Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
In situ tether formation from amines and alcohols enabling highly selective Tsuji–Trost allylation and olefin functionalization
Ugo
Orcel
and
Jérôme
Waser
 *
*
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 4306, 1015 Lausanne, Switzerland. E-mail: jerome.waser@epfl.ch
First published on 10th November 2016
Abstract
The use of tethers allows to overcome reactivity and selectivity issues often encountered with intermolecular reactions. Although tethers have been successfully applied for decades, their installation and removal usually requires additional steps. This minireview highlights the recent development of tethers that can be installed in situ on (homo)-allyl amines or alcohols for Tsuji–Trost allylation or double bond functionalization. In particular, the use of (hemi-)acetal tethers for highly regioselective and enantioselective Tsuji–Trost allylation was recently reported. Hydroamination of olefins starting from allylic amines could be achieved via a retro Cope-elimination using catalytic amount of an aldehyde for tether formation. Finally, bifunctionalizations of olefins were developed using either carbon dioxide or carbonyls/imines as tether precursors. These recent breakthroughs greatly enhanced the efficiency of the tethering approach for olefin functionalization, and will make it even more attractive for synthetic chemists in the future.
1. Introduction: the tethering approach for olefin functionalization
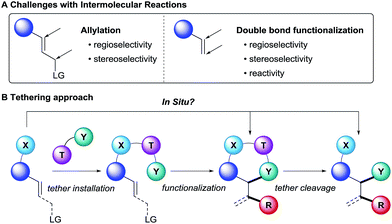
Olefins are key starting materials for the synthesis of valuable building blocks. Their broad availability as well as the extensive number of existing methods for their derivatization has made them essential in both academia and industry. Different strategies are commonly employed for the functionalization of either the α position or the double bond of alkenes. Intramolecular methods generally benefit from higher reactivity due to the lower entropies of activation. Furthermore, the regioselectivity and stereoselectivity can be more easily controlled based on the size and conformation of the formed ring. However, the substrates are often complex and accessed through long synthetic sequences. Furthermore, this strategy is limited to the synthesis of cyclic scaffolds with more narrow structural diversity, as normally it is difficult to change the innate regio- or stereo-selectivity induced by the ring size.Intermolecular reactions on the other hand are more versatile, since less functionalized and therefore readily available substrates can be employed. However, the transposition of known intramolecular reactions to fully intermolecular reactions can be a formidable challenge (Scheme 1A). Indeed, they are inherently more difficult to achieve since the high entropic cost to bring the reactants together lowers the reaction rate. Such energetic penalty can be overcome by using good leaving groups in the case of allylic functionalization, but it is particularly an issue when the modification of non-activated double bonds is considered. Furthermore, the stereo- and regioselectivity is more challenging to control because of the lack of cyclic transition state.
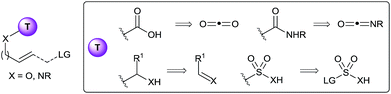
In this context, the use of tethers has emerged as an effective strategy to combine advantages of intra- and intermolecular reactions, such as simple starting materials, high reactivity and selectivity (Scheme 1B).1 Usually, the substrate has to bear a nucleophilic functionality, such as a hydroxy or an amino group, to attach the tether on it. Then the olefin functionalization step occurs in an intramolecular fashion. Finally, tether cleavage delivers the desired products. This approach requires the use of functionalized starting materials. When considering that many alcohols and amines in particular are broadly available from the biomass, this is not a strong limitation. As a consequence, the products synthesized via a tethering strategy are often highly functionalized.
Although tethers have been employed successfully for decades to address challenges of intermolecular reactions with alkenes, the multiple steps required to install and remove them have greatly limited their usefulness. Recently, new methods have emerged to circumvent this major drawback, in which the tethers are installed in situ onto alcohols or amines. This approach complements major progress realized recently in the use of removable or dynamic directing groups for the functionalization of olefins or allylation reactions.2 In order to achieve such performance, it is necessary to consider several criteria: (i) the tether installation must be fast and selective, (ii) excess of reagents and impurities should not impede the functionalization step, and (iii) final cleavage of the tether should be facile and as mild as possible.
Not surprisingly, researchers have focused on the in situ installation of well-established tethers (Fig. 1). For examples, carbonates and carbamates tethers have been intensively used for the synthesis of diols, aminoalcohols and diamines in both allylation reactions3 and the mono-4 and bi-functionalization5 of olefins. In principle, such tethers can be installed in situ by the use of either carbon dioxide or isocyanates as reagents. Prior to 2010, surprisingly few examples of such in situ tether formations had been reported. Using isocyanates, the palladium-catalyzed allylation for the synthesis of 1,2- or 1,3-oxazines was especially successful, starting either from epoxides/aziridines6 or carbonates7 as activated allyl alcohol equivalents. More rarely, the in situ formation of the carbamate directly from the free alcohol has also been applied.8 The use of carbon dioxide as tether precursor is even more challenging, and most success have been met for oxyhalogenation reactions.9 A disadvantage of the use of carbonyls as tethers is that cleavage can require relatively harsh reaction conditions. In this case, sp3 hybridized carbon tethers could be considered, as the obtained acetals, hemiaminals or aminals can be cleaved under milder conditions. Only rare examples of such tethers have been reported.10 Furthermore, in situ formation of the tether had never been achieved prior to 2010, even if aldehydes or imines would be potentially highly attractive precursors. Finally, heteroatom tethers, especially sulfonyl, have been also used highly successfully in the past, although again only using stepwise processes.11 Interestingly, a single example of the use of a boronate tether has also been reported by Cossy and co-workers in the Tsuji–Trost allylation.12
Recently, important progress has been realized for in situ tether formation, especially using sp3 hybridized carbon tethers obtained directly from the reaction of alcohols or amines with carbonyls or imines. This minireview will highlight selected recent examples which appeared since 2010. Progress with in situ tether formation in allylation reactions will be first presented, followed by the even more challenging mono- and bi-functionalization of the double bond of alkenes.
2. Tethers for regioselective Tsuji–Trost reactions
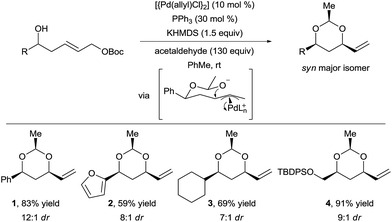
In 2012, Wang and Menche reported the use of acetaldehyde as tether for the synthesis of syn 1,3-diols via tandem hemiacetal formation and Tsuji–Trost reaction with a simple palladium catalyst (Scheme 2).13 This 3-step relay process involved (i) hemiacetal formation from a chiral homoallylic alcohols and acetaldehyde, (ii) palladium π-allyl complex formation, and (iii) intramolecular allylic substitution.The homoallylic alcohol directs and controls both the regio- and stereoselectivity of the C–O bond formation via a cyclic chair transition state. Notably, the nucleophilic attack occurs on the internal position of the π-allyl intermediate, which would have been challenging to achieve in an intermolecular reaction. Arenes, heteroarenes, and functionalized alkyl chains were tolerated to give products 1–4 in good yield and diastereoselectivity. This strategy not only allowed expanding the scope of O-nucleophiles in the Tsuji–Trost reaction, but also delivering complex chiral 1,3-diols in a straightforward manner from simple substrates. In 2015, Aponick and co-workers reported a similar strategy but starting directly from non-activated allylic alcohols using either a gold(III) or a bismuth(III) catalyst.14
In 2013, Menche and co-workers reported the extension of the palladium-catalyzed methodology to the formation of 1,3-aminoalcohols.15 They employed N-tosyl aldimines as nitrogen source. However, all four possible diastereoisomers were formed during the reaction, with an overall low syn/anti ratio, which decreases its synthetic utility.
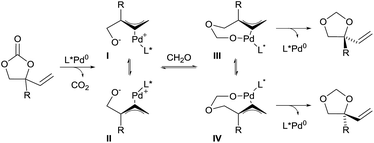
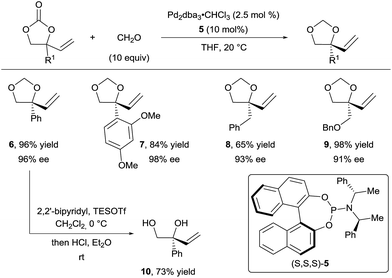
In 2014, Zhang and co-workers also demonstrated the utility of aldehydes and aldimines tethers for the enantioselective synthesis of 1,2-diols and amino alcohols starting from racemic vinylethylene carbonates.16 Their strategy took advantage of a Pd-catalyzed decarboxylation, which afforded zwitterionic allylpalladium intermediates I and II with concomitant loss of the stereochemical information of the starting material through fast isomerization of the π-allyl intermediates (Scheme 3). Subsequent trapping of the alkoxide with a carbonyl affords intermediates III and IV. In presence of a chiral ligand, nucleophilic attack of one of the intermediates is favored to give 5-membered heterocycles with high regio- and enantio-selectivity.
In their first report, they described the use of an excess of formaldehyde to form the tether (Scheme 4).16a They achieved excellent yields and enantioselectivities with a wide range of aryl (products 6 and 7) and alkyl (products 8 and 9) substituents using phosphoramidite ligand 5. Free diols such as 10 could be obtained in good yields upon acidic treatment of the acetals. In addition, several aldehydes, such as acetaldehyde and benzaldehyde derivatives, were efficient for this transformation, both in terms of yield and enantioselectivity. However, the diastereoselectivity observed for the additional stereocenter was low.
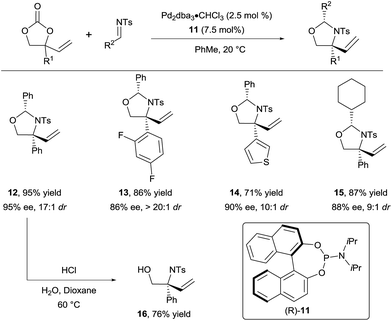
In 2015, they successfully extended this method to the use of isocyanates16b and aldimines16c to form respectively oxazolidinones and oxazolidines. For the latter, a variety of N-tosyl aldimines could be engaged efficiently in the reaction (Scheme 5). Products 12–15 were obtained in high diastereoselectivity and enantioselectivity using phosphoramidite 11 as ligand. Importantly, the hemiaminal group could be readily cleaved under acidic conditions to unveil free amino alcohols such as 16.
In summary, the Menche and the Zhang groups have demonstrated that tethers based on a sp3 hybridized carbon are highly efficient and practical for the synthesis of 1,2- and 1,3 amino alcohols and diols using the Tsuji–Trost allylation. High regio-, diastereo and enantio-selectivity could be achieved. Nevertheless, as olefins bearing a leaving group in α position are inherently activated substrates, it was not clear if such tethering strategies would also be successful in the more challenging functionalization of the π-bond of non-activated alkenes.
3. Tethers for olefin mono-functionalization
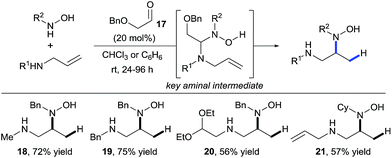
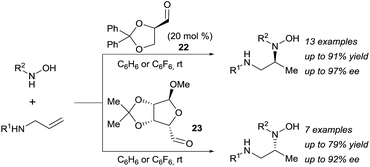
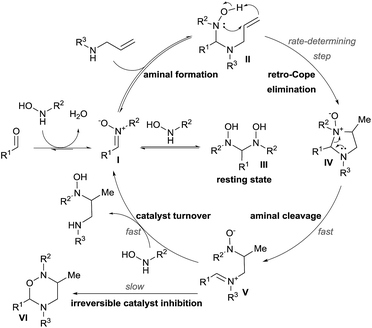
In 2011, building upon the work of Knight and coworkers on the stepwise retro Cope elimination,17 Beauchemin and co-workers reported the catalytic use of aldehydes as tether precursors for the metal-free synthesis of vicinal diamines.18 Their approach is based on a retro Cope elimination enabled by temporary intramolecularity. In their system, an allylic amine reacts with a nitrone generated in situ from an aldehyde and a hydroxylamine to deliver a key mixed aminal intermediate (Scheme 6). A Cope-type hydroamination of the olefin then occurs via a concerted, 5-membered cyclic transition state, leading to valuable 1,2-diamines products 18–21. While several aldehydes could be used in stoichiometric amount, protected α-hydroxy aldehydes such as 17 were identified as efficient catalysts for this transformation. The electron withdrawing ether group activates the aldehyde and therefore facilitates the formation of the key aminal intermediate. The key features of this work are: (i) the reaction is metal-free, (ii) occurs at room temperature and (iii) the aldehyde can be used in catalytic amount. Hence, the tether is both installed and cleaved during the course of the reaction.The potential of this approach was further demonstrated with the development of a catalytic enantioselective version (Scheme 7).18c Two readily available chiral aldehydes 22 and 23, derived from glyceraldehyde and mannose respectively, were found to be efficient both in term of reactivity and enantiocontrol. Furthermore, they allowed the formation of both enantiomers. A limitation of this work was the need of a relatively high loading of aldehyde and the sensitivity of the reaction towards steric hindrance. Indeed, amines bearing a bulky substituted at their allylic position and also internal olefins were poorly reactive under the developed conditions.
Mechanistic studies were then conducted, leading to a first proposal for the catalytic cycle (Scheme 8).18b The reaction starts with condensation of the hydroxylamine with the aldehyde to give nitrone I. Formation of aminal II followed by retro-Cope elimination leads then to cyclic aminal IV. C–N bond cleavage then gives iminium V. The catalytic cycle is completed by reaction with the hydroxylamine to free the hydroamination product.
Kinetic experiments revealed that the reaction was first order both in the allylamine and the aldehyde catalyst, but inverse first order in the hydroxylamine. The latter is explained by the formation of the non-productive aminal III by condensation of two molecules of hydroxylamine on the aldehyde. Inhibition of the catalyst occurs via the formation of oxadiazinanes VI, which were the products described by Knight and coworkers.17 The rate-determining step was established to be the retro-Cope elimination and the rate law of the reaction involved the formation of the aminal, as it occurs before this step.
These important findings enabled them to re-design their catalyst. Initial attempts to lower the activation energy for the hydroamination step were not fruitful, as the equilibrium to form II was more important. In particular, this step was very sensitive to steric effects and therefore formaldehyde was the best catalyst. Interestingly, the reaction could be performed using formalin in aqueous solution. The catalyst loading could be lowered to 5 mol% without erosion of the catalytic activity. The scope of the reaction could be expanded to branched allylamines, which delivered the corresponding diamines in high diastereoselectivity and good yields. Less reactive internal alkenes could also be successfully engaged in the reaction.18d
4. Tethers for olefin difunctionalization
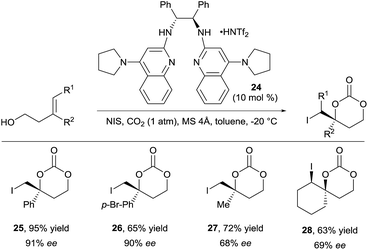
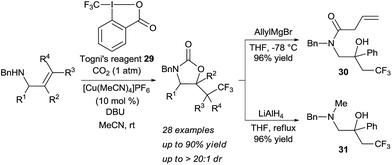
For a long time, difunctionalization of olefins via in situ tether formation has been limited to oxy- or amino-halogenation reactions using carbon dioxide.9 The obtained products are highly attractive for further functionalization and research in this area is therefore still ongoing. A highly efficient method for the oxy- and amino-iodation of both allylic alcohols and amines was developed by Minakata and co-workers using tert-butyl hypoiodite as iodine source with only one atmosphere of carbon dioxide.19 An important breakthrough was reported by Johnston and co-workers in 2015 with the development of the first enantioselective oxy-iodation using carbon dioxide for tether formation (Scheme 9).20 By using bisamidine catalyst 24 and N-iodo succinimide (NIS) as iodine source, benzylic carbonates 25 and 26 were obtained in high yield and enantioselectivity. Moderate enantioselectivity was obtained in case of aliphatic or trisubstituted olefins (products 27 and 28 respectively).Transformations beyond halogenations using carbon dioxide were limited for a long time to activated systems, such as allenes.21 Recently Yu and coworkers disclosed a Cu-catalyzed oxy-trifluoromethylation of allylamines using CO2 gas to form the tether, in which one C–O and one C–C bonds are formed (Scheme 10).22 The combination of a nucleophilic allylic amine, CO2 and Togni's reagent 29 in presence of a copper catalyst and a base led to the oxy-trifluoromethylation of the olefin in high yield and diastereoselectivity. Notably, the developed reaction allowed for mild conditions with CO2 at atmospheric pressure. Cleavage of the heterocycle was achieved either with the strong reducing agent LiAlH4 or allyl magnesium bromide to afford the corresponding vicinal amino alcohols such as 30 and 31 in high yield.
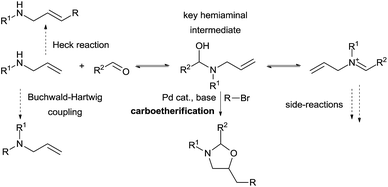
The use of other tether precursors beside CO2 has been less investigated. Inspired by the work of Beauchemin and co-workers involving aminal intermediates, our group aimed to develop a similar strategy for more complex olefin difunctionalization using a palladium catalyst (Scheme 11). In a first step we hypothesized that a simple allylamine and an aldehyde could in situ form a hemiaminal that could then undergo a palladium-catalyzed carboetherification reaction with an adequate electrophile. Many possible side reactions had to be prevented to make this process feasible. Quantitative formation of the tether is necessary to prevent direct Heck reactions, Buchwald-Hartwig coupling, or catalyst inhibition by the amine. Therefore, hemiaminal formation should be both kinetically rapid and thermodynamically favored over both the allylamine and the iminium, as the latter can lead to the formation of undesired aminals. Furthermore, the key hemiaminal intermediate must engage efficiently in the carbo-oxygenation reaction.
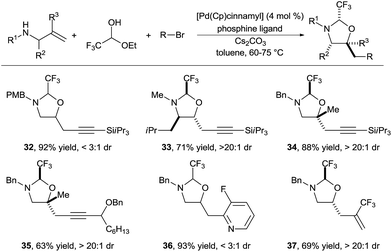
In 2015, we reported the first implementation of this strategy for the synthesis of amino alcohols (Scheme 12).23 The use of the highly electron deficient trifluoroacetaldehyde in its hemiacetal form in combination with electron-rich allylamines was essential to obtain high reactivity. This method allowed the introduction of alkynyl, aryl and vinyl groups in good to excellent yields (products 32–37). Depending of the substrate class, either biphosphines (such as DPEPhos or XANTPhos) or the monodentate trifurylphosphine were the best ligand on palladium. The reaction developed was completely regioselective, whereas the stereoselectivity was substrate dependent. Nevertheless, control of the stereochemistry at the hemiaminal center is less important, as the tether can be cleaved easily. In the case of α-substituted allylamines, the existing stereocenter controlled efficiently the formation of the two others stereocenters (product 33). Tertiary ether centers could be also formed in good yield (products 34 and 35).
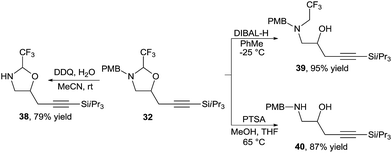
The oxazolidines could be easily transformed into other useful building blocks (Scheme 13). Orthogonal deprotection was possible to access either free amine 38 or free alcohol 39. The hemiaminal tether could be readily removed under acidic conditions to afford amino alcohol 40 in high yield.
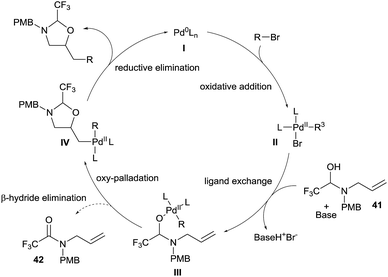
The speculative mechanism of this reaction, based on studies in our group and others,24 would start with the oxidative addition of an organohalide on a Pd(0) complex I to form complex II (Scheme 14). Base mediated ligand exchange on II with hemiaminal 41 would generate the Pd(II)-alkoxide III. At this point, either the productive key oxypalladation occurs to form intermediate IV, or β-hydride elimination is competitive and produces amide 42.25 Finally, reductive elimination allows the formation of the desired oxazolidine and re-generates the palladium(0) complex I.
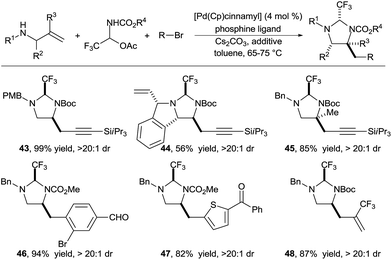
In 2016, we reported the extension of this strategy to the synthesis of diamines (Scheme 15).26 We identified carbamate N-protected trifluoroaldimines in their stable hemiaminal form as tether precursors of choice for this transformation. The trifluoromethyl group was again essential to ensure the fast formation of a very stable aminal, as well as full conversion for the Pd-catalyzed carboamination. This practical procedure allowed to efficiently access a wide range of functionalized diamines. A large variety of alkynyl, aryl and hetereoaryl groups could be introduced in high yield under mild conditions (products 43–48).
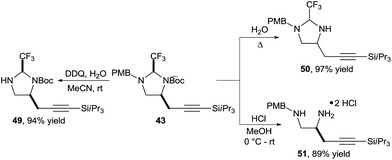
Also in this case, the obtained imidazolidines could be easily modified (Scheme 16). Selective Boc or PMB deprotection was possible to give amines 49 and 50 respectively. Alternatively, diamine 51 could be obtained under mild acidic conditions.
Our work consequently established the use of tethers derived from trifluoroacetaldehyde for the bifunctionalization of allyl amines. This type of tethers is expected to find many more application in the future, not only for olefin functionalization, but also for other types of transformations.
5. Conclusion and outlook
Tethering strategies have been used since a long time for the functionalization of olefins and in the Tsuji–Trost allylation reaction to overcome limitations of reactivity and selectivity inherent to intermolecular processes. Nevertheless, this approach traditionally involves multi-step procedures for tether installation and removal, which makes it synthetically less useful. Recent progress for in situ tether installation has begun to address this shortcoming, leading to an important increase in synthetic efficiency without jeopardizing selectivity and enhanced reactivity. The first example of catalytic tether formation, albeit for the moment limited to metal-free transformations, constitutes an even more efficient approach, and paves the way for future impressive breakthroughs.Acknowledgements
We thank EPFL and the Swiss National Science Foundation (grant number 200021_159920) for financial support.Notes and references
- (a) M. Bols and T. Skrydstrup, Chem. Rev., 1995, 95, 1253 CrossRef CAS; (b) L. Fensterbank, M. Malacria and S. M. Sieburth, Synthesis, 1997, 813 CrossRef CAS; (c) D. R. Gauthier, K. S. Zandi and K. J. Shea, Tetrahedron, 1998, 54, 2289 CrossRef CAS; (d) F. Diederich and P. J. Stang, Templated Organic Synthesis, Wiley-VCH, Chichester, UK, 2000 Search PubMed.
- G. Rousseau and B. Breit, Angew. Chem., Int. Ed., 2011, 50, 2450 CrossRef CAS PubMed.
- Selected examples: (a) T. Hayashi, A. Yamamoto and Y. Ito, Tetrahedron Lett., 1987, 28, 4837 CrossRef CAS; (b) T. Hayashi, A. Yamamoto and Y. Ito, Tetrahedron Lett., 1988, 29, 99 CrossRef CAS; (c) T. Oshitari, R. Akagi and T. Mandai, Synthesis, 2004, 2004, 1325 CrossRef; (d) K. J. Fraunhoffer and M. C. White, J. Am. Chem. Soc., 2007, 129, 7274 CrossRef CAS PubMed; (e) T. J. Osberger and M. C. White, J. Am. Chem. Soc., 2014, 136, 11176 CrossRef CAS PubMed; (f) Y. Z. Xie, K. Yu and Z. H. Gu, J. Org. Chem., 2014, 79, 1289 CrossRef CAS PubMed.
- Selected examples: (a) F. Nahra, F. Liron, G. Prestat, C. Mealli, A. Messaoudi and G. Poli, Chem.–Eur. J., 2009, 15, 11078 CrossRef CAS PubMed; (b) D. Atkinson, M. A. Kabeshov, M. Edgar and A. V. Malkov, Adv. Synth. Catal., 2011, 353, 3347 CrossRef CAS; (c) A. Joosten, A. K. Å. Persson, R. Millet, M. T. Johnson and J.-E. Bäckvall, Chem.–Eur. J., 2012, 18, 15151 CrossRef CAS PubMed; (d) L. Zhu, P. Xiong, Z.-Y. Mao, Y.-H. Wang, X. Yan, X. Lu and H.-C. Xu, Angew. Chem., Int. Ed., 2016, 55, 2226 CrossRef CAS PubMed; (e) P. Xiong, F. Xu, X.-Y. Qian, Y. Yohannes, J. Song, X. Lu and H.-C. Xu, Chem.–Eur. J., 2016, 22, 4379 CrossRef CAS PubMed.
- Selected examples: (a) P. A. Hunt, C. May and C. J. Moody, Tetrahedron Lett., 1988, 29, 3001 CrossRef CAS; (b) Y. Tamaru, H. Tanigawa, S. Itoh, M. Kimura, S. Tanaka, K. Fugami, T. Sekiyama and Z. I. Yoshida, Tetrahedron Lett., 1992, 33, 631 CrossRef CAS; (c) H. Harayama, A. Abe, T. Sakado, M. Kimura, K. Fugami, S. Tanaka and Y. Tamaru, J. Org. Chem., 1997, 62, 2113 CrossRef CAS PubMed; (d) T. J. Donohoe, P. D. Johnson, A. Cowley and M. Keenan, J. Am. Chem. Soc., 2002, 124, 12934 CrossRef CAS PubMed; (e) T. J. Donohoe, M. J. Chughtai, D. J. Klauber, D. Griffin and A. D. Campbell, J. Am. Chem. Soc., 2006, 128, 2514 CrossRef CAS PubMed; (f) E. J. Alexanian, C. Lee and E. J. Sorensen, J. Am. Chem. Soc., 2005, 127, 7690 CrossRef CAS PubMed; (g) J. A. Fritz, J. S. Nakhla and J. P. Wolfe, Org. Lett., 2006, 8, 2531 CrossRef CAS PubMed; (h) B. A. Hopkins and J. P. Wolfe, Angew. Chem., Int. Ed., 2012, 51, 9886 CrossRef CAS PubMed; (i) F. C. Sequeira, B. W. Turnpenny and S. R. Chemler, Angew. Chem., Int. Ed., 2010, 49, 6365 CrossRef CAS PubMed; (j) G. Zhang, Y. Luo, Y. Wang and L. Zhang, Angew. Chem., Int. Ed., 2011, 50, 4450 CrossRef CAS PubMed; (k) S. Nicolai, C. Piemontesi and J. Waser, Angew. Chem., Int. Ed., 2011, 50, 4680 CrossRef CAS PubMed; (l) G.-S. Liu, Y.-Q. Zhang, Y.-A. Yuan and H. Xu, J. Am. Chem. Soc., 2013, 135, 3343 CrossRef CAS PubMed; (m) T. Wu, J. Cheng, P. Chen and G. Liu, Chem. Commun., 2013, 49, 8707 RSC.
- (a) B. M. Trost and A. R. Sudhakar, J. Am. Chem. Soc., 1987, 109, 3792 CrossRef CAS; (b) B. M. Trost and A. R. Sudhakar, J. Am. Chem. Soc., 1988, 110, 7933 CrossRef CAS; (c) J.-O. Baeg, C. Bensimon and H. Alper, J. Am. Chem. Soc., 1995, 117, 4700 CrossRef CAS; (d) C. Larksarp and H. Alper, J. Am. Chem. Soc., 1997, 119, 3709 CrossRef CAS.
- (a) Y. Tamaru, T. Bando, Y. Kawamura, K. Okamura, Z. I. Yoshida and M. Shiro, J. Chem. Soc., Chem. Commun., 1992, 1498 RSC; (b) T. Bando, H. Harayama, Y. Fukazawa, M. Shiro, K. Fugami, S. Tanaka and Y. Tamaru, J. Org. Chem., 1994, 59, 1465 CrossRef CAS.
- (a) B. M. Trost and D. L. Van Vranken, J. Am. Chem. Soc., 1993, 115, 444 CrossRef CAS; (b) G. Broustal, X. Ariza, J.-M. Campagne, J. Garcia, Y. Georges, A. Marinetti and R. Robiette, Eur. J. Org. Chem., 2007, 2007, 4293 CrossRef.
- Selected examples: (a) T. Toda and Y. Kitagawa, Angew. Chem., Int. Ed., 1987, 26, 334 CrossRef; (b) E. García-Egido, I. Fernández and L. Muñoz, Synth. Commun., 2006, 36, 3029 CrossRef.
- (a) R. Amoroso, G. Cardillo and C. Tomasini, Tetrahedron Lett., 1991, 32, 1971 CrossRef CAS; (b) R. Amoroso, G. Cardillo and C. Tomasini, Heterocycles, 1992, 34, 349 CrossRef CAS; (c) R. Amoroso, G. Cardillo, C. Tomasini and P. Tortoreto, J. Org. Chem., 1992, 57, 1082 CrossRef CAS; (d) R. A. T. M. Van Benthem, H. Hiemstra and W. N. Speckamp, J. Org. Chem., 1992, 57, 6083 CrossRef CAS; (e) R. A. T. M. van Benthem, H. Hiemstra, G. R. Longarela and W. N. Speckamp, Tetrahedron Lett., 1994, 35, 9281 CrossRef CAS; (f) D. Yoo, S. Kwon and Y. G. Kim, Tetrahedron: Asymmetry, 2005, 16, 3762 CrossRef CAS; (g) S. Fustero, D. Jiménez, J. Moscardó, S. Catalán and C. del Pozo, Org. Lett., 2007, 9, 5283 CrossRef CAS PubMed; (h) A. B. Weinstein, D. P. Schuman, Z. X. Tan and S. S. Stahl, Angew. Chem., Int. Ed., 2013, 52, 11867 CrossRef CAS PubMed.
- Selected examples: (a) R. I. McDonald and S. S. Stahl, Angew. Chem., Int. Ed., 2010, 49, 5529 CrossRef CAS PubMed; (b) R. M. Fornwald, J. A. Fritz and J. P. Wolfe, Chem.–Eur. J., 2014, 20, 8782 CrossRef CAS PubMed; (c) Z. J. Garlets, K. R. Parenti and J. P. Wolfe, Chem.–Eur. J., 2016, 22, 5919 CrossRef CAS PubMed.
- J. Cluzeau, P. Capdevielle and J. Cossy, Tetrahedron Lett., 2005, 46, 6945 CrossRef CAS.
- L. Wang and D. Menche, Angew. Chem., Int. Ed., 2012, 51, 9425 CrossRef CAS PubMed.
- J. A. Goodwin, C. F. Ballesteros and A. Aponick, Org. Lett., 2015, 17, 5574 CrossRef CAS PubMed.
- B. Tang, L. Wang and D. Menche, Synlett, 2013, 24, 625 CrossRef CAS.
- (a) A. Khan, R. F. Zheng, Y. H. Kan, J. Ye, J. X. Xing and Y. J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 6439 CrossRef CAS PubMed; (b) A. Khan, J. Xing, J. Zhao, Y. Kan, W. Zhang and Y. J. Zhang, Chem.–Eur. J., 2015, 21, 120 CrossRef CAS PubMed; (c) L. Yang, A. Khan, R. Zheng, L. Y. Jin and Y. J. Zhang, Org. Lett., 2015, 17, 6230 CrossRef CAS PubMed; (d) A. Khan and Y. J. Zhang, Synlett, 2015, 26, 853 CrossRef CAS.
- (a) M. B. Gravestock, D. W. Knight and S. R. Thornton, J. Chem. Soc., Chem. Commun., 1993, 169 RSC; (b) K. E. Bell, M. P. Coogan, M. B. Gravestock, D. W. Knight and S. R. Thornton, Tetrahedron Lett., 1997, 38, 8545 CrossRef CAS; (c) M. B. Gravestock, D. W. Knight, K. M. A. Malik and S. R. Thorton, J. Chem. Soc., Perkin Trans. 1, 2000, 3292 RSC.
- (a) M. J. MacDonald, D. J. Schipper, P. J. Ng, J. Moran and A. M. Beauchemin, J. Am. Chem. Soc., 2011, 133, 20100 CrossRef CAS PubMed; (b) N. Guimond, M. J. MacDonald, V. Lemieux and A. M. Beauchemin, J. Am. Chem. Soc., 2012, 134, 16571 CrossRef CAS PubMed; (c) M. J. MacDonald, C. R. Hesp, D. J. Schipper, M. Pesant and A. M. Beauchemin, Chem.–Eur. J., 2013, 19, 2597 CrossRef CAS PubMed; (d) C. R. Hesp, M. J. MacDonald, M. M. Zahedi, D. A. Bilodeau, S. B. Zhao, M. Pesant and A. M. Beauchemin, Org. Lett., 2015, 17, 5136 CrossRef CAS PubMed.
- (a) S. Minakata, I. Sasaki and T. Ide, Angew. Chem., Int. Ed., 2010, 49, 1309 CrossRef CAS PubMed; (b) Y. Takeda, S. Okumura, S. Tone, I. Sasaki and S. Minakata, Org. Lett., 2012, 14, 4874 CrossRef CAS PubMed.
- B. A. Vara, T. J. Struble, W. Wang, M. C. Dobish and J. N. Johnston, J. Am. Chem. Soc., 2015, 137, 7302 CrossRef CAS PubMed.
- (a) G. F. Chen, C. L. Fu and S. M. Ma, Org. Lett., 2009, 11, 2900 CrossRef CAS PubMed; (b) S. H. Li, B. K. Y. Miao, W. M. Yuan and S. M. Ma, Org. Lett., 2013, 15, 977 CrossRef CAS PubMed; (c) S. H. Li, J. T. Ye, W. M. Yuan and S. M. Ma, Tetrahedron, 2013, 69, 10450 CrossRef CAS; (d) K. Yamashita, S. Hase, Y. Kayaki and T. Ikariya, Org. Lett., 2015, 17, 2334 CrossRef CAS PubMed.
- J.-H. Ye, L. Song, W.-J. Zhou, T. Ju, Z.-B. Yin, S.-S. Yan, Z. Zhang, J. Li and D.-G. Yu, Angew. Chem., Int. Ed., 2016, 55, 10022 CrossRef CAS PubMed.
- U. Orcel and J. Waser, Angew. Chem., Int. Ed., 2015, 54, 5250 CrossRef CAS PubMed.
- (a) J. D. Neukom, N. S. Perch and J. P. Wolfe, J. Am. Chem. Soc., 2010, 132, 6276 CrossRef CAS PubMed; (b) J. D. Neukom, N. S. Perch and J. P. Wolfe, Organometallics, 2011, 30, 1269 CrossRef CAS; (c) P. S. Hanley and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 6302 CrossRef CAS PubMed; (d) P. S. Hanley and J. F. Hartwig, J. Am. Chem. Soc., 2011, 133, 15661 CrossRef CAS PubMed; (e) S. Nicolai, R. Sedigh-Zadeh and J. Waser, J. Org. Chem., 2013, 78, 3783 CrossRef CAS PubMed.
- Amide 42 was not observed under the optimized reaction conditions. However amide side products could be isolated when the formation of six-membered rings was attempted.
- U. Orcel and J. Waser, Angew. Chem., Int. Ed., 2016, 55, 12881 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |