Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Steric ploy for alternating donor–acceptor co-assembly and cooperative supramolecular polymerization†
Saptarshi
Chakraborty
,
Haridas
Kar
,
Amrita
Sikder
and
Suhrit
Ghosh
*
Polymer Science Unit, Indian Association for the Cultivation of Science, Kolkata, India-700032. E-mail: psusg2@iacs.res.in
First published on 19th September 2016
Abstract
The presence of a bulky peripheral wedge destabilizes the homo-assembly of an amide functionalized acceptor (A) monomer and thereby enables the formation of an alternating supramolecular copolymer with an amide appended donor (D) monomer via the synergistic effect of H-bonding and the charge-transfer (CT) interaction with a remarkably high Ka of 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1. In sharp contrast, H-bonding driven homo-polymers of A and D are formed by just replacing the bulky chains of the A monomer with linear hydrocarbons. By taking advantage of the clear difference in the critical temperature for the onset of the AA or DD homo-assemblies and DA co-assembly (TDA ≫ TAA or TDD), the supramolecular polymerization pathway of the NDI-monomer could be fully diverted from isodesmic to cooperative in the presence of a small amount of DAN which helped the in situ production of nucleating sites involving the D–A CT-complex at a relatively higher temperature and the subsequent chain growth at TAA following the nucleation-elongation model.
000 M−1. In sharp contrast, H-bonding driven homo-polymers of A and D are formed by just replacing the bulky chains of the A monomer with linear hydrocarbons. By taking advantage of the clear difference in the critical temperature for the onset of the AA or DD homo-assemblies and DA co-assembly (TDA ≫ TAA or TDD), the supramolecular polymerization pathway of the NDI-monomer could be fully diverted from isodesmic to cooperative in the presence of a small amount of DAN which helped the in situ production of nucleating sites involving the D–A CT-complex at a relatively higher temperature and the subsequent chain growth at TAA following the nucleation-elongation model.
Introduction
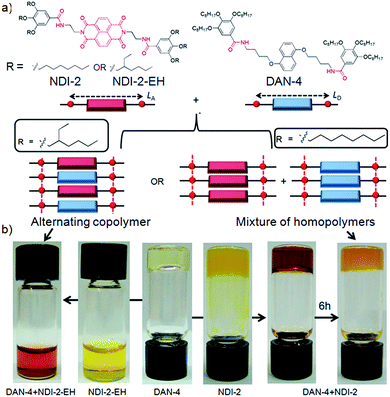
Organic donor (D)–acceptor (A) charge-transfer (CT)-complexes1 are of varied interest2 in the context of ambipolar charge transport,3a ferroelectricity,3b,c sensors,3d,e ion channels,3f–h photosystems,3i thermochromic material,3j foldamers,3k,l,6a,b gels,3m,n supra-amphiphiles,3o rotaxane,3p catenane3q and other supramolecular systems.2 However, the weak association constants ranging between 1–20 M−1 for most D–A pairs2 is the major obstacle to achieving extended stacking as desirable in optoelectronic applications.4 Emerging reports though suggest hydrogen-bond5a or halogen-bond5b promoted co-assembly can construct mixed D–A co-crystals with long range order; the manifestation of such ordered arrays in solution remains an uphill task.6 Recently we have reported the H-bonding promoted co-assembly of bis-amide-functionalized naphthalene-diimide (NDI) acceptor or dialkoxy-naphthalene (DAN) donor units7 with matching spacer lengths (LA and LD, respectively) between the two amide groups appended at either arm of the NDI and DAN chromophores. However even for the best match between LA and LD in NDI-2 + DAN-4 pair (Scheme 1a), the spontaneously formed CT-complex with intense red colour disappeared after ∼6 h (Scheme 1b) due to reorganization of the alternating D–A stack to individual homo-polymers of NDI and DAN.8 In a nutshell, while attempting to reinforce via H-bonding, we ended up fully sacrificing the inherent alternate stacking mode (though weak) of the DAN–NDI pair, which instead arrived at their self-sorted assembly.3j,9,10,20 This is because in the presence of the amide groups, the CT-interaction played a mere auxiliary role while the relatively stronger H-bonding preferred homo-assembly because in the alternating stack H-bonding had to be compromised due to the lack of a perfect match between LA and LD. From the classical copolymer equation11 it is learnt that to achieve an alternating copolymer between two monomers (M1 and M2), their monomer reactivity ratios (r1 and r2, respectively) should be close to zero. By definition r1 = k11/k12, where k11 and k12 represent the rate constants of the reactions involving monomer M1 adding to M1 or M2, respectively. Likewise r2 = k22/k21 for M2. Therefore to meet the criteria of r1 ∼ 0 and r2 ∼ 0, k11 and k22 should be close to zero implying that the propensity of homo-polymerization should be negligible for both the monomers M1 and M2. Thinking along a similar line, we envisioned lowering the propensity of the homo-assembly of DAN and/or NDI might enable the formation of a stable alternating D–A copolymer. Notably, the value of rDAN is a way lower as its homo-polymer is destabilized by electrostatic repulsion among the DAN chromophores.8 The challenge remains in lowering the value of rNDI without jeopardizing the CT-complex formation. We envisaged the presence of branched peripheral alkyl chains selectively in the NDI building block (NDI-2-EH, Scheme 1) would destabilize its homo-assembly by steric repulsion. On the other hand in an alternating NDI–DAN stack, steric crowding will be less pronounced due to the buffering by the less space consuming linear peripheral alkyl chains of the DAN units.Results and discussion
To test this hypothesis we have studied the co-assembly of NDI-2-EH + DAN-4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) which produces an intense red solution in methylcyclohexane (Scheme 1) indicating an alternating D–A stack which is also evidenced by the appearance of a prominent CT-absorption band (Fig. 1a). Notably in chloroform, a good solvent, no red colour or any CT-band was visible (Fig. 1a) suggesting that co-assembly is prominent in less polarizable solvent.4,7 In sharp contrast, NDI-2 + DAN-4 (1
1) which produces an intense red solution in methylcyclohexane (Scheme 1) indicating an alternating D–A stack which is also evidenced by the appearance of a prominent CT-absorption band (Fig. 1a). Notably in chloroform, a good solvent, no red colour or any CT-band was visible (Fig. 1a) suggesting that co-assembly is prominent in less polarizable solvent.4,7 In sharp contrast, NDI-2 + DAN-4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) spontaneously forms a red gel but gradually the color disappears after 6 h (Scheme 1b) suggesting reorganization of the CT-state to a segregated state without disrupting the gel phase. It is interesting to note that the initial intensity of the CT-band for NDI-2 + DAN-4 gel is almost identical to that of NDI-2-EH + DAN-4 sol (Fig. 1a) indicating an equal population of the CT-complex at the beginning. However after 6 h, the CT-band fully disappears in the former case with a concomitant increase in the baseline intensity, possibly arising out of scattering due to the structural inhomogeneity of the self-sorted state.12 In contrast, for the NDI-2-EH + DAN-4 pair, no change in the CT-band intensity was noticed even after 30 days (Fig. 1a) ascertaining stable alternating co-assembly. Likewise the UV-range of the spectra (recorded at a lower concentration) also exhibits a significant change in its nature over time revealing reorganization of the chromophores from an initial assembled state to a different state after 6 h in case of NDI-2 + DAN-4, while a lack of such spectral changes in the case of NDI-2-EH + DAN-4 (even after 7 days) confirms a stable mixed assembly (Fig. S1†). Job's plots (Fig. 1b and S2†) confirmed a 1
1) spontaneously forms a red gel but gradually the color disappears after 6 h (Scheme 1b) suggesting reorganization of the CT-state to a segregated state without disrupting the gel phase. It is interesting to note that the initial intensity of the CT-band for NDI-2 + DAN-4 gel is almost identical to that of NDI-2-EH + DAN-4 sol (Fig. 1a) indicating an equal population of the CT-complex at the beginning. However after 6 h, the CT-band fully disappears in the former case with a concomitant increase in the baseline intensity, possibly arising out of scattering due to the structural inhomogeneity of the self-sorted state.12 In contrast, for the NDI-2-EH + DAN-4 pair, no change in the CT-band intensity was noticed even after 30 days (Fig. 1a) ascertaining stable alternating co-assembly. Likewise the UV-range of the spectra (recorded at a lower concentration) also exhibits a significant change in its nature over time revealing reorganization of the chromophores from an initial assembled state to a different state after 6 h in case of NDI-2 + DAN-4, while a lack of such spectral changes in the case of NDI-2-EH + DAN-4 (even after 7 days) confirms a stable mixed assembly (Fig. S1†). Job's plots (Fig. 1b and S2†) confirmed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex which implies an alternating stack of the NDI and DAN chromophores. From the concentration dependent variation of the CT-band (Fig. 1c and d), the Ka was estimated (see ESI† for detailed calculation) to be 31
1 complex which implies an alternating stack of the NDI and DAN chromophores. From the concentration dependent variation of the CT-band (Fig. 1c and d), the Ka was estimated (see ESI† for detailed calculation) to be 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 which is by far the largest value reported for a NDI–DAN pair and one of the very few higher values reported for any intermolecular D–A complex.2 Transmission electron microscopy (TEM) (Fig. 1e) images reveal a fibrillar network for DAN-4 which is typical for a gelator while the non-gelator NDI-2-EH shows spherical particles of diameter ∼ 25 nm.13 Interestingly for their mixture, the self-identity of the individual components are fully lost as neither any fibrils nor any small particles are visible anymore, rather the mixed assembly produced particles with a ∼100 nm diameter confirming the absence of individual homo-aggregates in the mixture. In a curiosity driven experiment, we placed a solution of NDI-2-EH in methylcyclohexane on top of a preformed DAN-4 gel while we noticed that gradually the red color evolved with concomitant transformation of the gel phase to a homogeneous solution (Fig. S3†) indicating that even the pre-formed homo-aggregates when in contact can reorganize to an alternating D–A copolymer.
000 M−1 which is by far the largest value reported for a NDI–DAN pair and one of the very few higher values reported for any intermolecular D–A complex.2 Transmission electron microscopy (TEM) (Fig. 1e) images reveal a fibrillar network for DAN-4 which is typical for a gelator while the non-gelator NDI-2-EH shows spherical particles of diameter ∼ 25 nm.13 Interestingly for their mixture, the self-identity of the individual components are fully lost as neither any fibrils nor any small particles are visible anymore, rather the mixed assembly produced particles with a ∼100 nm diameter confirming the absence of individual homo-aggregates in the mixture. In a curiosity driven experiment, we placed a solution of NDI-2-EH in methylcyclohexane on top of a preformed DAN-4 gel while we noticed that gradually the red color evolved with concomitant transformation of the gel phase to a homogeneous solution (Fig. S3†) indicating that even the pre-formed homo-aggregates when in contact can reorganize to an alternating D–A copolymer.
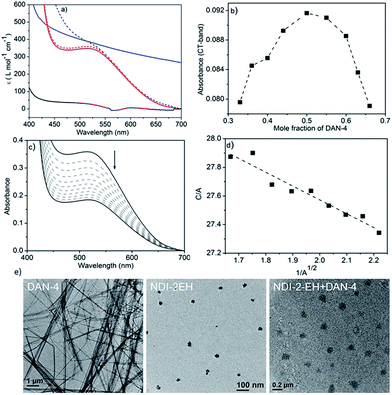 | ||
Fig. 1 (a) UV/Vis spectra (selected section) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DAN-4 + NDI-2-EH (red-dotted and solid lines show freshly prepared and aged samples after 30 days, respectively) and DAN-4 + NDI-2 (blue-dotted and solid lines show freshly prepared and aged samples after 6 h, respectively) in methylcyclohexane; black line shows DAN-4 + NDI-2-EH in CHCl3. In all experiments, the concentration of DAN = 10.0 mM and NDI = 10.0 mM. (b) Job's plot for DAN-4 + NDI-2-EH constructed from the CT-band intensity with different D/A ratios (total concentration fixed at 2.0 mM). (c) Concentration dependent UV/Vis spectra of DAN-4 + NDI-2-EH in methylcyclohexane (arrow indicates spectral change upon dilution) and (d) fitting of the data (eqn (1), ESI†) to estimate the association constant. C = 10 mM. (e) HRTEM images of different samples after drop-casting their methylcyclohexane solution on a carbon coated Cu grid. 1 DAN-4 + NDI-2-EH (red-dotted and solid lines show freshly prepared and aged samples after 30 days, respectively) and DAN-4 + NDI-2 (blue-dotted and solid lines show freshly prepared and aged samples after 6 h, respectively) in methylcyclohexane; black line shows DAN-4 + NDI-2-EH in CHCl3. In all experiments, the concentration of DAN = 10.0 mM and NDI = 10.0 mM. (b) Job's plot for DAN-4 + NDI-2-EH constructed from the CT-band intensity with different D/A ratios (total concentration fixed at 2.0 mM). (c) Concentration dependent UV/Vis spectra of DAN-4 + NDI-2-EH in methylcyclohexane (arrow indicates spectral change upon dilution) and (d) fitting of the data (eqn (1), ESI†) to estimate the association constant. C = 10 mM. (e) HRTEM images of different samples after drop-casting their methylcyclohexane solution on a carbon coated Cu grid. | ||
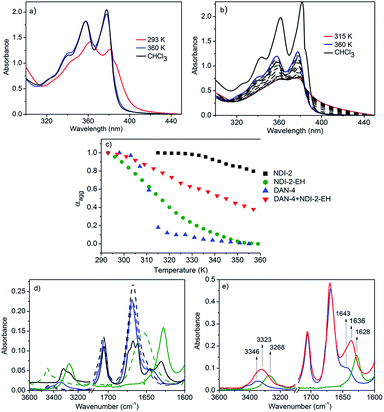
UV/Vis spectra of NDI-2-EH and NDI-2 (Fig. 2) suggest monomeric species in CHCl3 and aromatic interaction in methylcyclohexane at rt. Temperature dependent studies (Fig. 2a and b) show a significant difference; for NDI-2 self-assembly is highly stable and even at the highest tested temperature the spectrum in methylcyclohexane does not resemble that of the monomer in CHCl3. On the other hand for NDI-2-EH at a higher temperature the spectrum matches that for CHCl3 suggesting complete disassembly. Melting curves (Fig. 2c) clearly indicate a much reduced stability of NDI-2-EH (compared to NDI-2) which is now comparable to even DAN-4 (Fig. 2c and S4†). Such a significant difference is attributed to destabilization of the NDI-2-EH homo-assembly due to steric repulsion among the peripheral chains as otherwise both chromophores are identical. But now the stability of the NDI-2-EH + DAN-4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) alternating stack is significantly enhanced compared to the homo-assembly of either component as both electrostatic and steric repulsion could be avoided in an alternating stack.
1) alternating stack is significantly enhanced compared to the homo-assembly of either component as both electrostatic and steric repulsion could be avoided in an alternating stack.
In the FT-IR spectra (Fig. 2d) both NDI-2 and NDI-2-EH show two distinct peaks at 1666 and 1706 cm−1 which are assigned7 to the symmetric and asymmetric stretching of the imide carbonyl, respectively. As no separate peak is noticed for the amide-1 band, it is assumed that in the non-H-bonded state (in CHCl3) it merges with the symmetric stretching peak of the imide carbonyl. In methylcyclohexane distinct new peaks emerge at 1630 and 1643 cm−1for NDI-2 and NDI-2-EH, respectively, due to the shift of the amide-1 band as a consequence of H-bonding. But the significant difference in the peak positions clearly indicates much weaker H-bonding for NDI-2-EH which corroborates with the observed shifts for the N–H stretching band (3418 to 3320 cm−1 and 3418 to 3346 cm−1 for NDI-2 and NDI-2-EH, respectively) and supports the melting curves (Fig. 2c). Weaker H-bonding is attributed to the steric crowding among the peripheral chains for NDI-2-EH. Likewise for DAN-4, the amide-1 band shows a significant shift from 1653 cm−1 to 1628 cm−1 and the N–H stretching band from 3452 cm−1 to 3268 cm−1 confirming strong H-bonding. Now for the mixture of NDI-2-EH + DAN-4 (Fig. 2e), distinct peaks appear at 1636 cm−1 and 3323 cm−1 for the amide-1 and N–H stretching bands, respectively, which indicate relatively stronger H-bonding compared to the NDI-2-EH homo-assembly owing to the release of the steric strain in an alternating stack. By comparing the FT-IR and UV/Vis data, it appears albeit forming strong H-bonds, the self-assembly of DAN-4 is least stable. It indicates in addition to H-bonding, aromatic interaction also contributes to the overall stability which is least beneficial for the homo-assembly of DAN but most beneficial for alternating NDI–DAN stacking. Overall the spontaneously formed CT-state shifts to thermodynamic products (H-bonding driven homo-aggregates) for NDI-2 + DAN-4 but remains stable for the NDI-2-EH + DAN-4 pair as in this case both the homopolymers are destabilized by steric and electrostatic repulsion, respectively.
As an offshoot of the primary objective, we examined if such a kinetically trapped CT-complex could initiate cooperative supramolecular polymerization, which has captured immense attention in the recent past.14 We have already shown (Fig. 2c) that the temperature for the onset of homo-polymerization of NDI-2-EH (TA ∼ 343 K) or DAN-4 (TD ∼ 333 K) is significantly less compared to that for an alternating D–A stack (TDA > 353 K). Therefore we envisaged that during cooling of a hot solution NDI-2-EH + DAN-4, when temperature would reach TDA, only DA or ADA type species would be generated by H-bonding assisted CT-interaction if CA ≫ CD. Upon arrival at TA, the already formed CT-complex may act as the initiating site for the supramolecular polymerization15 of NDI-2-EH by a chain growth mechanism (Fig. 3a). To test this possibility we have probed the supramolecular polymerization of NDI-2-EH on its own and in the presence of 7–15% of DAN-4 by analyzing the nature of the respective cooling curves (Fig. 3b) generated from variable temperature UV/Vis studies. The cooling curve for NDI-2-EH fits well with the isodesmic model (Fig. S5†), similar to step-growth polymerization.16 But with 7% DAN-4 the curve starts deviating from a sigmoidal shape which becomes more prominent with 10% DAN-4 and does not change further with 15% DAN-4 (Fig. 3b). A much stiffer slope in the nucleation regime indicates cooperative self-assembly17 which was established by the satisfactory fit of the experimental data to the following eqn (1) and (2), representing the nucleation-elongation model.18
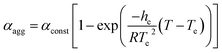 | (1) |
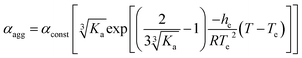 | (2) |
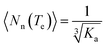 | (3) |
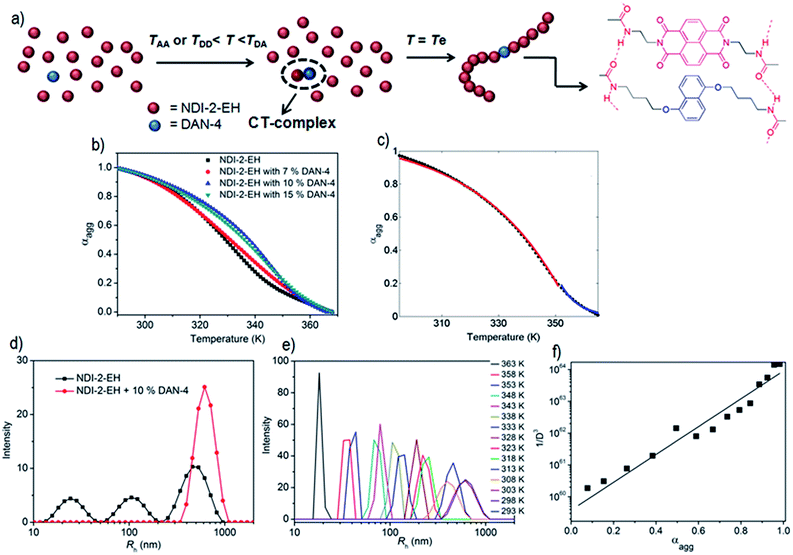 | ||
| Fig. 3 (a) Proposed model showing cooperative supramolecular polymerization of NDI-2-EH in the presence of 10% DAN-4. (b) Melting curve (derived from absorption at 395 nm) for NDI-2-EH in the absence and presence of DAN-4 in decane (C = 0.1 mM). (c) Fitting of elongation (red line) and nucleation (blue line) regime of the data for 10% DAN-4 in NDI-2-EH with eqn (1) and (2), respectively. (d) DLS data of supramolecular polymer formed by NDI-2-EH in the absence and presence of DAN-4. (e) Temperature-dependent DLS data for NDI-2-EH + 10% DAN-4 in decane and (f) the relationship of 1/D3 (calculated from Rh) with the mole fraction of the aggregate. | ||
Conclusion
This paper discloses two new findings: (i) a supramolecular strategy (based on steric factor) for stabilizing alternating donor (D)–acceptor (A) assembly and (ii) cooperative supramolecular polymerization of the A monomer only in the presence of a small amount of D, which helps in shaping the nucleus by an early formation of the CT-complex. While side chain (hydrocarbon, fluorocarbon or oligooxyethylene) immiscibility driven segregation3j,20 of π-systems or polymers is well known, it is less explored that two different hydrocarbons induce miscibility in an alternating sequence as demonstrated in this paper. This has far reaching consequences for controlling the sequence of dissimilar building blocks in multi-component self-assembly. Furthermore by taking advantage of the significantly higher stability of the alternating stack compared to individual homopolymers, it was possible to generate the CT-complex in situ in a mixture of A + D (CA ≫ CD) at a relatively higher temperature, which acted as the nucleus for subsequent homo-polymerization of A via a cooperative mechanism in contrast to its isodesmic self-assembly in the absence of D. Unlike the majority of examples of nucleation involving the monomer itself,14,17,21,22 initiation by a distinct molecular entity23 (DAN-4) opens up a world of opportunities for supramolecular block-copolymer24 stereo-selective supramolecular co-polymerization (by using D and A building blocks with enantiopure side chains instead of a racemic mixture as used in the present study)25 and sequencing in precision supramolecular copolymers similar to emerging reports on aperiodic covalent copolymers.26Acknowledgements
SC, HK and AS acknowledge CSIR, India, for a research fellowship. SG thanks CSIR for funding (02(0177)/14/EMR-II).Notes and references
- R. Foster, in Organic Charge-Transfer Complex, Academic Press, London, 1969 Search PubMed.
- For a recent review on supramolecular assembly based on CT-complexes see: A. Das and S. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 2038 CrossRef CAS PubMed.
- (a) A. A. Sagade, K. V. Rao, S. J. George, A. Datta and G. U. Kulkarni, Chem. Commun., 2013, 49, 5847 RSC; (b) S. Horiuchi, F. Ishii, R. Kumai, Y. Okimoto, H. Tachibana, N. Nagaosa and Y. Tokura, Nat. Mater., 2005, 4, 163 CrossRef CAS PubMed; (c) A. S. Tayi, A. K. Shveyd, A. C. H. Sue, J. M. Szarko, B. S. Rolczynski, D. Cao, T. J. Kennedy, A. A. Sarjeant, C. L. Stern, W. F. Paxton, W. Wu, S. K. Dey, A. C. Fahrenbach, J. R. Guest, H. Mohseni, L. X. Chen, K. L. Wang, J. F. Stoddart and S. I. Stupp, Nature, 2012, 488, 485 CrossRef CAS PubMed; (d) S. Hagihara, H. Tanaka and S. Matile, J. Org. Chem., 2008, 130, 5656 CAS; (e) A. Sandeep, V. K. Praveen, K. K. Kartha, V. Karunakaranab and A. Ajayaghosh, Chem. Sci., 2016, 7, 4460 RSC; (f) P. Talukdar, G. Bollot, J. Mareda, N. Sakai and S. Matile, J. Org. Chem., 2005, 127, 6528 CAS; (g) P. Talukdar, G. Bollot, J. Mareda, N. Sakai and S. Matile, Chem.–Eur. J., 2005, 11, 6525 CrossRef CAS PubMed; (h) S. Bhosale, A. L. Sisson, P. Talukdar, A. Fürstenberg, N. Banerji, E. Vauthey, G. Bollot, J. Mareda, C. Röger, F. Würthner, N. Sakai and S. Matile, Science, 2006, 313, 84 CrossRef CAS PubMed; (i) A. Bolag, J. López-Andarias, S. Lascano, S. Soleimanpour, C. Atienza, N. Sakai, N. Martín and S. Matile, Angew. Chem., Int. Ed., 2014, 53, 4890 CrossRef CAS PubMed; (j) P. M. Alvey, J. J. Reczek, V. Lynch and B. L. Iverson, J. Org. Chem., 2010, 75, 7682 CrossRef CAS PubMed; (k) R. S. Lokey and B. L. Iverson, Nature, 1995, 375, 303 CrossRef CAS; (l) J. Q. Nguyen and B. L. Iverson, J. Am. Chem. Soc., 1999, 121, 2639 CrossRef CAS; (m) P. Babu, N. M. Sangeetha, P. Vijaykumar, U. Maitra, K. Rissanen and A. R. Raju, Chem.–Eur. J., 2003, 9, 1922 CrossRef CAS PubMed; (n) C. Wang, D. Zang and D. Zhu, J. Am. Chem. Soc., 2005, 127, 17372 Search PubMed; (o) C. Wang, Z. Q. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608 CrossRef CAS PubMed; (p) S. A. Vignon, T. Jarrosson, T. Iijima, H.-R. Tseng, J. K. M. Sanders and J. F. Stoddart, J. Am. Chem. Soc., 2004, 126, 9884 CrossRef CAS PubMed; (q) H. Y. Au-Yeung, G. D. Pantos and J. K. M. Sanders, J. Am. Chem. Soc., 2008, 130, 12872 CrossRef PubMed.
- P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2011, 133, 20009 CrossRef CAS PubMed.
- (a) A. K. Blackburn, A. C.-H. Sue, A. K. Shveyd, D. Cao, A. Tayi, A. Narayanan, B. S. Rolczynski, J. M. Szarko, O. A. Bozdemir, R. Wakabayashi, J. A. Lehrman, B. Kahr, L. X. Chen, M. S. Nassar, S. I. Stupp and J. F. Stoddart, J. Am. Chem. Soc., 2014, 136, 17224 CrossRef CAS PubMed; (b) W. Zhu, R. Zheng, Y. Zhen, Z. Yu, H. Dong, H. Fu, Q. Shi and W. Hu, J. Am. Chem. Soc., 2015, 137, 11038 CrossRef CAS PubMed.
- (a) G. J. Gabriel and B. L. Iverson, J. Am. Chem. Soc., 2002, 124, 15174 CrossRef CAS PubMed; (b) S. De, D. Koley and S. Ramakrishnan, Macromolecules, 2010, 43, 3183 CrossRef CAS; (c) A. Das and S. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 1092 CrossRef CAS PubMed.
- A. Das and S. Ghosh, Chem. Commun., 2016, 52, 6860 RSC.
- (a) A. Das, M. R. Molla, A. Banerjee, A. Paul and S. Ghosh, Chem.–Eur. J., 2011, 17, 6061 CrossRef CAS PubMed; (b) C. R. Martinez and B. L. Iverson, Chem. Sci., 2012, 3, 2191 RSC.
- For a recent review on self-sorting see: M. M. S. Sempere, G. Fernández and F. Würthner, Chem. Rev., 2011, 111, 5784 CrossRef PubMed.
- For representative examples of self-sorting in different supramolecular systems see: (a) A. Wu and L. Isaacs, J. Am. Chem. Soc., 2003, 125, 4831 CrossRef CAS PubMed; (b) P. N. Taylor and H. L. Anderson, J. Am. Chem. Soc., 1999, 121, 11538 CrossRef CAS; (c) D. Ajami, J. L. Hou, T. J. Dale, E. Barrett and J. Rebek, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10430 CrossRef CAS PubMed; (d) M. R. Molla, A. Das and S. Ghosh, Chem.–Eur. J., 2010, 16, 10084 CrossRef CAS PubMed; (e) M. M. Safont-Sempere, P. Osswald, M. Stolte, M. Grüne, M. Renz, M. Kaupp, K. Radacki, H. Braunschweig and F. Würthner, J. Am. Chem. Soc., 2011, 133, 9580 CrossRef CAS PubMed; (f) Y. Rudzevich, V. Rudzevich, F. Klautzsch, C. A. Schalley and V. Böhmer, Angew. Chem., Int. Ed., 2009, 48, 3867 CrossRef CAS PubMed; (g) N. Tomimasu, A. Kanaya, Y. Takashima and H. Harada, J. Am. Chem. Soc., 2009, 131, 12339 CrossRef CAS PubMed; (h) K. Sugiyasu, S.-i. Kawano, N. Fujita and S. Shinkai, Chem. Mater., 2008, 20, 2863 CrossRef CAS; (i) N.-T. Lin, A. Vargas Jentzsch, L. Guénée, J.-M. Neudörfl, S. Aziz, A. Berkessel, E. Orentas, N. Sakai and S. Matile, Chem. Sci., 2012, 3, 1121 RSC; (j) Z. Xie, V. Stepanenko, K. Radacki and F. Würthner, Chem.–Eur. J., 2012, 18, 7060 CrossRef CAS PubMed; (k) S. Prasanthkumar, S. Ghosh, V. C. Nair, A. Saeki, S. Seki and A. Ajayaghosh, Angew. Chem., Int. Ed., 2015, 54, 946 CrossRef CAS PubMed; (l) B. Narayan, K. K. Bejagam, S. Balasubramanian and S. J. George, Angew. Chem., 2015, 127, 13245 CrossRef.
- G. Odian, Principles of Polymerization, Wiley, 4th edn, 2004 Search PubMed.
- The scattering arises due to the opaque nature of the self-sorted gel. The exact reason why the self-sorted gel is opaque in nature is not clear to us at the moment and a detailed investigation on that is out of the scope for the current manuscript but will be conducted in the future.
- NDI-2 forms a fibrillar network (data not shown) like DAN-4. From these results it is evident that alkyl chain packing plays a crucial role in fibrillar gelation in NDI-2 or DAN-4. In the case of NDI-2-EH or NDI-2-EH + DAN-4, such crystalline order is affected due to the presence of branched 2-EH chains which prohibits gelation. Macroscopic structural elucidation of these complex self-assembled systems is underway using X-ray scattering experiments which are beyond the scope of the present communication as it primarily highlights molecular assembly aspects.
- (a) F. Würthner, Nat. Chem., 2014, 6, 171 CrossRef PubMed; (b) D. van der Zwaag, T. F. A. de Greef and E. W. Meijer, Angew. Chem., Int. Ed., 2015, 54, 8334 CrossRef CAS PubMed; (c) R. Mukhopadhyay and A. Ajayaghosh, Science, 2015, 349, 241 CrossRef CAS PubMed.
- E. F. Niess, M. Maaloum, E. Buhler, I. Nyrkova and N. Giuseppone, Angew. Chem., Int. Ed., 2010, 49, 6974 CrossRef PubMed.
- Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC.
- C. Rest, R. Kandanelli and G. Fernández, Chem. Soc. Rev., 2015, 44, 2543 RSC.
- M. M. J. Smulders, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2008, 130, 606 CrossRef CAS PubMed.
- A. Polson, J. Phys. Colloid Chem., 1950, 54, 649 CrossRef CAS.
- (a) T. L. Benanti, P. Saejueng and D. Venkataraman, Chem. Commun., 2007, 692 RSC; (b) W.-S. Li, Y. Yamamoto, T. Fukushima, A. Saeki, S. Seki, S. Tagawa, H. Masunaga, S. Sasaki, M. Takata and T. Aida, J. Am. Chem. Soc., 2008, 130, 8886 CrossRef CAS PubMed; (c) M. Lista, J. Areephong, N. Sakai and S. Matile, J. Am. Chem. Soc., 2011, 133, 15228 CrossRef CAS PubMed; (d) J. Mandal, S. K. Prasad, D. S. S. Rao and S. Ramakrishnan, J. Am. Chem. Soc., 2014, 136, 2538 CrossRef CAS PubMed.
- (a) J. Q. Nguyen and B. L. Iverson, J. Am. Chem. Soc., 1999, 121, 2639 CrossRef CAS; (b) X. Wang, G. Guerin, H. Wang, Y. Wang, I. Manners and M. A. Winnik, Science, 2007, 317, 644 CrossRef CAS PubMed; (c) J. B. Gilroy, T. Gädt, G. R. Whittell, L. Chabanne, J. M. Mitchels, R. M. Richardson, M. A. Winnik and I. Manners, Nat. Chem., 2010, 2, 566 CrossRef CAS PubMed.
- (a) S. Ogi, K. Sugiyasu, S. Manna, S. Samitsu and M. Takeuchi, Nat. Chem., 2014, 6, 188 CrossRef CAS PubMed; (b) S. Ogi, V. Stepanenko, K. Sugiyasu, M. Takeuchi and F. Würthner, J. Am. Chem. Soc., 2015, 137, 3300 CrossRef CAS PubMed.
- J. Kang, D. Miyajima, T. Mori, Y. Inoue, Y. Itoh and T. Aida, Science, 2015, 347, 646 CrossRef CAS PubMed.
- (a) D. Görl, X. Zhang, V. Stepanenko and F. Würthner, Nat. Commun., 2015, 6, 7009 CrossRef PubMed; (b) X. Wang, G. Guerin, H. Wang, Y. Wang, I. Manners and M. A. Winnik, Science, 2007, 317, 644 CrossRef CAS PubMed.
- W. Zhang, W. Jin, T. Fukushima, T. Mori and T. Aida, J. Am. Chem. Soc., 2015, 137, 13792 CrossRef CAS PubMed.
- J.-F. Lutz, ACS Macro Lett., 2014, 3, 1020 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc02640k |
| This journal is © The Royal Society of Chemistry 2017 |