Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Denitrogenative Suzuki and carbonylative Suzuki coupling reactions of benzotriazoles with boronic acids†
Yuanhao
Wang
a,
Yunfei
Wu
b,
Yuanhe
Li
a and
Yefeng
Tang
 *ab
*ab
aSchool of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China. E-mail: yefengtang@tsinghua.edu.cn
bCollaborative Innovation Center for Biotherapy, State Key Laboratory of Biotherapy and Cancer Center, West China Medical School, Sichuan University, Chengdu 610041, China
First published on 15th March 2017
Abstract
Unprecedented palladium-catalyzed denitrogenative Suzuki and carbonylative Suzuki coupling reactions of benzotriazoles with boronic acids have been realized, which afforded structurally diverse ortho-amino-substituted biaryl and biaryl ketone derivatives. The key to this success is due to the development of a rationally designed strategy to achieve the ring opening of benzotriazoles with a synergistic activating–stabilizing effect, which enables the in situ generation of the corresponding ortho-amino-arenediazonium species. The present work opens up a new avenue to utilize benzotriazoles as synthetic equivalents of ortho-amino-arenediazoniums, which otherwise could not be directly accessed by existing synthetic methods.
Introduction
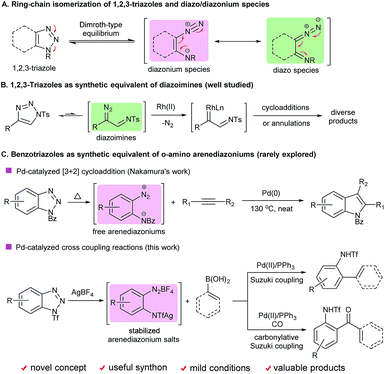
1,2,3-Triazoles are among the most important structural elements in modern chemical, biological and material sciences.1 One of their unique chemical properties is that they can undergo ring-chain isomerization to form the corresponding diazonium or diazo species via a Dimroth-type equilibrium (Scheme 1A).2 Considerable efforts have been devoted to the development of novel transformations based on this unique reactivity. As a paradigm, recently significant advances have been made in the applications of 1-sulfonyl-1,2,3-triazoles as synthetic equivalents of diazoimines in a broad range of intriguing reactions (Scheme 1B).3 In contrast, the ring-opening chemistry of benzotriazoles, a subset of 1,2,3-triazoles well known for their versatile reactivity,4 has remained underdeveloped, mainly due to their high stability and innate reluctance to undergo the ring-opening process.5 Historically, it was reported that benzotriazoles could undergo ring opening followed by denitrogenative cyclization upon photolysis6 or pyrolysis.7 However, those transformations require forcing conditions, and suffer from narrow substrate scope and moderate efficiency, and thus have rarely found applications in organic synthesis.In 2009, Nakamura and co-workers reported a novel palladium-catalyzed denitrogenative formal [3 + 2] cycloaddition of N-aroylbenzotriazoles with internal alkynes (Scheme 1C).8 It was assumed that the reaction proceeded via an ortho-amino-arenediazonium intermediate that was generated in situ through the ring opening of N-aroylbenzotriazole. This seminal discovery shed light on the feasibility of implementing the ring opening of benzotriazoles and transition-metal-catalyzed denitrogenative transformations in one pot. However, such potential has been overlooked by the synthetic community over the past several years, probably because both the demanding reaction conditions and moderate efficiency of the reaction make it less attractive from a practical point of view. Thus, the development of a more general, efficient and robust method to achieve the ring-opening chemistry of benzotriazoles remains an unmet challenge.
Our laboratory has been working on the development of novel reactions based on the ring-opening chemistry of 1,2,3-triazoles.9 Given that this type of chemistry remained underdeveloped for benzotriazoles, we initiated a program to confront this challenge. Notably, when our manuscript was in preparation, Glorius and co-workers published an elegant study on the subject, in which the first visible-light-promoted denitrogenative functionalization of benzotriazoles via aryl radical intermediates was realized.10 We herein report a different strategy to achieve the ring opening of benzotriazoles with a synergistic activating–stabilizing effect, which enables the in situ generation of an ortho-amino-arenediazonium species. As proof-of-concept cases, both palladium-catalyzed denitrogenative Suzuki and carbonylative Suzuki coupling reactions of benzotriazoles with boronic acids have been developed, giving rise to diverse ortho-amino-substituted biaryl and biaryl ketone derivatives. The present work opens up a new avenue to utilize benzotriazoles as synthetic equivalents of ortho-amino-arenediazoniums, which otherwise could not be accessed by existing synthetic methods.11
Results and discussion
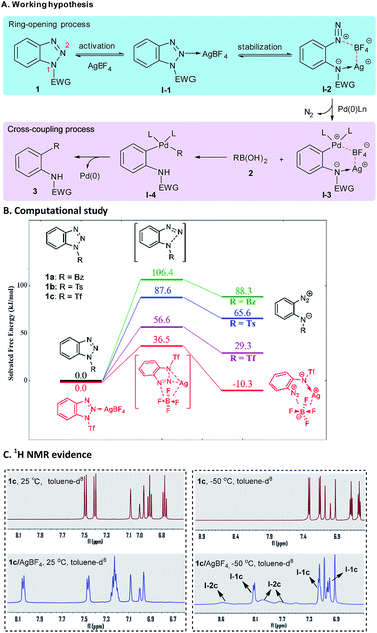
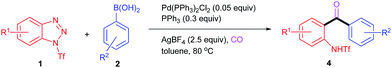
At the outset of our study, we were aware of two challenges associated with the project: (1) how to effect the ring opening of benzotriazoles under mild conditions and (2) how to combine the ring-opening process with other synthetically useful transformations. In terms of the first question, it is known that an electron-withdrawing N1-substituent could facilitate the ring opening of benzotriazoles.12 For example, Ziegler and co-workers reported that 1-nonafluorobutanesulfonyl-benzotriazole12c and 1-nitrobenzotriazole12f could undergo ring opening upon treatment with strong nucleophiles such as amines and deprotonated phenols. Nakamura and co-workers8 and Glorius and co-workers10 utilized 1-aroylbenzotriazoles as effective substrates in their studies. To obtain deeper insight into the role of the N1-substituent, we conducted a computational study with three representative benzotriazoles (1a–c) as model substrates (Scheme 2B). The results revealed that the electron-withdrawing N1-substituent exerts a beneficial effect on the ring opening of benzotriazoles by lowering both the activation free energy (ΔG≠) and Gibbs free energy difference (ΔG). Not surprisingly, such an activating effect positively correlates with the electron-withdrawing capability of N1-substituents (Tf > Ts > Bz). Nevertheless, in all cases, the ring opening of benzotriazoles is a thermodynamically unfavorable process, and thus the resulting zwitterionic diazoniums readily return to their ring-closed forms. To overcome this problem, we planned to use an additive such as AgBF4 to further promote the ring-opening process of benzotriazole by (1) activating the N1–N2 bond through the formation of a complex of benzotriazole–AgBF4 (I-1), and (2) stabilizing the ring-opening product through the formation of an arenediazonium tetrafluoroborate species (I-2). Encouragingly, the synergistic activating–stabilizing effect of AgBF4 was supported by the calculation results when using 1c as the model substrate. Indeed, both ΔG≠ and ΔG of the ring-opening process notably decreased in the presence of AgBF4. As a result, the ring-opening/ring-closing equilibrium shifted in the desired direction. More convincing evidence was obtained from the extensive spectroscopic study performed by us. As shown in Scheme 2C, the chemical shifts of 1c (toluene-d8, 25 °C) notably move downfield in the presence of equimolar AgBF4, thus implying that there exists a strong coordination effect between AgBF4 and 1c. Moreover, the variable temperature 1H NMR experiments showed that a new group of broad signals appeared when the 1H NMR spectrum was recorded at −50 °C, which might be due to the putative ring-opening species I-2c. Of note is the fact that a similar phenomenon was also observed in the variable temperature 19F NMR experiments (for details, see ESI†).As to the second question, we envisaged that since arenediazonium tetrafluoroborates have been proven to be versatile precursors in organic synthesis,13 it was feasible to combine the ring-opening chemistry of benzotriazoles with various other synthetically useful transformations, such as the palladium-catalyzed denitrogenative Suzuki coupling reaction. Indeed, Bohle et al. proved that the ring-opening forms of benzotriazoles could be trapped by coordination to a suitable organometallic complex.14 In the current scenario, the in situ generated ortho-amino-arenediazonium tetrafluoroborate (I-2) would undergo oxidative addition with Pd(0) to give an organopalladium complex (I-3) which, after transmetalation with boronic acid (2) and reductive elimination, could advance to the final product (3) (Scheme 2A).
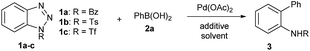
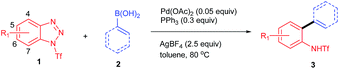
We commenced the study by treating 1-benzoylbenzotriazole (1a) and phenylboronic acid (2a) with AgBF4 (2.5 equiv.) and Pd(OAc)2 (0.05 equiv.) in toluene at 100 °C for 12 h. To our disappointment, no reaction occurred under the reaction conditions (entry 1, Table 1). We further evaluated benzotriazoles 1b and 1c in the reaction. Gratifyingly, while 1b also failed to give a promising result (entry 2), 1c displayed superior reactivity by affording the desired product 3a in 58% isolated yield (entry 3). Encouraged by this outcome, we proceeded to optimize the reaction using 1c as the substrate. First of all, several other additives, including LiBF4, AgSbF6 and AgOTf, were utilized in the reactions, however, none of them gave improved results over AgBF4 (entries 4–6), which indicated that both of the counter ions of AgBF4 played crucial roles in promoting the reaction. Next, a simple evaluation of the solvent effect was conducted, however it failed to give promising outcomes (entries 7–9). Gratifyingly, we found that the usage of PPh3 as an additive could notably improve the reaction by affording 3a in an excellent yield (entry 10). Comparably, the other commonly used phosphine ligand, dppf, afforded only a moderate yield (entry 11). Interestingly, although Pd(PPh3)4 proved to be an effective catalyst for the transformation, it gave a decreased yield (entry 12). Also of note is the fact that lowering the reaction temperature to 80 °C had no side-effects on the reaction (entry 12). However, a poor yield of 3a was obtained when the reaction was performed with reduced equivalents of or in the absence of AgBF4 (entries 14–15).
| Entry | Triazole | Solvent | Additive | T (°C) | Yield of 3 |
|---|---|---|---|---|---|
| a Reaction conditions: 1a–c (0.30 mmol), 2a (0.45 mmol), Pd(OAc)2 (0.015 mmol), PPh3 (0.09 mmol) and AgBF4 (0.75 mmol) in the solvent (3.0 mL). b Isolated yield. c Pd(PPh3)4 (5 mol%) was used. d 1.0 equiv. of AgBF4 (0.30 mmol) was used. n.r. = no reaction. Bz = benzoyl, Ts = p-toluenesulfonyl, Tf = trifluoromethanesulfonyl, dppf = 1,1′-bis(diphenylphosphino)ferrocene. | |||||
| 1 | 1a | Toluene | AgBF4 | 100 | n.r. |
| 2 | 1b | Toluene | AgBF4 | 100 | n.r. |
| 3 | 1c | Toluene | AgBF4 | 100 | 58% |
| 4 | 1c | Toluene | LiBF4 | 100 | n.r. |
| 5 | 1c | Toluene | AgSbF6 | 100 | 18% |
| 6 | 1c | Toluene | AgOTf | 100 | 27% |
| 7 | 1c | CH3CN | AgBF4 | 100 | 17% |
| 8 | 1c | 1,4-Dioxane | AgBF4 | 100 | 25% |
| 9 | 1c | DMF | AgBF4 | 100 | n.r. |
| 10 | 1c | Toluene | AgBF4/PPh3 | 100 | 92% |
| 11 | 1c | Toluene | AgBF4/dppf | 100 | 39% |
| 12c | 1c | Toluene | AgBF4/PPh3 | 100 | 68% |
| 13 | 1c | Toluene | AgBF4/PPh3 | 80 | 94% |
| 14d | 1c | Toluene | AgBF4/PPh3 | 80 | 22% |
| 15 | 1c | Toluene | PPh3 | 80 | 13% |
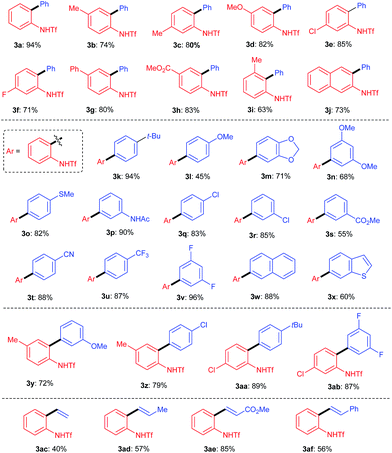
Having determined the optimal conditions, we next investigated the substrate scope of the reaction. First, a variety of substituted benzotriazoles were evaluated using 2a as a reactant. Pleasingly, all of the examined benzotriazoles bearing either electron-donating or -withdrawing substituents were proven to be suitable substrates by affording the corresponding products (3b–3i, Table 2) in good to excellent yields. Generally, the 5- and 6-substituted benzotriazoles gave slightly higher yields than the 4-substituted one (e.g.3b and 3cvs.3i). Moreover, the naphtha[2,3-d]-1,2,3-triazole was also amenable to the reaction, thus indicating that the reaction could be extended to the use of other aromatic ring-fused 1,2,3-triazole derivatives. Next, the scope of boronic acids was evaluated using 1c as a reactant. As shown, an array of substituted phenylboronic acids worked well to efficiently give the corresponding products, regardless of the steric or electronic bias imposed by the substrates. Also of note is the fact that the ester, amide and cyano functional groups remained unchanged during the reactions, thus demonstrating good functionality tolerance. Some other aryl boronic acids were also amenable to the reactions, as witnessed by the reactions leading to 3w and 3x. Not surprisingly, the transformation could be applied to the synthesis of biaryl derivatives containing two substituted aromatic rings (e.g.3y–ab). Last but not least, vinylboronic acids were also proven to be effective substrates for the reactions leading to 3ac–af, thus demonstrating their application in the synthesis of ortho-amino-substituted styrene derivatives.
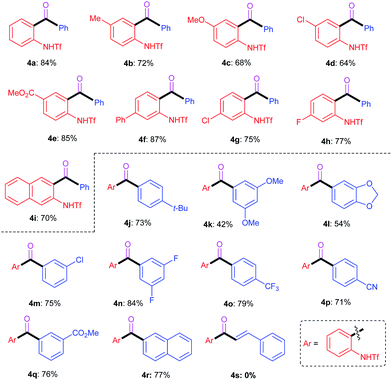
To further demonstrate the synthetic utility of the ring-opening chemistry, we successfully developed an intriguing carbonylative Suzuki coupling reaction, which provided a new method to access ortho-amino-substituted biaryl ketone derivatives. As shown in Table 3, a slightly different catalytic system (AgBF4, Pd(PPh3)2Cl2, toluene, CO, 80 °C) was utilized in the carbonylative Suzuki coupling reactions. Generally, the transformations exhibited moderate substrate scopes with regard to both the benzotriazole and aryl boronic acid reactants. Most of the reactions proceeded smoothly to furnish the corresponding products in good to excellent yields, regardless of the electronic properties and substituent patterns of the substrates. However, different from the aforementioned Suzuki coupling reactions, the vinyl boronic acids failed to give the desired products (e.g.4s) under the studied conditions.
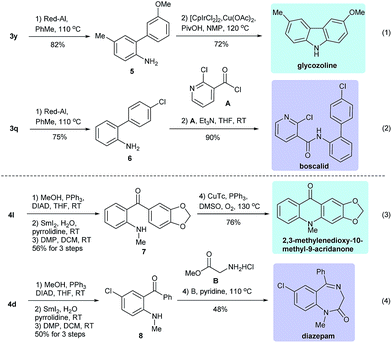
To exemplify the power of the above reactions, we converted some of the resulting products into bioactive natural products and drugs. For example, the Suzuki coupling product 3y could undergo deprotection (Red-Al, toluene, reflux, 82%) followed by an intramolecular Ir-catalyzed annulation15 to give the carbazole alkaloid glycozoline,16 which exhibits antibiotic and antifungal properties (eqn (1), Scheme 3). Similarly, 3q could undergo sequential deprotection and condensation with 2-chloronicotinoyl chloride to provide boscalid,17 a fungicide marketed by BASF company (eqn (2)). For the carbonylative Suzuki coupling product 4l, it could be converted to 7 in three steps (methylation, SmI2 mediated reductive deprotection and Dess–Martin oxidation), which then underwent Cu-catalyzed intramolecular C–H bond activation/C–N bond formation18 to give the alkaloid 2,3-methylenedioxy-10-methyl-9-acridanone (eqn (3)).19 The same protocol was also utilized to convert 4d to 8, which, after condensation with glycine methyl ester, gave diazepam, a well-known drug for treating anxiety and epilepsy (eqn (4)).20
Conclusions
In summary, we developed a new strategy to achieve the ring opening of benzotriazole with a synergistic activating–stabilizing effect, which enables the facile generation of a versatile ortho-amino-arenediazonium species. As proof-of-concept examples, palladium-catalyzed denitrogenative Suzuki and carbonylative Suzuki coupling reactions of benzotriazoles with boronic acids have been achieved. The great synthetic potential of the developed chemistry was demonstrated by its application in the synthesis of bioactive natural products and drugs. We anticipated that the novel concept presented in this work may inspire the development of more mechanistically interesting and synthetically useful transformations. Related studies on this subject are currently underway in our laboratory and the progress will be communicated in due course.Acknowledgements
We gratefully acknowledge the financial support from the National Science Foundation of China (21572112) and Beijing Natural Science Foundation (2172026).Notes and references
- (a) Chemistry of 1,2,3-triazoles, ed. W. Dehaen and V. A. Bakulev, Springer International Publishing, 2015 Search PubMed; (b) J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249 RSC; (c) S. G. Agalave, S. R. Maujan and V. S. Pore, Chem.–Asian J., 2011, 6, 2696 CrossRef CAS PubMed; (d) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed.
- (a) V. Bakulev, W. Dehaen and T. Beryozkina, Thermal Rearrangements and Transformations of 1,2,3-Triazoles, in Chemistry of 1,2,3-triazoles. Springer International Publishing, 2015, p. 1 Search PubMed; (b) E. S. H. E. Ashry, S. Nadeem, M. R. Shah and Y. E. Kilanyd, Adv. Heterocycl. Chem., 2010, 101, 161 CrossRef. For selected examples, see: (c) E. Lieber, T. S. Chao and C. N. R. Rao, J. Org. Chem., 1957, 22, 654 CrossRef CAS; (d) M. E. Hermes and F. D. Marsh, J. Am. Chem. Soc., 1967, 89, 4760 CrossRef CAS; (e) G. J. Lábbé, J. Heterocycl. Chem., 1984, 21, 627 CrossRef.
- For leading reviews, see: (a) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862 CrossRef CAS PubMed; (b) A. V. Gulevich and V. Gevorgyan, Angew. Chem., Int. Ed., 2013, 52, 1371 CrossRef CAS PubMed; (c) H. M. L. Davies and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151 RSC; (d) T. Miura, T. Tanaka, T. Biyajima, A. Yada and M. Murakami, Angew. Chem., Int. Ed., 2013, 52, 3883 CrossRef CAS PubMed; (e) E. E. Schultz, V. N. G. Lindsay and R. Sarpong, Angew. Chem., Int. Ed., 2014, 53, 9904 CrossRef CAS PubMed; (f) B. T. Parr, S. A. Green and H. M. J. Davies, J. Am. Chem. Soc., 2013, 135, 4716 CrossRef CAS PubMed; (g) S. Chuprakov, B. T. Worrell, N. Selander, R. K. Sit and V. V. Fokin, J. Am. Chem. Soc., 2014, 136, 195 CrossRef CAS PubMed; (h) J. Yang, C. Zhu, X. Tang and M. Shi, Angew. Chem., Int. Ed., 2014, 53, 5142 CAS; (i) B. Chattopadhyay and V. Gevorgyan, Org. Lett., 2011, 13, 3746 CrossRef CAS PubMed; (j) D. Yadagiri, A. C. S. Reddy and P. Anbarasan, Chem. Sci., 2016, 7, 5934 RSC; (k) D. Yadagiri and P. Anbarasan, Chem. Sci., 2015, 6, 5847 RSC; (l) E. Lee, T. Ryu, E. Shin, J.-Y. Son, W. Choi and P. H. Lee, Org. Lett., 2015, 17, 2470 CrossRef CAS PubMed; (m) T. Miura, M. Yamauchi and M. Murakami, Org. Lett., 2008, 10, 3085 CrossRef CAS PubMed; (n) T. Miura, M. Yamauchi and M. Murakami, Chem. Commun., 2009, 1470 RSC.
- (a) A. R. Katritzky and S. Rachwal, Chem. Rev., 2011, 111, 7063 CrossRef CAS PubMed; (b) A. R. Katritzky and S. Rachwal, Chem. Rev., 2010, 110, 1564 CrossRef CAS PubMed.
- (a) A. R. Katritzky and B. Yang, J. Org. Chem., 1998, 63, 1467 CrossRef CAS; (b) Y. Su, J. L. Petersen, T. L. Gregg and X. Shi, Org. Lett., 2015, 17, 1208 CrossRef CAS PubMed; (c) T. Kim and K. Kim, Tetrahedron Lett., 2010, 51, 868 CrossRef CAS; (d) D. Kumar, D. B. B. Mishra and V. K. Tiwari, J. Org. Chem., 2014, 79, 251 CrossRef CAS PubMed; (e) D. Kumar, A. Mishra, B. B. Mishra, S. Bhattacharya and V. K. Tiwari, J. Org. Chem., 2013, 78, 899 CrossRef CAS PubMed; (f) S. Battula, A. Kumar, A. P. Gupta and Q. N. Ahmed, Org. Lett., 2015, 17, 5562 CrossRef CAS PubMed.
- (a) E. M. Burgess, R. Caithers and L. McCullagh, J. Am. Chem. Soc., 1968, 90, 1923 CrossRef CAS; (b) P. A. Wender and C. B. Cooper, Tetrahedron, 1986, 42, 2985 CrossRef CAS; (c) J. K. Dutton, D. P. M. Pleynet and A. P. Johnson, Tetrahedron, 1999, 55, 11927 CrossRef CAS; (d) N. A. Al-Jalal, M. R. Ibrahim, N. A. Al-Awadi, M. H. Elnagdi and Y. A. Ibrahim, Molecules, 2014, 19, 20695 CrossRef PubMed; (e) J. K. Dutton, D. P. M. Pleynet and A. P. Johnson, Tetrahedron, 1999, 55, 11927 CrossRef CAS.
- (a) H. Al-Awadi, M. R. Ibrahim, N. A. Al-Awadi and Y. A. Ibrahim, J. Heterocycl. Chem., 2008, 45, 723 CrossRef CAS; (b) N. A. Al-Awadi, B. J. George, H. H. Dib, M. R. Ibrahim, Y. A. Ibrahim and O. M. E. El-Dusouqui, Tetrahedron, 2005, 61, 8257 CrossRef CAS; (c) R. A. Aitken, I. M. Fairhurst, A. Ford, P. E. Y. Milne, D. W. Russell and M. Whittaker, J. Chem. Soc., Perkin Trans. 1, 1997, 3107 RSC; (d) S. J. Barker and R. C. Storr, J. Chem. Soc., Perkin Trans. 1, 1990, 485 RSC.
- I. Nakamura, T. Nemoto, N. Shiraiwa and M. Terada, Org. Lett., 2009, 11, 1055 CrossRef CAS PubMed.
- (a) H. Shang, Y. H. Wang, Y. Tian, J. Feng and Y. F. Tang, Angew. Chem., Int. Ed., 2014, 53, 5662 CrossRef CAS PubMed; (b) Y. Tian, Y. H. Wang, H. Shang, X. D. Xu and Y. F. Tang, Org. Biomol. Chem., 2015, 13, 612 RSC; (c) J. Feng, Y. H. Wang, Q. G. Li, R. W. Jiang and Y. F. Tang, Tetrahedron Lett., 2014, 55, 6455 CrossRef CAS; (d) Y. H. Wang, X. Q. Lei and Y. F. Tang, Synlett, 2015, 26, 2051 CrossRef; (e) Y. H. Wang, X. Q. Lei and Y. F. Tang, Chem. Commun., 2015, 51, 4507 RSC; (f) X. Q. Lei, L. B. Li, Y. B. He and Y. F. Tang, Org. Lett., 2015, 17, 5224 CrossRef CAS PubMed; (g) X. Q. Lei, M. H. Gao and Y. F. Tang, Org. Lett., 2016, 18, 4990 CrossRef CAS PubMed.
- M. Teders, A. Gómez-Suárez, L. Pitzer, M. N. Hopkinson and F. Glorius, Angew. Chem., Int. Ed., 2017, 56, 902 CrossRef CAS PubMed.
- As far as we know, there is no method for the preparation of ortho-amino-arenediazoniums. Indeed, diazotization of 1,2-diaminobenzenes and related derivatives generally results in the formation of the corresponding benzotriazoles instead of ortho-amino-arenediazoniums. For examples, see: (a) L. F. Fieser and E. L. Martin, J. Am. Chem. Soc., 1935, 57, 1835 CrossRef CAS; (b) J. Hu, M. R. Whittaker, H. Duong, Y. Li, C. Boyer and T. P. Davis, Angew. Chem., Int. Ed., 2014, 53, 7779 CrossRef CAS PubMed; (c) C. J. Perry, K. Holding and E. Tyrrell, Synth. Commun., 2008, 38, 3354 CrossRef CAS.
- (a) C. L. Habraken, C. Erkelens, J. R. Mellema and P. Cohen-Fernandes, J. Org. Chem., 1984, 49, 2197 CrossRef CAS; (b) A. R. Katritzky, F. B. Ji, W. Q. Fan, J. K. Gallos, J. V. Greenhill and R. W. King, J. Org. Chem., 1992, 57, 190 CrossRef CAS; (c) X. A. Àlvarez Micó, T. Ziegler and L. R. Subramanian, Angew. Chem., Int. Ed., 2004, 43, 1400 CrossRef PubMed; (d) A. R. Katritzky, R. Akue-Gedu and A. V. Vakulenko, ARKIVOC, 2007, 5 Search PubMed; (e) A. R. Katritzky, L. Khelashvili, K. N. B. Le, P. P. Mohapatra and P. J. Steel, J. Org. Chem., 2007, 72, 5805 CrossRef CAS PubMed; (f) M. Uhde, M. U. Anwar and T. Ziegler, Synth. Commun., 2008, 38, 881 CrossRef CAS.
- For leading reviews, see: (a) C. Galli, Chem. Rev., 1988, 88, 765 CrossRef CAS; (b) A. Roglands, A. Pla-Quintana and M. Moreno-Manas, Chem. Rev., 2006, 106, 4622 CrossRef PubMed; (c) H. Bonin, E. Fouquet and F. X. Felpin, Adv. Synth. Catal., 2011, 353, 3063 CrossRef CAS. For inspiring examples, see: (d) S. Darses, T. Jeffery, J.-P. Genet, J.-L. Brayer and J.-P. Demoute, Tetrahedron Lett., 1996, 37, 3857 CrossRef CAS; (e) S. Sengupta and S. Bhattacharyya, J. Org. Chem., 1997, 62, 3405 CrossRef CAS PubMed; (f) J. T. Kuethe and K. G. Childers, Adv. Synth. Catal., 2008, 350, 1577 CrossRef CAS; (g) M. Dai, B. Liang, C. Wang, J. Chen and Z. Yang, Org. Lett., 2004, 6, 221 CrossRef CAS PubMed.
- D. S. Bohle, Z. Chua and I. Perepichka, ChemPlusChem, 2013, 78, 1304 CrossRef CAS.
- C. Suzuki, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2015, 17, 1597 CrossRef CAS PubMed.
- (a) D. P. Chakraborty, Tetrahedron Lett., 1966, 6, 661 CrossRef; (b) A. K. Chakravarty, T. Sarkar, K. Masuda and K. Shiojima, Phytochemistry, 1999, 50, 1263 CrossRef CAS; (c) M. S. Naykode, V. T. Humne and P. D. Lokhande, J. Org. Chem., 2015, 80, 2392 CrossRef CAS PubMed.
- (a) F.-X. Felpin, E. Fouquet and C. Zakri, Adv. Synth. Catal., 2009, 351, 649 CrossRef CAS; (b) Y. Ye, L. Ma, Z. Dai, Y. Xiao, Y. Zhang, D. Li, J. Wang and H. Zhu, J. Agric. Food Chem., 2014, 62, 4063 CrossRef CAS PubMed.
- J. Huang, C. Wan, M. Xu and Q. Zhu, Eur. J. Org. Chem., 2013, 1876 CrossRef CAS.
- (a) M. Feng, B. Tang, N. Wang, H. Xu and X. Jiang, Angew. Chem., Int. Ed., 2015, 54, 14960 CrossRef CAS PubMed; (b) G. M. Coppola and H. F. Schuster, J. Heterocycl. Chem., 1989, 26, 957 CrossRef CAS.
- N. E. Calcaterra and J. C. Barrow, ACS Chem. Neurosci., 2014, 5, 253 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc00367f |
| This journal is © The Royal Society of Chemistry 2017 |