66.3 |
| 6 | Cl | 100 | 5 | 2.6 | 78.0 |
| 7 | Cl | 150 | 5 | 41.3 | 72.5 |
| 8 | Cl | 200 | 5 | 90.1 | 97.3 |
| 9 | Br | 150 | 7.5 | 49 | 81.9 |
| 10 | Br | 150 | 10 | 91 | 92.4 |
| 11 | Br | 200 | 7.5 | 95 | 95.0 |
| 12 | Br | 200 | 10 | 98 | 95.3 |
With the temperature data in hand, we then screened residence times (RT) at 150 and 200 °C, first for bromodiphenylmethane, using off-line MS qualitatively as a means to monitor product formation, followed by both MS and 1H NMR analysis to calculate a relative conversion. The same trends were observed with both techniques.
Diphenhydramine is commonly administered orally as a hydrochloride salt. We therefore pursued chlorodiphenylmethane as an alternative starting material for this process to allow us to isolate diphenhydramine directly as the hydrochloride salt. To accomplish this optimization rapidly with a minimal amount of material, we developed an off-line quantitative MS method using serial dilution and diphenhydramine-D3 for monitoring product yield (Fig. S4†). The temperature screen was repeated and a more extensive residence time screen was conducted. The quantitative MS data confirmed the temperature dependence previously observed, and revealed a strong RT dependence for the formation of diphenhydramine, with the yield increasing with RT up to 60 seconds, followed by a leveling off at residence times up to 600 seconds (Fig. 2). The highest yield achieved was 0.5 M, which corresponds to a production rate of 128 mg of DPH per hour. Assuming second order kinetics, this is roughly eight times faster than the previously published continuous flow synthesis.10
The detailed structural information that MS and MS/MS can rapidly provide make MS a powerful tool for reaction monitoring and process optimization. The formation of multiple N-oxide byproducts is observed in the mass spectra and their structures have been confirmed with MS/MS (Fig. 3 and S5†).44 Furthermore, the formation of these byproducts is dependent on the residence time of the reagents. This information is summarized in Fig. 3 and S5.†
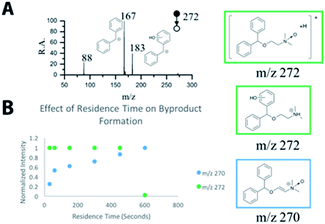 | ||
| Fig. 3 Byproduct formation (A) MS/MS of m/z 272 shows presence of isomeric ions; (B) MS follows the effect of residence time on byproduct formation. | ||
On-line MS reaction monitoring
The use of off-line and slow analytical methods are common bottlenecks in flow reaction optimization. Therefore, the development of an on-line reaction monitoring system using MS represents a significant advance in the capability to rapidly optimize reaction conditions. We demonstrate here the ability to monitor and optimize the synthesis of diphenhydramine from chlorodiphenylmethane and dimethylaminoethanol using on-line MS. The setup for these experiments uses the 19.5 μL SOR chip with the outlet connected to a 2 μL injection valve that allowed the product to be collected, sent to waste, or sent to the MS. As the material emerging from the reactor was approximately 1.0 M, it was necessary to include an acetonitrile in-line dilution step after the injector valve. In order to enhance the rate of data acquisition and reduce the amount of material wasted when collecting MS data, N2(g) segmented droplets were created using an electronic pressure regulator (EPR) to rapidly deliver droplets from the reactor to the MS. This provided μL sized droplets that were approximately 0.01 M in reaction mixture. These droplets traveled a distance of roughly 3 m, utilizing 150 μL of tubing volume to the mass spectrometer. The use of air-segmentation allowed data collection within 30 seconds with minimal waste generation. A video microscope along with an optical sensor was used in-line in order to monitor the droplets. The MS analysis was conducted using an inductive ionization source (iESI-MS).35,37 These data are summarized in Fig. 4. At a residence time of 5.0 minutes, we demonstrated the same temperature dependence that we observed using off-line MS. No significant product is detected at lower temperatures, and an increase in product formation is seen at 150 °C, followed by a significant increase at 200 °C. The peak at m/z 550 is a gas phase dimer of the DPH product ([2 DPH + 2H + Cl]+).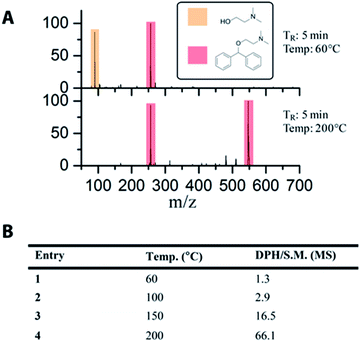 | ||
| Fig. 4 (A) Sample mass spectra collected on-line. (B) Calculated conversions from MS data collected on-line demonstrate temperature dependence of the reaction. S.M. refers to DMAE. | ||
Continuous-flow crystallization of DPH
In order to develop a completely continuous and automated process, we designed a system capable of seamlessly transitioning between synthesis, analytics, and ultimately purification of the final product. The purification system has been designed as a flexible miniaturized pharmaceutical platform, so that the appropriate purification module, such as extraction and/or crystallization, can be implemented easily and optimized depending on the synthesis being conducted. For the synthesis of diphenhydramine from chlorodiphenylmethane, we have utilized an air-segmented flow crystallizer in addition to the on-line MS capabilities described above. Crystallization in segmented droplets allows for the continuous crystallization of material at a controlled crystal size without fouling.20,45 A full engineering diagram of the experimental setup is detailed in Fig. 5.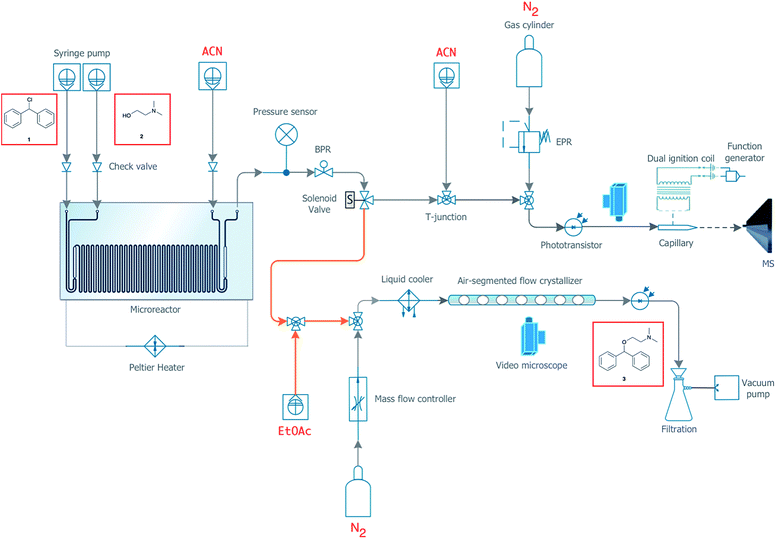 | ||
| Fig. 5 Engineering details for the integrated synthesis and purification system. Orange lines represent a tubular heater (Fig. S8†). | ||
Again using a 19.5 μL reactor chip, the outlet was fed into a valve capable of splitting the flow between collection, waste, MS, and the air-segmented flow crystallizer. As shown in Fig. 5, the MiniPharm consists of PFA tubing connected in a T-junction to a syringe pump filled with EtOAc, the outlet of the microreactor, and a 0.3 m length of PFA tubing where mixing takes place by diffusion. The EtOAc served as the anti-solvent prior to droplet formation. At this point, there is a high potential for crystal formation that could block the tubing shortly after anti-solvent mixing (Fig. S6 and S7†). We have, therefore, implemented cut-to-length tubing heaters before and after the T-junction developed in-house to keep the material solubilized and prevent fouling from occurring (Fig. S8†). This tubing section is followed by another T-junction, which is connected to the mass-flow controller and a 1.2 m length of PFA tubing where the crystallization takes place. The mass-flow controller is used to modulate the precise flow rate of N2(g) and in turn create a uniform distribution of N2(g) segmented droplets. Currently, it takes three residence times for the droplets to reach a uniform distribution with low variance (Fig. S9†). This can be improved upon by using a BPR at the crystallizer's outlet in the future. The controlled and accelerated formation of N2(g) segmented crystals was subsequently induced by immersing a section of the tubing into a dry ice bath. The temperature was monitored and maintained at −20 °C at the interface between the dry ice bath and the tubing. Here, uniform crystals are formed while fouling is prevented due to precise supersaturation within each segmented droplet.
Multiple analytical techniques were used as PAT to monitor this process, and a LabView based GUI was developed for process integration and automation. In addition to on-line MS, off-line MS based quantitation was also utilized during crystallization experiments in order to monitor product yield and ensure effective crystallization. A phototransistor was also placed in-line to monitor droplet formation. The use of the mass flow controller resulted in a uniform droplet distribution that allowed us to control crystal size and prevent fouling of the tubing (Fig. 6C and S9†). The phototransistor was also effective, as shown in Fig. 6B, in detecting crystal formation, and a video microscope was incorporated in-line for the air-segmented flow crystallizer to visualize crystal formation (Fig. 6A). The output was collected by filtration and analyzed by nESI-MS and 1HNMR (Fig. S10 and S11†).
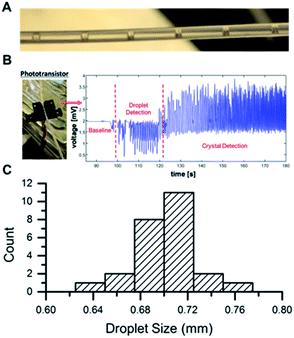 | ||
| Fig. 6 (A) Droplet train from microreactor. (B) Droplet detection using LED phototransistor, (C) droplet size distribution. | ||
Droplet and flow synthesis of DPH from benzhydrol
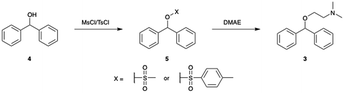
In an effort to develop a process utilizing commodity starting materials, we sought to replace the halo-diphenylmethane with the significantly cheaper diphenylmethanol (benzhydrol). Using benzhydrol as a starting material for the synthesis of diphenhydramine has been explored previously in continuous-flow through a p-toluene sulfonic acid catalyzed condensation reaction with dimethylaminoethanol.10 We pursued a different approach and created a better leaving group by reacting benzhydrol with methanesulfonyl chloride (MsCl) or p-toluenesulfonyl chloride (TsCl) (Scheme 2).A major component of our analytic-directed continuous synthesis system is a reaction screening module which takes advantage of reaction acceleration in charged microdroplets.38 A representation of this module is shown in Fig. 1A. When starting the synthesis of a new target molecule, there is a vast number of options not only for routes, but also for solvents and conditions to conduct each organic transformation. The droplet methodology provides for rapid screening of reaction pathways and solvents to guide the subsequent continuous-flow optimization. Droplet reactions can be conducted either by spraying directly into the MS (on-line) or by spraying onto a substrate such as glass wool followed by dissolution and off-line analysis. The off-line method is beneficial as it allows one to collect more material for analysis, provides the opportunity to telescope multiple synthetic steps, and avoids solvent effects during ionization by dissolution into a common solvent for analysis. We employed this approach to rapidly screen solvents for the synthesis of diphenhydramine via the mesylation or tosylation of benzhydrol to form 5, followed by treatment with dimethylaminoethanol to make DPH (3). Information obtained from these experiments was then compared to the data obtained from continuous-flow reactions.
Prior to telescoping the two reaction steps for this synthesis, the mesylation of benzhydrol was first performed in both the droplet reactor and in a single microfluidic chip. When benzhydrol and methanesulfonyl chloride were sprayed for 5 minutes in the off-line droplet reactor, the dibenzylic carbocation (m/z 167) was observed in positive mode and the two-electron reduction product of the mesylate (m/z 263) was observed in negative mode. In a 5 μL microfluidic reactor at 200 °C, MS analysis showed that significantly more product was formed at a residence time of 30 seconds than at a residence time of 60 seconds (Fig. S12 and S13†). This was likely due to degradation of the mesylated benzhydrol on the chip after it was formed. NMR and MS evidence suggested that the mesylate degraded under these conditions over the course of a day.
Telescoping this two-step synthesis in the droplet reactor was accomplished by spraying benzhydrol and MsCl/TsCl onto glass wool for 5 minutes, re-dissolving the collected material and then spraying that solution again with DMAE. These reactions were sprayed in ACN, DMF, and toluene while the analysis was conducted in ACN for all cases. For TsCl, the droplet reactor indicated product formation for all solvents tested with the reaction in toluene having the highest conversion (Fig. S14†).
Fouling issues in the microfluidic reactor caused us to abandon this route. Droplet reactions occur in an open environment, therefore potential information about component insolubility is not obtained. However, the large number of synthetic routes available suggests that a subset of positive droplet reactions will meet the requirement of providing a successful flow synthesis.
With this information in hand, we then sought to optimize the telescoped two-step synthesis of diphenhydramine from benzhydrol in a microfluidic reactor. This was first attempted using a single 15 μL reactor chip that had a third injection port at 5 μL, effectively providing a 5 μL reactor for the first step and a 10 μL reactor for the second step. A large number of conditions were attempted using this system, including residence time screens at 150 and 200 °C. All of these conditions failed, however, due to fouling of the chip shortly after starting the reaction. Triethylamine (TEA) was then incorporated into the reaction mixture in an attempt to improve the solubility of the process. While the addition of TEA maintained solubility throughout the course of the reaction, the conversion to DPH remained low for all conditions attempted (Fig. S15†).
In an effort to obtain conditions that were compatible with continuous-flow, five different bases were screened in small batches for solubility and product formation using both methanesulfonyl chloride (MsCl) and p-toluenesulfonyl chloride (TsCl). These experiments were conducted by both adding the base in the first step of the synthesis as well as during the second step to explore different possible configurations for the microfluidic setup. While 2,6-lutidine, DMAP, and DABCO all exhibited conditions where the reactants appeared to remain soluble while also forming product, fouling of the reactor chip remained a major issue that prevented any of these conditions from being optimized in continuous-flow.
The system was then reconfigured to allow this synthesis to be conducted in two separate reactor chips. To accomplish this, a device of multiple heating units that could be independently controlled was designed and fabricated (Fig. S16†). A 5 μL chip was employed for the first step and a 1 μL chip was used for the second step. The purpose behind this design was an attempt to drive the second step of the synthesis at high temperatures in a short residence time to prevent fouling of the chip. The synthesis was screened in ACN, DMF, and toluene at a variety of temperatures, residence times, and stoichiometries. The concentrations of all reagents were kept at 0.5 M. Off-line MS was again used to monitor this synthesis qualitatively, and a subset of conditions were examined quantitatively in the same manner that was done for the synthesis from chlorodiphenylmethane. Qualitative conversions were calculated by considering starting materials, intermediates, and byproducts. The experiments that were analyzed quantitatively are summarized in Table 2, and the full dataset is detailed in the ESI (Table S1†).
| Entry | Temp. (°C) (1/2) | RT (min) | Stoich. (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Solvent | Conv. (%) | Yield (%) |
|---|---|---|---|---|---|---|
| a Qualitative calculation of conversion from MS. b Quantitative calculation of yield from MS. | ||||||
| 1 | 150/200 | 0.50/0.067 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ACN | 32.1 | — |
| 2 | 150/200 | 1.0/0.13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ACN | 20.2 | 4.85 |
| 3 | 150/200 | 2.5/0.33 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ACN | 54.9 | 5.63 |
| 4 | 150/200 | 5.0/0.66 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ACN | 55.2 | 34.5 |
| 5 | 150/175 | 2.5/0.33 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DMF | 47.5 | — |
| 6 | 150/175 | 5.0/0.67 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DMF | 41.5 | 5.93 |
| 7 | 150/175 | 2.5/0.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMF | 43.0 | 6.79 |
| 8 | 150/175 | 5.0/0.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DMF | 49.6 | 8.73 |
| 9 | 150/200 | 1.0/0.13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene | 12.0 | — |
| 10 | 150/200 | 1.0/0.10 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene | 17.1 | 0.15 |
| 11 | 150/200 | 1.0/0.08 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene | 6.85 | 0.14 |
| 12 | 150/200 | 1.0/0.67 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene | 23.1 | 0.29 |
The results obtained from the solvent screen that was done with the droplet reactor are consistent with the findings from the microfluidic reactor. The droplet reactor indicated product formation was possible with all solvents, but ACN is significantly better than DMF and toluene. Due to solubility concerns, DMF was initially screened for this system as it was the most effective at preventing fouling of the reactor chip. A large number of conditions were, therefore, explored in DMF, but the presence of byproducts that resulted from solvent degradation prevented the synthesis of DPH in a yield over 9%. Fouling issues in toluene prevented the screening of many different conditions, and those conditions where solubility was retained all had yields of less than 1%. ACN also had significant issues with precipitation and clogging of the reactor that prevented a large screen of conditions. However, at 150 and 200 °C and RT of 5.0 and 0.66 minutes in the first and second chips, respectively, the highest yielding condition of 34.5% was achieved (Table 2, entry 4).
Conclusions
Diphenhydramine was synthesized via multiple routes using both a microfluidic system and an accelerated droplet reactor. The synthesis from the reaction of bromo- and chlorodiphenylmethane with dimethylaminoethanol was first optimized in the microfluidic system by screening temperatures and residence times. ESI-MS was used as a process analytical tool (PAT) both qualitatively and quantitatively to facilitate the optimization process. These studies led to an optimal condition from chlorodiphenylmethane in acetonitrile at 200 °C with a residence time of only 1 minute to produce DPH at a rate of 128 mg h−1. The ability to monitor this system in real time using ESI-MS was also demonstrated using N2(g) segmented droplets and an inductive ionization. These tools were then integrated with a MiniPharm platform and DPH was successfully crystallized continuously in N2(g) segmented droplets without fouling. The size of the droplets, and therefore the crystals, was effectively controlled with a mass flow controller and a temperature bath. The droplets and crystals are monitored using an in-line phototransistor and a video microscope, which will be integrated with an image processing algorithm for real-time droplet size distribution calculation in the future.The synthesis of DPH from benzhydrol using methanesulfonyl and p-toluenesulfonyl chloride was also accomplished. This synthesis was demonstrated in both the microfluidic system and an accelerated droplet reactor which established its potential to be used as a rapid reaction screening tool. A variety of solvents and conditions were screened in both reactor systems, using both qualitative and quantitative MS to monitor these processes. The trends in product formation with varying solvent were established using the droplet reactor, and these were shown to be consistent with the findings from microfluidic reactions. Fouling issues required the development of a two-chip system, and MS quantitation revealed that ACN was the best solvent for this synthesis.
This study describes our initial efforts towards the development of an automated organic synthesis system directed by mass spectrometry. The design of this system includes using MS as not only a PAT, but also as a way to accelerate reactions for high throughput route screening as well as scalable manufacturing. Future plans include the integration of a design of experiments approach to allow for automated optimization of a process. The accomplishments demonstrated here are significant steps towards the development of such a system. Increasing the speed of both route screening and route optimization, along with increasing the scope of chemistry for both the accelerated reaction screening and the microfluidic reaction and purification are critical next steps.
Acknowledgements
We gratefully acknowledge the financial support of DARPA (award no. W911NF-16-2-0020) and the technical assistance provided by Ryan Hilger of the Amy Facility at Purdue University, Department of Chemistry, and Adam Hollerbach.Notes and references
- S. D. Schaber, D. I. Gerogiorgis, R. Ramachandran, J. M. B. Evans, P. I. Barton and B. L. Trout, Ind. Eng. Chem. Res., 2011, 50, 10083–10092 CrossRef CAS.
- B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed. Engl., 2015, 54, 6688–6728 CrossRef CAS PubMed.
- K. F. Jensen, B. J. Reizman and S. G. Newman, Lab Chip, 2014, 14, 3206–3212 RSC.
- K. S. Elvira, X. C. I. Solvas, R. C. R. Wootton and A. J. deMello, Nat. Chem., 2013, 5, 905–915 CrossRef CAS PubMed.
- H. Kim, K. I. Min, K. Inoue, D. J. Im, D. P. Kim and J. Yoshida, Science, 2016, 352, 691–694 CrossRef CAS PubMed.
- N. Kockmann, M. Gottsponer, B. Zimmermann and D. M. Roberge, Chem.–Eur. J., 2008, 14, 7470–7477 CrossRef CAS PubMed.
- Y. Zhang, S. C. Born and K. F. Jensen, Org. Process Res. Dev., 2014, 18, 1476–1481 CrossRef CAS.
- J. P. McMullen and K. F. Jensen, Annu. Rev. Anal. Chem., 2010, 3, 19–42 CrossRef CAS PubMed.
- P. L. Mills, D. J. Quiram and J. F. Ryley, Chem. Eng. Sci., 2007, 62, 6992–7010 CrossRef CAS.
- D. R. Snead and T. F. Jamison, Chem. Sci., 2013, 4, 2822–2827 RSC.
- A. Adamo, R. L. Beingessner, M. Behnam, J. Chen, T. F. Jamison, K. F. Jensen, J. C. Monbaliu, A. S. Myerson, E. M. Revalor, D. R. Snead, T. Stelzer, N. Weeranoppanant, S. Y. Wong and P. Zhang, Science, 2016, 352, 61–67 CrossRef CAS PubMed.
- A. R. Bogdan, S. L. Poe, D. C. Kubis, S. J. Broadwater and D. T. McQuade, Angew. Chem., Int. Ed. Engl., 2009, 48, 8547–8550 CrossRef CAS PubMed.
- D. R. Snead and T. F. Jamison, Angew. Chem., Int. Ed. Engl., 2015, 54, 983–987 CrossRef CAS PubMed.
- F. Lima, M. A. Kabeshov, D. N. Tran, C. Battilocchio, J. Sedelmeier, G. Sedelmeier, B. Schenkel and S. V. Ley, Angew. Chem., Int. Ed. Engl., 2016, 55, 14085–14089 CrossRef CAS PubMed.
- S. V. F. Hansen, Z. E. Wilson, T. Ulven and S. V. Ley, React. Chem. Eng., 2016, 1, 280–287 CAS.
- U. Gross, P. Koos, M. O'Brien, A. Polyzos and S. V. Ley, Eur. J. Org. Chem., 2014, 6418–6430, DOI:10.1002/ejoc.201402804.
- T. Fukuyama, Y. Fujita, M. A. Rashid and I. Ryu, Org. Lett., 2016, 18, 5444–5446 CrossRef CAS PubMed.
- T. Arai, H. Tateno, K. Nakabayashi, T. Kashiwagi and M. Atobe, Chem. Commun., 2015, 51, 4891–4894 RSC.
- J. G. Kralj, H. R. Sahoo and K. F. Jensen, Lab Chip, 2007, 7, 256–263 RSC.
- P. Neugebauer and J. G. Khinast, Cryst. Growth Des., 2015, 15, 1089–1095 CAS.
- J. W. Lee, Z. Horváth, A. G. O'Brien, P. H. Seeberger and A. Seidel-Morgenstern, Chem. Eng. J., 2014, 251, 355–370 CrossRef CAS.
- V. Sans and L. Cronin, Chem. Soc. Rev., 2016, 45, 2032–2043 RSC.
- B. J. Reizman, Y. M. Wang, S. L. Buchwald and K. F. Jensen, React. Chem. Eng., 2016, 1, 658–666 CAS.
- J. P. McMullen, M. T. Stone, S. L. Buchwald and K. F. Jensen, Angew. Chem., Int. Ed. Engl., 2010, 49, 7076–7080 CrossRef CAS PubMed.
- V. Sans, L. Porwol, V. Dragone and L. Cronin, Chem. Sci., 2015, 6, 1258–1264 RSC.
- H. Lu, M. A. Schmidt and K. F. Jensen, Lab Chip, 2001, 1, 22–28 RSC.
- T. A. Hamlin and N. E. Leadbeater, Beilstein J. Org. Chem., 2013, 9, 1843–1852 CrossRef PubMed.
- C. F. Carter, H. Lange, S. V. Ley, I. R. Baxendale, B. Wittkamp, J. G. Goode and N. L. Gaunt, Org. Process Res. Dev., 2010, 14, 393–404 CrossRef CAS.
- S. Newton, C. F. Carter, C. M. Pearson, L. D. Alves, H. Lange, P. Thansandote and S. V. Ley, Angew. Chem., Int. Ed. Engl., 2014, 53, 4915–4920 CrossRef CAS PubMed.
- J. J. Haven, J. Vandenbergh and T. Junkers, Chem. Commun., 2015, 51, 4611–4614 RSC.
- J. S. Mathieson, M. H. Rosnes, V. Sans, P. J. Kitson and L. Cronin, Beilstein J. Nanotechnol., 2013, 4, 285–291 CrossRef PubMed.
- B. V. Silva, F. A. Violante, A. C. Pinto and L. S. Santos, Rapid Commun. Mass Spectrom., 2011, 25, 423–428 CrossRef CAS PubMed.
- K. L. Vikse, M. P. Woods and J. S. McIndoe, Organometallics, 2010, 29, 6615–6618 CrossRef CAS.
- A. J. Ingram, C. L. Boeser and R. N. Zare, Chem. Sci., 2016, 7, 39–55 RSC.
- X. Yan, E. Sokol, X. Li, G. Li, S. Xu and R. G. Cooks, Angew. Chem., Int. Ed. Engl., 2014, 126, 6041–6045 CrossRef.
- K. L. Vikse, M. A. Henderson, A. G. Oliver and J. S. McIndoe, Chem. Commun., 2010, 46, 7412–7414 RSC.
- G. Huang, G. Li and R. G. Cooks, Angew. Chem., Int. Ed. Engl., 2011, 50, 9907–9910 CrossRef CAS PubMed.
- R. M. Bain, C. J. Pulliam and R. G. Cooks, Chem. Sci., 2015, 6, 397–401 RSC.
- M. Wleklinski, C. E. Falcone, B. P. Loren, Z. Jaman, K. Iyer, H. S. Ewan, S.-H. Hyun, D. H. Thompson and R. G. Cooks, Eur. J. Org. Chem., 2016, 2016, 5480–5484 CrossRef CAS.
- X. Yan, R. M. Bain and R. G. Cooks, Angew. Chem., Int. Ed. Engl., 2016, 55, 12960–12972 CrossRef CAS PubMed.
- R. M. Bain, C. J. Pulliam, S. T. Ayrton, K. Bain and R. G. Cooks, Rapid Commun. Mass Spectrom., 2016, 30, 1875–1878 CrossRef CAS PubMed.
- Y. Li, X. Yan and R. G. Cooks, Angew. Chem., Int. Ed. Engl., 2016, 55, 3433–3437 CrossRef CAS PubMed.
- C. P. Holvey, D. M. Roberge, M. Gottsponer, N. Kockmann and A. Macchi, Chem. Eng. Process., 2011, 50, 1069–1075 CrossRef CAS.
- A. Baldacci, F. Prost and W. Thormann, Electrophoresis, 2004, 25, 1607–1614 CrossRef CAS PubMed.
- J. Lu, J. D. Litster and Z. K. Nagy, Cryst. Growth Des., 2015, 15, 3645–3651 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR spectra of selected product, mass spectra of selected products, crystallization information, and experimental procedures are supplied. See DOI: 10.1039/c7sc00905d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |