Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optical control of AMPA receptors using a photoswitchable quinoxaline-2,3-dione antagonist†
David M.
Barber‡
a,
Shu-An
Liu‡
a,
Kevin
Gottschling
b,
Martin
Sumser
a,
Michael
Hollmann
b and
Dirk
Trauner
*a
aDepartment of Chemistry and Center for Integrated Protein Science, Ludwig Maximilians University Munich, Butenandtstraße 5-13, 81377 Munich, Germany. E-mail: dirk.trauner@lmu.de
bDepartment of Biochemistry I – Receptor Biochemistry, Ruhr-Universität-Bochum, Bochum 44780, Germany
First published on 24th August 2016
Abstract
AMPA receptors respond to the neurotransmitter glutamate and play a critical role in excitatory neurotransmission. They have been implicated in several psychiatric disorders and have rich pharmacology. Antagonists of AMPA receptors have been explored as drugs and one has even reached the clinic. We now introduce a freely diffusible photoswitchable antagonist that is selective for AMPA receptors and endows them with light-sensitivity. Our photoswitch, ShuBQX-3, is active in its dark-adapted trans-isoform but is significantly less active as its cis-isoform. ShuBQX-3 exhibits a remarkable red-shifting of its photoswitching properties through interactions with the AMPA receptor ligand binding site. Since it can be used to control action potential firing with light, it could emerge as a powerful tool for studying synaptic transmission with high spatial and temporal precision.
Introduction
Photopharmacology is the attempt to endow biological targets with light sensitivity using small photoswitchable molecules.1 It has been applied to a wide variety of molecular targets, including ion channels,2 G-protein coupled receptors (GPCRs)3 and enzymes.4 As such, it has enabled the light-dependent control of diverse cellular processes, such as proliferation5 and neuronal excitability.6The ionotropic glutamate receptors (iGluRs) are attractive targets for photopharmacology due to their fundamental roles in excitatory neurotransmission7 and their involvement in neurodegenerative conditions and psychiatric disorders.8 The iGluRs are natively gated by the neurotransmitter glutamate and are divided into three subclasses due to their individual responses to selective agonists: AMPA receptors (GluAs), kainate receptors (GluKs) and NMDA receptors (GluNs).9
To date, all three of the iGluR subtypes have been addressed with photopharmacology, using freely diffusible photoswitchable agonists.10 Although synthetic agonists for neurotransmitter receptors are powerful tools, they do create a non-physiological situation that can complicate the analyses of neural networks. This is perhaps the reason why antagonists of glutamatergic signaling are more widely used in neuroscience and why they have undergone extensive development as drugs to treat psychiatric diseases.11 It would be advantageous to use light to precisely control glutamate receptor antagonists and target their actions to specific locations. To that end, a few caged antagonists of iGluRs have been disclosed12 but their activation is irreversible. This prompted us to develop a photoswitchable antagonist that can be reversibly turned on and off. Our studies resulted in a quinoxaline-2,3-dione derivative, termed ShuBQX-3,§ that enables the optical control of AMPA receptor-mediated action potential firing of hippocampal neurons.
Results and discussion
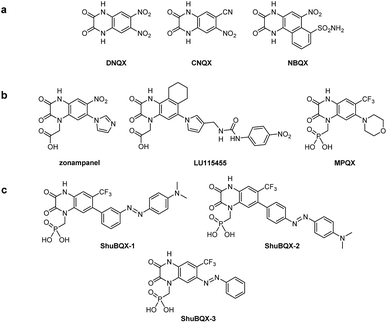
Our design of ShuBQX-3 was based on the vast array of antagonists for AMPA receptors that have been developed.13 These encompass compounds that exhibit non-competitive antagonism, such as perampanel,14 which is clinically used, as well as those that compete for the glutamate binding site. Competitive AMPA receptor antagonists that contain the quinoxaline-2,3-dione motif (Fig. 1a and b), are an extremely well developed family of antagonists and have undergone extensive structure–activity relationship (SAR) studies.15Starting from the parent compound DNQX, a wide array of more selective and more soluble derivatives have been developed, including CNQX and NBQX (Fig. 1a). To increase solubility, carboxylate or phosphonate moieties were introduced on the nitrogen in position 4, which gave rise to zonampanel, LU115455 and MPQX (fanampanel) (Fig. 1b).14
We postulated that an azobenzene moiety could be accommodated in the 6-position of the quinoxaline-2,3-dione core as sterically bulky substituents are tolerated in this position. Our design hypothesis was also supported by a X-ray crystal structure of the compound MPQX bound to a GluA2 receptor.16 The crystal structure strongly suggested that a reasonable amount of steric bulk could be accommodated in the 6-position of the quinoxaline-2,3-dione without interfering with the binding of the antagonist. On the other hand, we concluded that photoisomerization of such a moiety would change the affinity of a ligand bound to the clamshell-like ligand binding domain. At the very least, this could be mediated by a reorganization of the solvation sphere upon photoisomerization. We also added the phosphonic acid side chain from the antagonist MPQX as it dramatically improves the solubility of the compound in aqueous solutions.17 When considering all of our strategic aspects, the photoswitchable antagonists ShuBQX-1, ShuBQX-2 and ShuBQX-3 were designed (Fig. 1c).
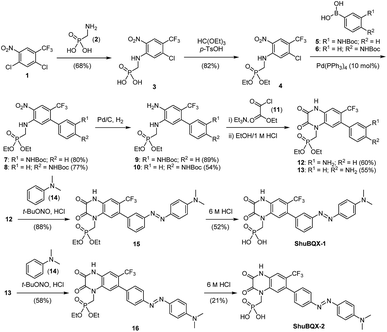
We then set about preparing our target photoswitches, initially focusing on ShuBQX-1 and ShuBQX-2 (Scheme 1). Starting from dichloride 1 and aminomethylphosphonic acid (2) a SNAr reaction furnished phosphonic acid 3, which was protected to afford phosphonate ester 4. Suzuki coupling reactions using boronic acids 5 and 6, bearing Boc-protected anilines in the meta- and para-positions, respectively, were performed to provide biaryls 7 and 8. Subsequent reduction of the nitro group followed by cyclization with ethyl chlorooxoacetate (11) afforded the desired meta- and para-amino substituted quinoxaline-2,3-diones 12 and 13. Quinoxaline 12 was then converted to meta-azobenzene 15 in an azo-coupling reaction with N,N-dimethylaniline (14) and ShuBQX-1 was furnished after deprotection of the phosphonate ester using 6 M HCl followed by reverse phase chromatography. The preparation of ShuBQX-2 followed the same azo-coupling and deprotection procedures as ShuBQX-1, with moderate yields of the desired products being obtained in both reactions.
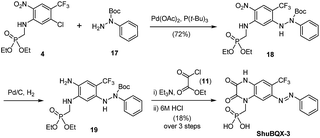
We next accomplished the synthesis of ShuBQX-3 starting from phosphonate ester 4 (Scheme 2). A Buchwald–Hartwig cross-coupling of N-Boc protected hydrazine 17 with phosphonate ester 4 afforded protected hydrazine 18 in 72% yield. Reduction of the nitro group followed by cyclization to the quinoxaline-2,3-dione using ethyl chlorooxoacetate (11) and subsequent deprotection of the phosphonate ester gave ShuBQX-3 in 18% yield (over three steps).
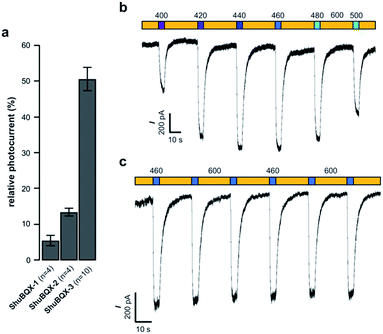
With our small family of soluble, photochromic ligands in hand, we first deduced the optimum photoswitching wavelengths using UV-Vis spectroscopy (Fig. S1†) and then determined their functional properties as light-controllable AMPA receptor antagonists. Whole-cell patch-clamp electrophysiology of HEK293T cells expressing GluA1-L497Y receptors (a non-desensitizing AMPA receptor mutant)18 found that the photochromic ligands ShuBQX-1 (5 μM) and ShuBQX-2 (5 μM) are good antagonists of AMPA receptors in the presence of glutamate (300 μM). However, upon photoisomerization using blue (460 nm) and green (560 nm) light only a small change in AMPA receptor antagonism was observed (Fig. 2a). We then evaluated ShuBQX-3 (5 μM) and discovered that it is an excellent photoswitchable antagonist of AMPA receptors.
First, we determined the IC50 value of ShuBQX-3 in the absence of light and in the presence of glutamate (300 μM). It was found to be 3.1 μM (Fig. S3†). A very similar value (IC50 = 3.3 μM) was obtained when illuminating ShuBQX-3 with orange light (600 nm). At this wavelength, the photoswitch largely resides in the trans-form. Under blue light illumination (460 nm), which favors the cis-state of ShuBQX-3, the antagonist was significantly less potent than in the dark-adapted state (Fig. 2). Finally, experiments conducted using ShuBQX-3 with differing concentrations of glutamate (100 μM and 1 mM) confirmed that it is a competitive antagonist of GluA1 receptors (Fig. S4†). Further studies on the biological activity of ShuBQX-3 using patch-clamp electrophysiology showed that glutamate-induced currents are completely blocked in its trans-form. Upon illumination with 460 nm light, 50% of the glutamate-induced current (compared to the current induced in the absence of ShuBQX-3) could be released (Fig. 2a). We then determined the action spectrum of ShuBQX-3 in the presence of glutamate. When the wavelength was switched between orange light (600 nm) and different wavelengths of purple blue and green light (400–500 nm), we observed large differences in current (Fig. 2b). The maximum inward current was consistently observed when illuminating with 440 nm or 460 nm (Fig. S5†) whereas minimum inward currents were observed using long wavelength light or in the dark. Reversible photoactivation of AMPA receptors with ShuBQX-3 is robust and could be repeated over many times without any significant loss of receptor antagonism (Fig. 2c). Highly reproducible photoswitching of ShuBQX-3 was also obtained when operating in current-clamp mode (Fig. S6†).
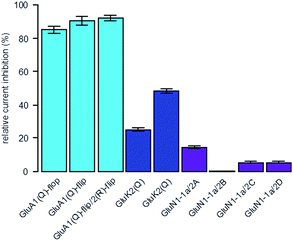
To determine the selectivity profile of ShuBQX-3 (5 μM) in its dark state, we evaluated its effects on Xenopus oocytes expressing a variety of glutamate receptors (Fig. 3). ShuBQX-3 (5 μM) showed excellent antagonism of all GluA1-containing receptors that were tested (85–93%). By contrast, the amount of antagonism observed at this concentration on the GluK2 receptor was significantly reduced (25%) when inducing the current using glutamate (100 μM). Reducing the concentration of glutamate (30 μM) increased the amount of antagonism exhibited by ShuBQX-3 at the GluK2 receptor (49%). Thus, indicating that the dark state of ShuBQX-3 is a competitive antagonist of the GluK2 receptor. Additionally, ShuBQX-3 (5 μM) was evaluated against several GluN1-1a-containing receptors. ShuBQX-3 (5 μM) displayed minimal antagonism at the GluN1-1a-containing glutamate receptors, demonstrating that ShuBQX-3 is partially selective for AMPA receptors over kainate, whilst having significantly reduced levels of antagonism at NMDA receptors.
With the full evaluation of ShuBQX-3 in transfected HEK293T cells and Xenopus oocytes complete, we set out to demonstrate that ShuBQX-3 could control native AMPA receptors in excitable cells. For these experiments we used acute mouse brain slice preparations and whole cell patch-clamp electrophysiology of hippocampal CA1 neurons. Pleasingly, when ShuBQX-3 (10 μM) and glutamate (100 μM) were locally applied in a brain slice preparation, the induced action potential firing of a single neuron could be effectively controlled by switching between blue (460 nm) and orange (620 nm) light (Fig. 4). The NMDA-receptor antagonist AP-5 (50 μM) was locally added to ensure that the action potential firing was not caused by any interactions between the NMDA receptors and glutamate. Additionally, we were able to optically control hippocampal CA1 neurons when using AMPA as the agonist (Fig. S7†). Demonstrating that ShuBQX-3 is selective for AMPA receptors.
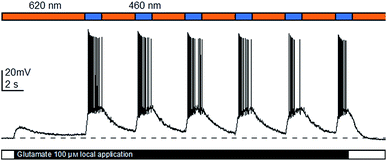 | ||
| Fig. 4 Optical control of action potential firing in hippocampal CA1 neurons using ShuBQX-3 (10 μM) in the presence of glutamate (100 μM) and AP-5 (50 μM). | ||
The action spectrum of ShuBQX-3 is noteworthy, as we observed a significant difference between the optimal photoswitching wavelengths established by the UV-Vis experiments and the physiological patch-clamp experiments. In our UV-Vis experiments, a solution of ShuBQX-3 (50 μM in DMSO) displayed optimal photoswitching at 380 nm (trans to cis) and 460 nm (cis to trans), with a λmax = 365 nm for the trans-isomer (Fig. 5a, S1†). However, in the patch-clamp experiments in HEK293T cells expressing the GluA1 receptor, ShuBQX-3 exhibited a bathochromic shift in its action spectrum, with optimal photoswitching now taking place when illuminating with 460 nm and 600 nm light.19 In an attempt to ascertain why this bathochromic shift was occurring, we consulted the X-ray crystal structure of the non-photoswitchable antagonist MPQX bound to a GluA2 receptor (Fig. 5c).16 The structure features prominent interaction between the guanidinium moiety of arginine R-485, which is also involved in glutamate binding, and the quinoxaline-2,3-dione core of MPQX. We therefore postulated that this interaction could have an effect on the photoswitching properties of ShuBQX-3 when it is bound to the GluA1 receptor.
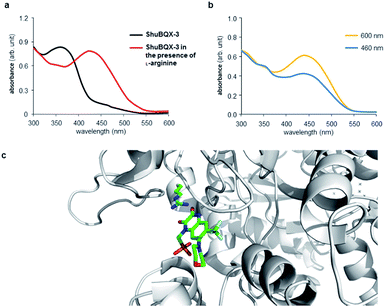 | ||
| Fig. 5 UV-Vis analysis of the photoswitching properties of ShuBQX-3. (a) UV-Vis spectrum showing ShuBQX-3 (50 μM in DMSO) and the bathochromic shift in the presence of 1 mM L-arginine. (b) UV-Vis spectrum ShuBQX-3 (50 μM) in DMSO in the presence of 1 mM L-arginine when illuminated with 460 nm and 600 nm light. (c) X-ray structure of MPQX bound to a GluA2 receptor ligand binding domain, showing the interaction between a conserved arginine and the quinoxaline-2,3-dione.16 | ||
To probe this possible interaction, we took the UV-Vis solution of ShuBQX-3 (50 μM in DMSO) and doped it with L-arginine. Indeed, a bathochromic shift of 75 nm (λmax = 440 nm) in the UV-Vis spectrum of ShuBQX-3 was observed (Fig. 5a) with a 20-fold excess of L-arginine. The optimum photoswitching wavelengths of ShuBQX-3 were now demonstrated to be 460 nm and 600 nm (Fig. 5b). When L-arginine was replaced by guanidine, a similar bathochromic shift was observed (Fig. S8†), substantiating our hypothesis. UV-Vis experiments conducted with the diethyl phosphonate of ShuBQX-3 showed that the phosphonic acid side chain is not crucial for the interaction (Fig. S9†). We also measured the thermal relaxation rate of ShuBQX-3 in the presence of L-arginine (τ = 1.68 min) and found it to be faster than the rate of ShuBQX-3 alone (τ = 7.37 min). This is in accordance to the rate acceleration of red-shifted azobenzenes in comparison to their non red-shifted analogues.20 To the best of our knowledge, this is the first example of an azobenzene-based photoswitch to exhibit such red-shifting properties. Control experiments using ShuBQX-3 (50 μM) dissolved in Ringer's solution showed almost no change in the absorption maximum (Fig. S1†). Our findings suggest that the interaction between R-485 and the quinoxaline-2,3-dione is responsible for the bathochromic shift in the action spectrum of ShuBQX-3.
Conclusions
In summary, we have developed a photochromic antagonist that permits the precise optical control of AMPA receptors. Our photoswitch, ShuBQX-3, is active as its trans-isomer and is converted to its cis-isomer using blue light illumination enabled by red-shifting upon binding to AMPA receptors. ShuBQX-3 expands the photopharmacology of iGluRs and demonstrates that potent photoswitchable antagonists for these receptors can be developed. We envision that ShuBQX-3 will be an important tool for studying the function of AMPA receptors in vivo.Live subject statement
All animal procedures were performed in accordance with the guidelines of the Regierung Oberbayern/the Tierschutzgesetz (TierSchG) and the TierschutzVersuchstierverordnung (TierSchVersV).Acknowledgements
D. M. B. gratefully acknowledges the European Commission for a Marie Skłodowska-Curie Intra-European Fellowship (PIEF-GA-2013-627990). S. L. thanks the Studienstiftung des deutschen Volkes for a PhD studentship. D. T. thanks the Munich Centre for Integrated Protein Science (CIPSM) and the European Research Council (Advanced Grant 268795). K. G. was funded by the International Graduate School of Neuroscience (IGSN) at the Ruhr University Bochum and the RUB Research School. We thank Dr Laura Salonen for initial studies toward the synthesis of ShuBQX-1 and Luis de la Osa de la Rosa for technical assistance. In addition, we are grateful to Dr Johannes Broichhagen for helpful discussions and Philipp Leippe for assistance with the UV-Vis experiments.Notes and references
- (a) T. Fehrentz, M. Schönberger and D. Trauner, Angew. Chem., Int. Ed., 2011, 50, 12156–12182 CrossRef CAS PubMed; (b) A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC; (c) C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Angew. Chem., Int. Ed., 2012, 51, 8446–8476 CrossRef CAS PubMed; (d) W. Szymański, J. M. Beierle, H. A. V. Kistemaker, W. A. Velema and B. L. Feringa, Chem. Rev., 2013, 113, 6114–6178 CrossRef PubMed; (e) W. A. Velema, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 2178–2191 CrossRef CAS PubMed.
- (a) M. Schönberger, M. Althaus, M. Fronius, W. Clauss and D. Trauner, Nat. Chem., 2014, 6, 712–719 Search PubMed; (b) J. Broichhagen, M. Schönberger, S. C. Cork, J. A. Frank, P. Marchetti, M. Bugliani, A. M. J. Shapiro, S. Trapp, G. A. Rutter, D. J. Hodson and D. Trauner, Nat. Commun., 2014, 5, 5116 CrossRef CAS PubMed; (c) A. Damijonaitis, J. Broichhagen, T. Urushima, K. Hüll, J. Nagpal, L. Laprell, M. Schönberger, D. H. Woodmansee, A. Rafiq, M. P. Sumser, W. Kummer, A. Gottschalk and D. Trauner, ACS Chem. Neurosci., 2015, 6, 701–707 CrossRef CAS PubMed.
- (a) M. Schönberger and D. Trauner, Angew. Chem., Int. Ed., 2014, 53, 3264–3267 CrossRef PubMed; (b) S. Pittolo, X. Gómez-Santacana, K. Eckelt, X. Rovira, J. Dalton, C. Goudet, J.-P. Pin, A. Llobet, J. Giraldo, A. Llebaria and P. Gorostiza, Nat. Chem. Biol., 2014, 10, 813–815 CrossRef CAS PubMed.
- (a) C. Falenczyk, M. Schiedel, B. Karaman, T. Rumpf, N. Kuzmanovic, M. Grotli, W. Sippl, M. Jung and B. Konig, Chem. Sci., 2014, 5, 4794–4799 RSC; (b) B. Reisinger, N. Kuzmanovic, P. Löffler, R. Merkl, B. König and R. Sterner, Angew. Chem., Int. Ed., 2014, 53, 595–598 CrossRef CAS PubMed; (c) X. Chen, S. Wehle, N. Kuzmanovic, B. Merget, U. Holzgrabe, B. König, C. A. Sotriffer and M. Decker, ACS Chem. Neurosci., 2014, 5, 377–389 CrossRef CAS PubMed; (d) J. Broichhagen, I. Jurastow, K. Iwan, W. Kummer and D. Trauner, Angew. Chem., Int. Ed., 2014, 53, 7657–7660 CrossRef CAS PubMed.
- M. Borowiak, W. Nahaboo, M. Reynders, K. Nekolla, P. Jalinot, J. Hasserodt, M. Rehberg, M. Delattre, S. Zahler, A. Vollmar, D. Trauner and O. Thorn-Seshold, Cell, 2015, 162, 403–411 CrossRef CAS PubMed.
- (a) A. Polosukhina, J. Litt, I. Tochitsky, J. Nemargut, Y. Sychev, I. De Kouchkovsky, T. Huang, K. Borges, D. Trauner, R. N. Van Gelder and R. H. Kramer, Neuron, 2012, 75, 271–282 CrossRef CAS PubMed; (b) A. Mourot, T. Fehrentz, Y. Le Feuvre, C. M. Smith, C. Herold, D. Dalkara, F. Nagy, D. Trauner and R. H. Kramer, Nat. Methods, 2012, 9, 396–402 CrossRef CAS PubMed; (c) I. Tochitsky, A. Polosukhina, V. E. Degtyar, N. Gallerani, C. M. Smith, A. Friedman, R. N. Van Gelder, D. Trauner, D. Kaufer and R. H. Kramer, Neuron, 2014, 81, 800–813 CrossRef CAS PubMed.
- S. F. Traynelis, L. P. Wollmuth, C. J. McBain, F. S. Menniti, K. M. Vance, K. K. Ogden, K. B. Hansen, H. Yuan, S. J. Myers and R. Dingledine, Pharmacol. Rev., 2010, 62, 405–496 CrossRef CAS PubMed.
- (a) S. A. Lipton, Nat. Rev. Drug Discovery, 2006, 5, 160–170 CrossRef CAS PubMed; (b) A. Alt, E. S. Nisenbaum, D. Bleakman and J. M. Witkin, Biochem. Pharmacol., 2006, 71, 1273–1288 CrossRef CAS PubMed; (c) D. E. Jane, D. Lodge and G. L. Collingridge, Neuropharmacology, 2009, 56, 90–113 CrossRef CAS PubMed.
- R. W. Gereau and G. T. Swanson, The Glutamate Receptors, Humana Press, Totowa, 2008 Search PubMed.
- (a) A. Reiner, J. Levitz and E. Y. Isacoff, Curr. Opin. Pharmacol., 2015, 20, 135–143 CrossRef CAS PubMed; (b) M. Volgraf, P. Gorostiza, S. Szobota, M. R. Helix, E. Y. Isacoff and D. Trauner, J. Am. Chem. Soc., 2007, 129, 260–261 CrossRef CAS PubMed; (c) P. Stawski, M. Sumser and D. Trauner, Angew. Chem., Int. Ed., 2012, 51, 5748–5751 CrossRef CAS PubMed; (d) L. Laprell, E. Repak, V. Franckevicius, F. Hartrampf, J. Terhag, M. Hollmann, M. Sumser, N. Rebola, D. A. DiGregorio and D. Trauner, Nat. Commun., 2015, 6, 8076 CrossRef CAS PubMed.
- P. K. Y. Chang, D. Verbich and R. A. McKinney, Eur. J. Neurosci., 2012, 35, 1908–1916 CrossRef PubMed.
- (a) K. P. S. J. Murphy, G. P. Reid, D. R. Trentham and T. V. P. Bliss, J. Physiol., 1997, 504, 379–385 CrossRef CAS PubMed; (b) J. E. Reeve, M. M. Kohl, A. Rodríguez-Moreno, O. Paulsen and H. L. Anderson, Commun. Integr. Biol., 2012, 5, 240–242 CrossRef CAS PubMed; (c) J. J. Chambers, H. Gouda, D. M. Young, I. D. Kuntz and P. M. England, J. Am. Chem. Soc., 2004, 126, 13886–13887 CrossRef CAS PubMed.
- (a) S. S. Nikam and B. E. Kornberg, Curr. Med. Chem., 2001, 8, 155–170 CrossRef CAS PubMed; (b) P. Stawski, H. Janovjak and D. Trauner, Bioorg. Med. Chem., 2010, 18, 7759–7772 CrossRef CAS PubMed.
- S. Hibi, K. Ueno, S. Nagato, K. Kawano, K. Ito, Y. Norimine, O. Takenaka, T. Hanada and M. Yonaga, J. Med. Chem., 2012, 55, 10584–10600 CrossRef CAS PubMed.
- D. Catarzi, V. Colotta and F. Varano, Med. Res. Rev., 2007, 27, 239–278 CrossRef CAS PubMed.
- A. I. Sobolevsky, M. P. Rosconi and E. Gouaux, Nature, 2009, 462, 745–756 CrossRef CAS PubMed.
- L. Turski, A. Huth, M. Sheardown, F. McDonald, R. Neuhaus, H. H. Schneider, U. Dirnagl, F. Wiegand, P. Jacobsen and E. Ottow, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 10960–10965 CrossRef CAS.
- Y. Stern-Bach, S. Russo, M. Neuman and C. Rosenmund, Neuron, 1998, 21, 907–918 CrossRef CAS PubMed.
- We also conducted patch-clamp experiments in which ShuBQX-1 and ShuBQX-2 were irradiated with varying wavelengths of light but no bathochromic shift was observed for either of these compounds (Fig. S2†).
- J. García-Amorós and D. Velasco, Beilstein J. Org. Chem., 2012, 8, 1003–1017 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterisation data. See DOI: 10.1039/c6sc01621a |
| ‡ These authors contributed equally. |
| § ShuBQX is a combination of the names of the authors D. M. B. and S. L. and the antagonist MPQX. |
| This journal is © The Royal Society of Chemistry 2017 |