Open Access Article
Open Access ArticleIon-pair recognition by a neutral [2]rotaxane based on a bis-calix[4]pyrrole cyclic component†
J. Ramón
Romero
a,
Gemma
Aragay
 a and
Pablo
Ballester
a and
Pablo
Ballester
 *ab
*ab
aInstitute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Avgda. Països Catalans 16, 43007 Tarragona, Spain. E-mail: pballester@iciq.es
bICREA, Passeig Lluís Companys, 23, 08010 Barcelona, Spain
First published on 31st August 2016
Abstract
In this work, we report our investigations on the synthesis of a [2]rotaxane based on a bis(calix[4]pyrrole) cyclic component and a 3,5-bis-amidepyridyl-N-oxide derivative axle. We isolated the [2]rotaxane in a significant 50% yield through an optimized “in situ” capping strategy using the copper(I)-catalyzed azide–alkyne cycloaddition reaction. The synthetic precursor of the [2]rotaxane, featuring [2]pseudorotaxane topology, could be quantitatively assembled in solution in the presence of one equivalent of tetrabutylammonium chloride or cyanate salts producing a four-particle aggregate. However, we observed that the addition of the salt was deleterious not only for the isolation of the [2]rotaxane in its pure form but, more important, for the optimal performance of the copper catalyst. We probed the interaction of the prepared [2]rotaxane with tetraalkylammonium salts of chloride, nitrate and cyanate anions by means of 1H NMR titrations and ITC experiments. We show that in chloroform solution the [2]rotaxane functions as an efficient heteroditopic receptor for the salts forming thermodynamically and kinetically highly stable ion-paired complexes with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. At millimolar concentration and using 1H NMR spectroscopy we observed that the addition of more than 1 equiv. of the salt induced the gradual disassembly of the 1
1 stoichiometry. At millimolar concentration and using 1H NMR spectroscopy we observed that the addition of more than 1 equiv. of the salt induced the gradual disassembly of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of the [2]rotaxane and the concomitant formation of higher stoichiometry aggregates i.e. 2
1 complex of the [2]rotaxane and the concomitant formation of higher stoichiometry aggregates i.e. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes.
1 complexes.
Introduction
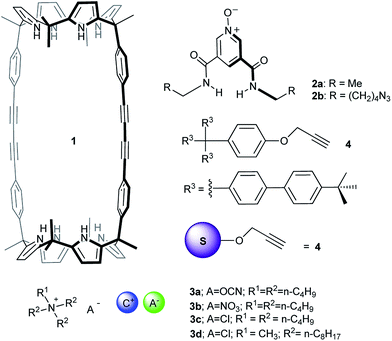
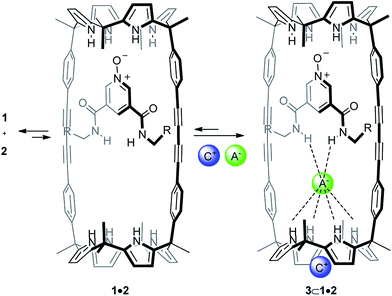
Anion recognition is an important and established field in supramolecular chemistry owing to the fundamental role that negatively charged species play in chemical, biological and environmental processes.1–3 Mechanically interlocked molecules such as rotaxanes or catenanes present unique three-dimensional cavities for anion recognition that resemble the preorganized pocket of anion binding proteins in Nature.4–6The use of anions as templates in the preparation of interlocked structures has been widely described7 and is known to generate topologically unique cavities for anion recognition.8–10 However, the majority of anion-template strategies for the assembly of interlocked structures combined strong ion-pairing of the linear axle with hydrogen bonding recognition of the anion by the macrocyclic component.11–13 In 2012, we reported the use of polyatomic anions for the quantitative assembly of [2]pseudorotaxane-like architectures without involving ion-pairing in the linear component.14 Our approach exploited the exceptional recognition properties exhibited by a neutral interwoven self-assembled receptor 1·2a towards ion-pairs (Fig. 1 and Scheme 1). In chloroform, at millimolar concentration, an equimolar mixture of bis-calix[4]pyrrole macrocycle 1 and linear bis-amidepyridyl-N-oxide 2a produced the 1·2a receptor in a partial extent.15 The linear axle threaded the cyclic component by establishing four convergent hydrogen bonds between the oxygen atom of 2a and the pyrrole NHs of one calix[4]pyrrole unit in 1 (Fig. 1). Remarkably, the addition of 1 equiv. of certain polyatomic anions (A−) as tetrabutylammonium salts (+NBu4) to the two-components' mixture induced the quantitative assembly of four-particle aggregates ANBu4⊂1·2a with [2]pseudorotaxane-like topology (Scheme 1). The resulting complexes displayed a cyclic-component separated binding mode of the ion-pair. The anion was included in the polar cavity of the interwoven receptor in which six NHs converged (two from bound bis-amide and four from the opposing calix[4]pyrrole cap of the cyclic component). While the cation was located in the shallow, electron-rich cavity defined by the cone conformation of the calix[4]pyrrole, opposed to the bound anion.
Almost coinciding with our report, Beer and co-workers16 described the synthesis of neutral [2]rotaxanes that recognized halide anions in aqueous mixtures containing a bis-amidepyridyl-N-oxide axle derivative closely related to 2a. The employed strategy for the assembly and thermodynamic stabilization of the synthetic intermediate displaying pseudorotaxane topology was very similar to ours. They used a subsequent capping approach relying on the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction to produce the [2]rotaxane. The CuAAC has been extensively and successfully applied in the synthesis of mechanically interlocked molecules i.e. [2]rotaxanes.17–21
Inspired by these findings, herein, we describe the synthesis of the neutral [2]rotaxane 5 (Scheme 2). The employed capping strategy was based on the CuAAC reaction mentioned above. Surprisingly to us, the use of anion templation was detrimental for the synthesis of 5. The absence of templating anions (i.e. Cl−, OCN−) resulted in higher yields of the [2]rotaxane 5. We also report preliminary results obtained in the binding studies of the mechanically interlocked host 5 with a series of tetraalkylammonium salts containing hydrogen-bonding anions. In chloroform solution, [2]rotaxane 5 acted as a heteroditopic receptor of ion-pairs.22,23 It formed kinetically and thermodynamically highly stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes. The ion-pair was bound in a cyclic-component separated binding mode. The binding affinity of the supramolecular host 5 for the ion-pairs was regulated by the nature of both the anion and the cation.
1 complexes. The ion-pair was bound in a cyclic-component separated binding mode. The binding affinity of the supramolecular host 5 for the ion-pairs was regulated by the nature of both the anion and the cation.
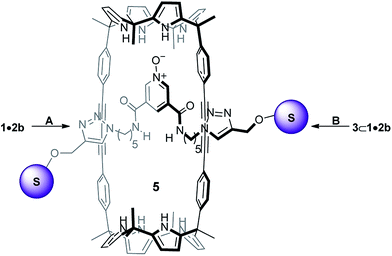 | ||
| Scheme 2 Synthetic schemes for the preparation of [2]rotaxane 5. A and B reaction conditions: 4; DIPEA, [Cu(CH3CN)4]PF6 and TBTA in CH2Cl2, 5 h at R.T. | ||
Results and discussion
The syntheses of the [2]rotaxane's molecular components (i.e. macrocycle 1,14 linear component 2b and stopper 4,24Fig. 1) were performed by following or adapting synthetic procedures described in literature (see ESI† for experimental details). The selection of the tris(biphenyl) stopper 4 was not trivial and derived from multiple unsuccessful attempts to isolate the [2]rotaxane when using smaller terphenyl stoppers.Previously, we reported that in chloroform solution macrotricycle 1 and N-oxide 2a formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with [2]pseudorotaxane topology (Scheme 1). At room temperature, the 1H NMR spectrum of the equimolar mixture (1 mM) of 1 and 2a showed broadening beyond detection for some of the signals of the protons of the two binding partners. Based on 1H NMR titrations we determined that the stability constant of the 1·2a complex was 800 M−1 at 298 K.14 Taken together, these results indicate that at this concentration only around 30% of the binding partners are involved in the interwoven 1·2a or 1·2b complexes and that the equilibrium process is intermediate/fast on the proton chemical shift timescale.
1 complex with [2]pseudorotaxane topology (Scheme 1). At room temperature, the 1H NMR spectrum of the equimolar mixture (1 mM) of 1 and 2a showed broadening beyond detection for some of the signals of the protons of the two binding partners. Based on 1H NMR titrations we determined that the stability constant of the 1·2a complex was 800 M−1 at 298 K.14 Taken together, these results indicate that at this concentration only around 30% of the binding partners are involved in the interwoven 1·2a or 1·2b complexes and that the equilibrium process is intermediate/fast on the proton chemical shift timescale.
[2]Rotaxane 5 was initially prepared by adding to the 1 mM equimolar mixture of 1 and 2b in dichloromethane solution, 2 equiv. of the alkyne-functionalized tris(biphenyl) stopper 4, 4 equiv. of diisopropyl ethyl amine (DIPEA), and a catalytic amount (5%) of [Cu(CH3CN)4]PF6 and tris(benzyltriazoylmethyl)amine (TBTA) (Scheme 2A, entry a from Table 1). The mixture was stirred at room temperature for 5 hours. [2]Rotaxane 5 was isolated as a white solid in 10% yield after silica column chromatography purification of the crude reaction mixture using AcOEt/CH2Cl2 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 as eluent.
70 as eluent.
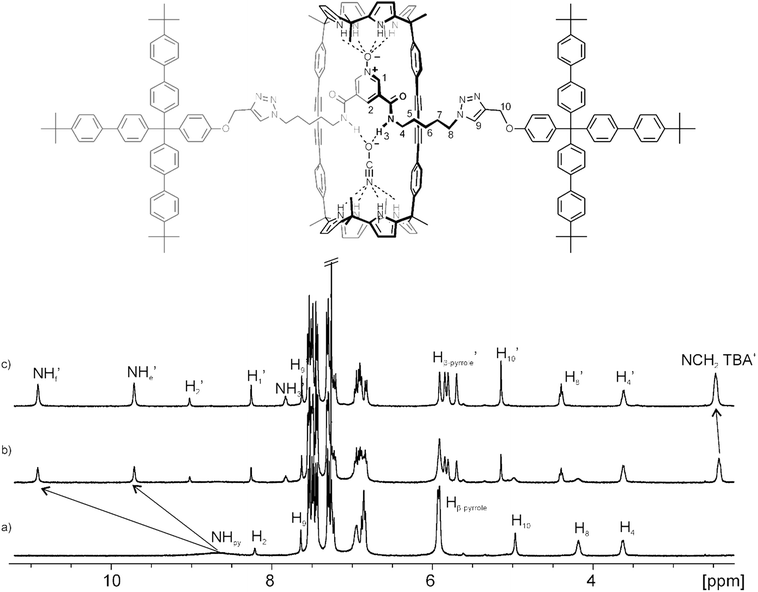
Rotaxane 5 was characterized by a complete set of high-resolution spectra (1D and 2D NMR spectroscopy and ESI-HRMS+ spectrometry) (see ESI†). The 1H NMR spectrum of 5 (Fig. 2a) in CDCl3 solution displayed both broad and sharp, well-defined signals that were assigned to the protons of its two components, macrocycle and axle, using 2D NMR experiments (ESI†). In particular, the sharp signals observed in the region of 7.5 to 7.2 ppm were attributed to protons of the bulky tris(biphenyl) stopper groups of the axle. The pyrrole NHs resonated at δ = 8.7 ppm as a unique broad signal, which is downfield shifted in comparison to the NHs in the free macrocycle (Fig. S9†). On the one hand, these observations suggested the existence of hydrogen-bonding interactions between the pyrrole NHs and the oxygen atom of the pyridyl-N-oxide group in the threaded linear component. On the other hand, because the cyclic component 1 possesses two identical calix[4]pyrrole binding sites, it must be involved in a dynamic process that most likely encompasses the pirouetting of the pyridyl-N-oxide axle within the macrocycle cavity. At room temperature, this dynamic process is fast on the chemical shift timescale providing a single set of signals for the protons of 1 although some of them appear as broad signals i.e. NHs.‡ The aromatic proton of triazole rings resonated as a sharp singlet at δ = 7.6 ppm confirming the covalent connection between the stoppers and the linear axle.
We also performed Diffusion Ordered Spectroscopy (DOSY) NMR experiments using a millimolar solution of 5 in CDCl3. Despite the size difference between the two molecular components of 5, the diffusion constant values calculated for the decay of the proton signals' intensities in any of them were identical. This result indicated that the two components were involved in the formation of the interlocked supramolecular species 5. The diffusion constant determined for 5 was 4.7 × 10−10 m2 s−1 (Fig. S17†).
Aiming at improving the yield of [2]rotaxane 5, we considered to undertake its synthesis in the presence of 1 equiv. of tetrabutylammonium cyanate 3a (OCNTBA) (Scheme 2B, entry b Table 1). We have showed that the addition of 1 equiv. of OCNTBA to a 1 mM equimolar mixture of 1 and 2a in CDCl3 solution yielded the quantitative assembly of the 3a⊂1·2a complex (Ka ∼ 1011 M−2) featuring [2]pseudorotaxane topology (Scheme 1).14 This result highlighted the important role played by the ion-pair in templating and stabilizing the interwoven structure of the complex formed between 1 and 2a in solution. We expected that the concentration increase experienced by the synthetic intermediate with [2]pseudorotaxane topology (from 30% to almost 100% in the presence of an equimolar amount of 3a) could be translated in a significant improvement of the isolated yield of [2]rotaxane 5.
Although we were aware of literature precedents alerting on the inhibitory effects of halides in CuAAC reaction rates in DMSO,25 aqueous26 and buffered systems,27 we repeated the threading and CuAAC stoppering reactions in the presence of 1 equiv. of OCNTBA 3a. We sought to perform this reaction in a NMR tube with the goal of analyzing the initial assembly of the synthetic [2]pseudorotaxane precursor 3a⊂1·2b in the presence of the catalytic system (5% TBTA and Cu(I) salt), as well as the evolution of the CuAAC reaction by 1H NMR spectroscopy. We observed that the addition of the catalytic system to a 1 mM equimolar mixture of 1, 2b and OCNTBA 3a and 2 equiv. of the tris(biphenyl) stopper 4 had a negligible effect on the assembled 3a⊂1·2b complex in chloroform solution. Five hours after the addition of the Hünig's base (4 equiv.), the 1H NMR spectrum of the reaction mixture revealed that the signal corresponding to terminal alkyne protons of 4 and the methylene protons alpha to unreacted azide groups in 2b were still visible. The analysis of the mixture at longer times evidenced that the reaction was not evolving any further. We obtained very similar results when we replaced the tetrabutylammonium cyanate salt by methyltrioctylammonium chloride 3d. These findings suggested that the salts, used as templates for the assembly of the [2]pseudorotaxanes, had a significant effect in the formation's rate of the mechanical bond.
In order to gain some insight on the effect played by the tetralkylammonium salts in the CuAAC reaction's rate we performed additional control experiments. First, we determined that the CuAAC reaction of diazide 2b with 2 equiv. of stopper 4 in CH2Cl2 solution in the absence of macrocycle 1 was completed after 3 hours. Conversely, the same reaction in the presence of 1 equiv. of ion-pairs OCNTBA 3a or ClMTOA 3d did not take place even after a period of 24 hours. All together, these results supported that the presence of a great excess of tetralkylammonium salts of coordinating anions, like cyanate or chloride caused the inhibition and deactivation of the copper(I) catalytic system possibly by competing with the activation of the azide through coordination with the metal center. As mentioned above, the presence of the cyclic component 1 in the mixture of reactants 2b, 3a and 4 allowed the CuAAC reaction to evolve but to a partial extent. Most likely, in this latter case, the complexation of the ion-pair exerted by the self-assembled [2]pseudorotaxanes and the emerging [2]rotaxane significantly reduced the amount of free anions (salt) in solution. Moreover, the isolation of [2]rotaxane 5 from the crude reaction mixtures obtained using the ion-pair template strategy was not exempt of complications. Surprisingly, the fractions isolated from the column chromatography purification of 5 consisted mainly of mixtures of the free receptor, free linear component and the ion-paired complex, 3⊂5.§¶.
Considering the synthetic limitations encountered in the use of ion-pairs as templates for the synthesis of the mechanically interlocked receptor 5 (i.e. purification problems and catalyst deactivation), we explored alternative methodologies that could improve its isolated yield (entries c and d from Table 1). A tenfold increase in the concentration of reactants ([2b] = 14 mM and [4] = 28 mM) and the cyclic component ([1] = 14 mM) maintaining a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio almost tripled the yield of the isolated [2]rotaxane 5 (27%). Doubling the stoichiometric ratio of reactants (2b and 4) with respect to the cyclic component (1) and using the latter at a concentration of 8 mM in order to assure the solubility of all compounds, allowed us to isolate the [2]rotaxane 5 in a significant 50% yield. We used the catalytic system in a 5% load with respect to the concentration of the linear component and all reactions were completed in less than 5 hours. Most likely, the higher concentration attained for the [2]pseudorotaxane precursor in the more concentrated solutions was responsible for the observed increase in isolated yields of 5.
1 stoichiometric ratio almost tripled the yield of the isolated [2]rotaxane 5 (27%). Doubling the stoichiometric ratio of reactants (2b and 4) with respect to the cyclic component (1) and using the latter at a concentration of 8 mM in order to assure the solubility of all compounds, allowed us to isolate the [2]rotaxane 5 in a significant 50% yield. We used the catalytic system in a 5% load with respect to the concentration of the linear component and all reactions were completed in less than 5 hours. Most likely, the higher concentration attained for the [2]pseudorotaxane precursor in the more concentrated solutions was responsible for the observed increase in isolated yields of 5.
Next, we probed the interaction of [2]rotaxane 5 with anions in CDCl3 solution by means of 1H NMR titration experiments. The addition of 0.5 equiv. of OCNTBA 3a to a millimolar solution of 5 (Fig. 2b) produced the observation of separate signals for the hydrogen atoms in the bound and free rotaxane indicating that the binding equilibrium was slow on the 1H NMR timescale. When 1 equiv. of OCNTBA 3a was added (Fig. 2c), only the signals corresponding to the protons in the bound rotaxane were detected. This observation supported the formation of a complex with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and an association constant larger than 104 M−1.
1 stoichiometry and an association constant larger than 104 M−1.
In the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, all the proton signals related to 5 were sharp and well-defined suggesting that the putative pirouetting process of the axle component within the macrocycle was slow on the chemical shift timescale. The slow dynamics of this process was also evidenced by the observation of two separate and highly downfield shifted proton signals corresponding to the pyrrole NHs of 1. This observation was in agreement with their involvement in hydrogen bonding interactions with two different acceptor moieties (i.e. one hemisphere binds the N-oxide unit of the axle and the other binds the cyanate anion). The β-pyrrole protons resonated as two sets of two signals in the range of 5.9 to 5.7 ppm. This finding also supported the existence of two chemically non-equivalent hemispheres in the resulting 1
1 complex, all the proton signals related to 5 were sharp and well-defined suggesting that the putative pirouetting process of the axle component within the macrocycle was slow on the chemical shift timescale. The slow dynamics of this process was also evidenced by the observation of two separate and highly downfield shifted proton signals corresponding to the pyrrole NHs of 1. This observation was in agreement with their involvement in hydrogen bonding interactions with two different acceptor moieties (i.e. one hemisphere binds the N-oxide unit of the axle and the other binds the cyanate anion). The β-pyrrole protons resonated as two sets of two signals in the range of 5.9 to 5.7 ppm. This finding also supported the existence of two chemically non-equivalent hemispheres in the resulting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. The amide protons (NH′3) of the linear component in the 1
1 complex. The amide protons (NH′3) of the linear component in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex appeared as a singlet at δ = 7.8 ppm. Finally, in the initial stage of the titration, the signal for the methylene protons alpha to the nitrogen atom of the TBA cation experienced an upfield shift with respect to the cation in the free OCNTBA salt, 3a. However, the incremental addition of 3a produced a gradual downfield shift of this signal, which became more evident when more than 1 equiv. of the salt was added. These observations supported the binding of the TBA cation in the electron-rich and shallow aromatic cavity opposed to the bound cyanate and defined by the calix[4]pyrrole core in cone conformation. In short, [2]rotaxane 5 acted as a heteroditopic receptor for the recognition of OCNTBA in CDCl3 solution and the resulting 1
1 complex appeared as a singlet at δ = 7.8 ppm. Finally, in the initial stage of the titration, the signal for the methylene protons alpha to the nitrogen atom of the TBA cation experienced an upfield shift with respect to the cation in the free OCNTBA salt, 3a. However, the incremental addition of 3a produced a gradual downfield shift of this signal, which became more evident when more than 1 equiv. of the salt was added. These observations supported the binding of the TBA cation in the electron-rich and shallow aromatic cavity opposed to the bound cyanate and defined by the calix[4]pyrrole core in cone conformation. In short, [2]rotaxane 5 acted as a heteroditopic receptor for the recognition of OCNTBA in CDCl3 solution and the resulting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex displayed a host-separated ion-pair binding geometry. Remarkably, the inspection of different exchange dynamics on the chemical shift timescale between free and bound 5 (slow) and between free and bound TBA cation (fast) was indicative of two dissimilar exchange mechanisms. Because the anion is included and hydrogen bonded in the polar cavity defined by the interlocked structure, the exchange between free and bound host requires the cleavage of multiple hydrogen bonds and a conformational reorganization. Both processes are typically associated with energetically demanding transition states. Conversely, the exchange between the free and bound TBA cation can occur without such strict energetic requirements owing to the exposed nature of its binding mode.
1 complex displayed a host-separated ion-pair binding geometry. Remarkably, the inspection of different exchange dynamics on the chemical shift timescale between free and bound 5 (slow) and between free and bound TBA cation (fast) was indicative of two dissimilar exchange mechanisms. Because the anion is included and hydrogen bonded in the polar cavity defined by the interlocked structure, the exchange between free and bound host requires the cleavage of multiple hydrogen bonds and a conformational reorganization. Both processes are typically associated with energetically demanding transition states. Conversely, the exchange between the free and bound TBA cation can occur without such strict energetic requirements owing to the exposed nature of its binding mode.
A 1H–1H ROESY experiment performed on a millimolar CDCl3 solution containing the quantitatively formed 3a⊂5 complex revealed the presence of cross-peaks due to through-space proximity between the H1ortho-protons of the pyridyl-N-oxide unit in the linear axle and the calix[4]pyrrole NHs, He, of the cyclic component hemisphere that are hydrogen bonded (Fig. S25†). Other cross peaks due to through-space proximity were observed between the calix[4]pyrrole β-protons of the cyclic component's hemisphere to which the cyanate is hydrogen bonded, and the methylene protons alpha to the N atom of the TBA cation (NCH2 TBA′, Fig. S26†). This observation is in complete agreement with the macrocycle-separated binding mode assigned to OCNTBA 3a in the 3a⊂5 ion-paired complex. Altogether, the above ROESY spectroscopic observations provided additional support to the interlocked nature and binding mode assigned to the 3a⊂5 complex.
The results of a DOSY experiment allowed us to assign very similar diffusion constants for the signals of the bound receptor 5 and the ones corresponding to the bound TBA cation (Fig. S18†). This result also supported the formation of the ion-paired 3a⊂5 complex in CDCl3 solution. We determined the diffusion constant for 3a⊂5 complex as 4.0 × 10−10 m2 s−1. This value is slightly smaller than the one measured above for the free [2]rotaxane 5 (4.7 × 10−10 m2 s−1) and it's in agreement with the gain in hydrodynamic radii for the 3a⊂5 complex produced by the peripheral complexation of the TBA cation.
We also performed 1H NMR titration experiments with 3b (NO3TBA) and 3c (ClTBA) obtaining similar results (Fig. S23 and S22,† respectively). In the case of the nitrate anion, at 298 K the chemical exchange between the free and bound [2]rotaxane 5 was intermediate on the chemical shift timescale producing broad bands for some of its proton signals. At low temperature (263 K) an equimolar mixture of 5 and NO3TBA 3b produced a single set of sharp proton signals that were indicative for the quantitative formation of the 3b⊂5 complex (Fig. S28†).
The accurate determination of the binding constant values for the ion-paired interlocked complexes 3⊂5 was undertaken using isothermal titration calorimetry (ITC) experiments. We performed the titration experiments at 298 K by the incremental addition of a chloroform solution containing the ion-pair (3a–c) to a solution of the rotaxane 5 in the same solvent. We observed a regular heat release upon salt addition that was caused by the exothermicity of the binding process.
All ITC experiments showed good fits to the theoretical binding isotherm for the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (Fig. S35 and S36†).||
1 complex (Fig. S35 and S36†).||
Table 2 summarizes the results derived from the ITC experiments. The obtained results demonstrated the high selectivity exhibited by [2]rotaxane 5 in the complexation of the cyanate tetrabutylammonium salt 3a in front of the nitrate 3b or chloride 3c tetrabutylammonium counterparts (i.e. K3a⊂5 is almost 20-fold larger than K3b⊂5 and K3c⊂5).
| K a × 10−5 | ΔG | ΔH | TΔS | |
|---|---|---|---|---|
| 3a | 7.9 ± 0.2 | −8.0 ± 0.02 | −11.7 ± 1.7 | −3.7 ± 1.7 |
| 3b | 0.4 ± 0.1 | −6.3 ± 0.1 | −10.7 ± 2.1 | −4.4 ± 2.1 |
| 3c | 0.5 ± 0.2 | −6.4 ± 0.2 | −6.3 ± 0.4 | 0.1 ± 0.4 |
| 3d | 158 ± 16 | −9.8 ± 0.06 | −9.6 ± 0.3 | 0.2 ± 0.3 |
Most likely, the observed differences derived from the better shape and size complementarity that existed between the cyanate anion and the polar three-dimensional cylindrical cavity contained in the [2]rotaxane topology of the heteroditopic receptor 5. Indeed, simple molecular modelling studies showed that the binding pocket of 5, featuring six convergent hydrogen-bond donors, is highly complementary in shape and size to the cyanate anion (Fig. 3).** The smaller and spherical chloride anion and the trigonal and planar nitrate are considerably worst fits to the cylindrical internal cavity of 5 equipped with six hydrogen bond donor sites.
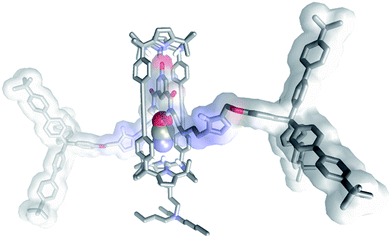 | ||
| Fig. 3 Energy minimized structure (MM3) of the 3a⊂5 complex. The included anion is displayed in CPK representation. Non-polar hydrogens were removed for clarity.** | ||
We were interested in providing further evidence on the functioning of [2]rotaxane 5 as a heteroditopic receptor for ion-pairs in chloroform solution. The methyl trioctylammonium cation (i.e. MTOA+) is known to be a better fit than the TBA cation for the electron rich and shallow cup defined by the calix[4]pyrroles in cone conformation.28,29 The stability constant measured for the 3d⊂5 complex was three orders of magnitude larger than for the 3c⊂5 counterpart with the only difference between the two complexes being the nature of the cation of the ion-pair: MTOA in 3d and TBA in 3c. This result proved the functioning of [2]rotaxane 5 as a heteroditopic receptor of organic ion-pairs in chloroform solution. The noticeable affinity and selectivity exhibited by 5 in the recognition of ClMTOA 3d directly derived from the better cation-receptor complementarity.
In non-polar solvents, anion binding by synthetic receptors is mainly dominated by the use of hydrogen-bonding interactions. The formation of hydrogen bonds with anions in non-polar solvents is associated with a high enthalpy gain. In this line, Table 1 shows that for all the studied anions, the binding processes in chloroform solution are enthalpy driven and highly exothermic. Remarkably, the binding processes leading to complexes 3a⊂5 and 3b⊂5, with OCNTBA and NO3TBA, respectively, did not obey the enthalpy–entropy compensation principle.30 Unexpectedly, we also determined that the formation of complexes with the chloride anion, 3c⊂5 and 3d⊂5, is not significantly opposed by entropy. Taken in concert, these thermodynamic observations are quite significant because the binding processes occur in a nonpolar solvent (chloroform) that minimizes solvation effects and better approximates to a gas phase host–guest complexation process. We are convinced that the use of ion-pairs as guest complicated the analysis of the enthalpic and entropic terms using a simplistic 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding process in the gas phase, where enthalpically more favourable interactions should led to thermodynamically more stable complexes. We concluded that the solvation/desolvation effects occurring during the binding processes are important and difficult to predict. This is related to the deep inclusion geometry of the anion and the separated binding mode of the ion-pair being present in the formed complexes. Nevertheless, the thermodynamic signatures of the complexation processes augur well for the application of the reported interlocked receptor 5 in the recognition of anions in aqueous media.
1 binding process in the gas phase, where enthalpically more favourable interactions should led to thermodynamically more stable complexes. We concluded that the solvation/desolvation effects occurring during the binding processes are important and difficult to predict. This is related to the deep inclusion geometry of the anion and the separated binding mode of the ion-pair being present in the formed complexes. Nevertheless, the thermodynamic signatures of the complexation processes augur well for the application of the reported interlocked receptor 5 in the recognition of anions in aqueous media.
The assessment of the relative thermodynamic stabilities of ion-paired complexes formed with receptors featuring [2]pseudorotaxane and [2]rotaxane topology is complicated owing to their different stoichiometries (number of involved components). In our case, we considered that a sensible solution to address this problem would consist in determining the required amounts of salt that have to be added to 1 mM solutions of the supramolecular 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion-paired complexes, either with [2]rotaxane or [2]pseudorotaxane topologies, in order to induce the observation of higher stoichiometry complexes with respect to the salt, as well as the complete disassembly of the parent 1
1 ion-paired complexes, either with [2]rotaxane or [2]pseudorotaxane topologies, in order to induce the observation of higher stoichiometry complexes with respect to the salt, as well as the complete disassembly of the parent 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (Fig. 4). In other words, we were interested in forcing the replacement of the pyridine-N-oxide unit of the axle, which is ditopically bound to the macrocycle and the anion in the 1
1 complex (Fig. 4). In other words, we were interested in forcing the replacement of the pyridine-N-oxide unit of the axle, which is ditopically bound to the macrocycle and the anion in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, by a second ion-pair that will coordinate the cyclic component in a receptor separated binding mode (Fig. 4). In this vein, we previously reported that macrocycle 1 coordinated ClMTOA through a non-cooperative process yielding a dimeric ion-paired aggregate (3d)2⊂1. Both ion pairs were bound to the tetratopic macrocycle 1 and displayed receptor-separated binding geometry.29
1 complex, by a second ion-pair that will coordinate the cyclic component in a receptor separated binding mode (Fig. 4). In this vein, we previously reported that macrocycle 1 coordinated ClMTOA through a non-cooperative process yielding a dimeric ion-paired aggregate (3d)2⊂1. Both ion pairs were bound to the tetratopic macrocycle 1 and displayed receptor-separated binding geometry.29
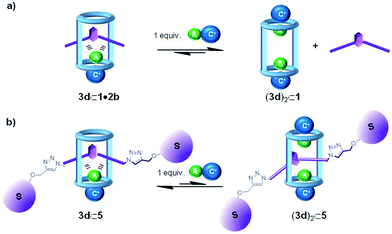 | ||
Fig. 4 Schematic cartoon of the plausible equilibria involved in the disassembly of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion-paired complexes of pseudorotaxane (a) and rotaxane (b) structures. 1 ion-paired complexes of pseudorotaxane (a) and rotaxane (b) structures. | ||
In the case of the [2]pseudorotaxane complex 3d⊂1·2b (1 mM), the addition of slightly more than 1 equiv. of the ClMTOA ion-pair induced the initial disassembly of the interlocked structure and the concomitant formation of higher order aggregates (i.e. (3d)2⊂1 complex). The disassembly process was monitored using 1H NMR spectroscopy by following the intensity changes of diagnostic proton signals of the two species that were involved in a slow chemical exchange on the proton chemical shift timescale (Fig. S33†). The proton signals corresponding to 3d⊂1·2b, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with [2]pseudorotaxane topology, disappeared completely after the addition of 2 equiv. of ClMTOA 3d. In contrast, in the mechanically locked 3d⊂5 complex, even after the addition 8 equiv. of ClMTOA ion-pair 3d we could observe the characteristic proton signals of the 1
1 complex with [2]pseudorotaxane topology, disappeared completely after the addition of 2 equiv. of ClMTOA 3d. In contrast, in the mechanically locked 3d⊂5 complex, even after the addition 8 equiv. of ClMTOA ion-pair 3d we could observe the characteristic proton signals of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with [2]rotaxane topology (Fig. S34†). These results suggested that the increase in local concentration of the pyridyl-N-oxide unit exerted by the mechanical bond of 5 provided a measurable thermodynamic stabilization to its ditopic interaction with one calix[4]pyrrole hemisphere of the cyclic component and the bound chloride. Nonetheless, the emergence of a new set of proton signals in the 1H NMR spectrum of a 1 mM CDCl3 of the 3d⊂5 complex became evident after addition of more than an extra equiv. of ClMTOA 3d. We assigned this new set of proton signals to a higher stoichiometry complex between 3d and 5 (i.e. 2
1 complex with [2]rotaxane topology (Fig. S34†). These results suggested that the increase in local concentration of the pyridyl-N-oxide unit exerted by the mechanical bond of 5 provided a measurable thermodynamic stabilization to its ditopic interaction with one calix[4]pyrrole hemisphere of the cyclic component and the bound chloride. Nonetheless, the emergence of a new set of proton signals in the 1H NMR spectrum of a 1 mM CDCl3 of the 3d⊂5 complex became evident after addition of more than an extra equiv. of ClMTOA 3d. We assigned this new set of proton signals to a higher stoichiometry complex between 3d and 5 (i.e. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for which we have not fully determined its structure. Upon incremental addition of 3d, the new set of signals grew in intensity at the expenses of those assigned to the 3d⊂5 complex.
1) for which we have not fully determined its structure. Upon incremental addition of 3d, the new set of signals grew in intensity at the expenses of those assigned to the 3d⊂5 complex.
Conclusions
In summary, we describe a synthetic methodology for the preparation of [2]rotaxane 5 in a remarkable 50% isolated yield. We conclude that the use of anion templation in the preparation of interlocked structures may limit the application of the CuACC reaction in the capping step. In our specific case, we found that the use of ion-pairs as templates for the quantitative self-assembly of the synthetic precursor displaying [2]pseudorotaxane topology was associated with two synthetic limitations: (a) difficulties in the isolation of the final [2]rotaxane 5 free from the ion-pair used as template and (b) deactivation of the Cu(I) catalytic process employed to promote the CuAAC reaction in the capping step. In chloroform solution, [2]rotaxane 5 functioned as an efficient heteroditopic receptor for tetraalkylammonium salts, 3a–d, containing mono- (Cl−) or polyatomic anions (NO3− or −OCN). In all cases, we observed the formation of kinetically and thermodynamically stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion-paired complexes featuring cyclic-component separated binding mode. The determined Ka values indicated that [2]rotaxane 5 was selective for the complexation of cyanate vs. chloride or nitrate if the anion precursors were TBA salts. This selectivity was attributed to the superior complementarity that existed between the cylindrical polar cavity of the interlocked receptor and the polyatomic linear geometry of the cyanate anion. Remarkably, the change of the TBA cation for MTOA in the chloride ion-pair produced an increase in binding affinity of almost three orders of magnitude. This result highlighted the functioning of 5 as a heteroditopic receptor for ion-pairs and supported the better fit of the MTOA cation in the electron-rich and shallow cavity opposed to the bound anion that defined the cone conformation of the calix[4]pyrrole.
1 ion-paired complexes featuring cyclic-component separated binding mode. The determined Ka values indicated that [2]rotaxane 5 was selective for the complexation of cyanate vs. chloride or nitrate if the anion precursors were TBA salts. This selectivity was attributed to the superior complementarity that existed between the cylindrical polar cavity of the interlocked receptor and the polyatomic linear geometry of the cyanate anion. Remarkably, the change of the TBA cation for MTOA in the chloride ion-pair produced an increase in binding affinity of almost three orders of magnitude. This result highlighted the functioning of 5 as a heteroditopic receptor for ion-pairs and supported the better fit of the MTOA cation in the electron-rich and shallow cavity opposed to the bound anion that defined the cone conformation of the calix[4]pyrrole.
Unfortunately, the accurate comparison of the thermodynamic stabilities of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion paired complexes with receptors featuring [2]rotaxane and [2]pseudorotaxane topologies is challenging. In any case, we assigned a higher thermodynamic stability to the 1
1 ion paired complexes with receptors featuring [2]rotaxane and [2]pseudorotaxane topologies is challenging. In any case, we assigned a higher thermodynamic stability to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion-paired complexes resulting from the [2]rotaxane 5 receptor with respect to the ones derived from the assembled [2]pseudorotaxane counterpart. We based this conclusion on the salt's excess that is required to produce aggregates of higher stoichiometry (i.e. 2
1 ion-paired complexes resulting from the [2]rotaxane 5 receptor with respect to the ones derived from the assembled [2]pseudorotaxane counterpart. We based this conclusion on the salt's excess that is required to produce aggregates of higher stoichiometry (i.e. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes). We are aware that this reasoning is limited and does not take into account the difference in thermodynamic stability of the higher stoichiometry produced complexes.
1 complexes). We are aware that this reasoning is limited and does not take into account the difference in thermodynamic stability of the higher stoichiometry produced complexes.
The reported findings augur well for the future application of water soluble analogues of [2]rotaxane 5 as effective anion receptors in aqueous media6 and we expect to report on the issue in due time.
Acknowledgements
The authors thank Gobierno de España MINECO for the project CTQ2014-56295-R, Severo Ochoa Excellence Accreditation (2014-2018 SEV-2013-0319), FEDER funds (project CTQ2014-56295-R) and the ICIQ Foundation for funding. J. R. R. thanks MINECO for an FPI fellowship.Notes and references
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- V. B. Bregovic, N. Basaric and K. Mlinaric-Majerski, Coord. Chem. Rev., 2015, 295, 80–124 CrossRef.
- P. A. Gale, N. Busschaert, C. J. E. Haynes, L. E. Karagiannidis and I. L. Kirby, Chem. Soc. Rev., 2014, 43, 205–241 RSC.
- A. Caballero, F. Zapata and P. D. Beer, Coord. Chem. Rev., 2013, 257, 2434–2455 CrossRef CAS.
- M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015, 115, 7398–7501 CrossRef CAS PubMed.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 1974–1987 CrossRef CAS PubMed.
- G. M. Hubner, J. Glaser, C. Seel and F. Vogtle, Angew. Chem., Int. Ed., 1999, 38, 383–386 CrossRef CAS.
- M. S. Vickers and P. D. Beer, Chem. Soc. Rev., 2007, 36, 211–225 RSC.
- P. D. Beer, M. R. Sambrook and D. Curiel, Chem. Commun., 2006, 2105–2117 RSC.
- M. K. Chae, J.-m Suk and K.-S. Jeong, Tetrahedron Lett., 2010, 51, 4240–4242 CrossRef CAS.
- A. Brown and P. D. Beer, Dalton Trans., 2012, 41, 118–129 RSC.
- M. Rezanka, M. J. Langton and P. D. Beer, Chem. Commun., 2015, 51, 4499–4502 RSC.
- M. D. Lankshear, N. H. Evans, S. R. Bayly and P. D. Beer, Chem.–Eur. J., 2007, 13, 3861–3870 CrossRef CAS PubMed.
- V. Valderrey, E. C. Escudero-Adán and P. Ballester, J. Am. Chem. Soc., 2012, 134, 10733–10736 CrossRef CAS PubMed.
- For other examples of [2]rotaxanes and pseudorotaxanes that use pyridine-N-oxide motifs in the axle component see: (a) R. C. Knighton and P. D. Beer, Chem. Commun., 2014, 50, 1540–1542 RSC; (b) L. M. Hancock and P. D. Beer, Chem. Commun., 2011, 47, 6012–6014 RSC; (c) M. J. Chen, S. J. Han, L. S. Jiang, S. G. Zhou, F. Jiang, Z. K. Xu, J. D. Liang and S. H. Zhang, Chem. Commun., 2010, 46, 3932–3934 RSC; (d) F. Zapata, O. A. Blackburn, M. J. Langton, S. Faulkner and P. D. Beer, Chem. Commun., 2013, 49, 8157–8159 RSC.
- J. M. Mercurio, F. Tyrrell, J. Cookson and P. D. Beer, Chem. Commun., 2013, 49, 10793–10795 RSC.
- K. D. Hanni and D. A. Leigh, Chem. Soc. Rev., 2010, 39, 1240–1251 RSC.
- J. Y. C. Lim, M. J. Cunningham, J. J. Davis and P. D. Beer, Dalton Trans., 2014, 43, 17274–17282 RSC.
- C. Ke, R. A. Smaldone, T. Kikuchi, H. Li, A. P. Davis and J. F. Stoddart, Angew. Chem., Int. Ed., 2013, 52, 381–387 CrossRef CAS PubMed.
- O. Š. Miljanić, W. R. Dichtel, I. Aprahamian, R. D. Rohde, H. D. Agnew, J. R. Heath and J. Fraser Stoddart, QSAR Comb. Sci., 2007, 26, 1165–1174 Search PubMed.
- V. Aucagne, J. Berná, J. D. Crowley, S. M. Goldup, K. D. Hänni, D. A. Leigh, P. J. Lusby, V. E. Ronaldson, A. M. Z. Slawin, A. Viterisi and D. B. Walker, J. Am. Chem. Soc., 2007, 129, 11950–11963 CrossRef CAS PubMed.
- S. K. Kim and J. L. Sessler, Chem. Soc. Rev., 2010, 39, 3784–3809 RSC.
- A. J. McConnell and P. D. Beer, Angew. Chem., Int. Ed., 2012, 51, 5052–5061 CrossRef CAS PubMed.
- G. H. Clever and M. Shionoya, Chem.–Eur. J., 2010, 16, 11792–11796 CrossRef CAS PubMed.
- S. Lal and S. Diez-Gonzalez, J. Org. Chem., 2011, 76, 2367–2373 CrossRef CAS PubMed.
- R. M. Moorman, M. B. Collier, B. H. Frohock, M. D. Womble and J. M. Chalker, Org. Biomol. Chem., 2015, 13, 1974–1978 CAS.
- V. O. Rodionov, S. I. Presolski, D. D. Diaz, V. V. Fokin and M. G. Finn, J. Am. Chem. Soc., 2007, 129, 12705–12712 CrossRef CAS PubMed.
- D. E. Gross, F. P. Schmidtchen, W. Antonius, P. A. Gale, V. M. Lynch and J. L. Sessler, Chem.–Eur. J., 2008, 14, 7822–7827 CrossRef CAS PubMed.
- V. Valderrey, E. C. Escudero-Adan and P. Ballester, Angew. Chem., Int. Ed., 2013, 52, 6898–6902 CrossRef CAS PubMed.
- J. D. Dunitz, Chem. Biol., 1995, 2, 709–712 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, compounds characterization, 1H titrations, ITC data. See DOI: 10.1039/c6sc03554j |
| ‡ Variable temperature 1H NMR experiments performed using a CDCl3 solution of rotaxane 5 showed that at low temperatures many of its broad proton signals split into two or more sharper signals (Fig. S16†). Most likely, this is due to the change in the rate of the pirouetting process experienced by the interlocked linear component of 5. The process is fast on the chemical shift time scale at 298 K but becomes slow at temperatures close to 233 K. |
| § Successive water washes did not allow the extraction of the salt from the host cavity. |
¶ Column chromatography performed on an equimolar mixture of rotaxane 5 and ion-pair OCNTBA 3a using a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mixture of AcOEt 7 mixture of AcOEt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 as eluent, did not allow the isolation of the pure free rotaxane but mixtures of rotaxane with different amounts of the ion-pair. CH2Cl2 as eluent, did not allow the isolation of the pure free rotaxane but mixtures of rotaxane with different amounts of the ion-pair. |
|| The low concentration used in the ITC experiments and the reduced excess of added tetralkylammonium salts (up to 2 equiv.) made that the presence of species with stoichiometries higher than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was negligible. 1 was negligible. |
| ** The energy minimized structure was obtained using the software package Fujitsu Scigress Version 2.2.0. The structure was optimized by performing a geometry calculation using the implemented molecular mechanics force field with augmented MM3 parameters. |
| This journal is © The Royal Society of Chemistry 2017 |