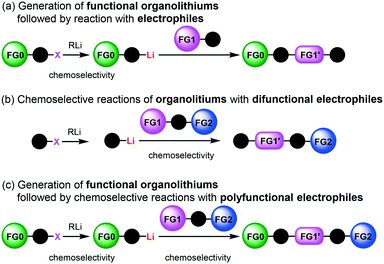
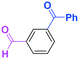
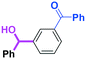
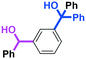
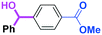
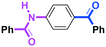
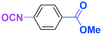
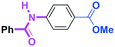
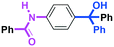
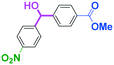
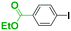
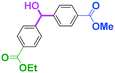
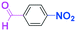
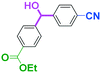
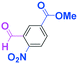
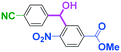
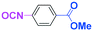
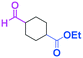
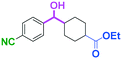
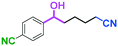
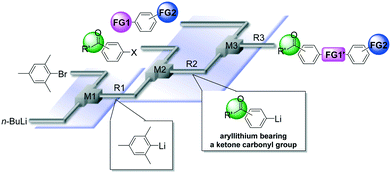
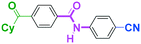
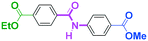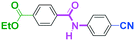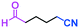Micromixing enables chemoselective reactions of difunctional electrophiles with functional aryllithiums†
Aiichiro
Nagaki
 *a,
Satoshi
Ishiuchi
a,
Keita
Imai
a,
Kengo
Sasatsuki
a,
Yuichi
Nakahara
b and
Jun-ichi
Yoshida
*a
*a,
Satoshi
Ishiuchi
a,
Keita
Imai
a,
Kengo
Sasatsuki
a,
Yuichi
Nakahara
b and
Jun-ichi
Yoshida
*a
aDepartment of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: yoshida@sbchem.kyoto-u.ac.jp; anagaki@sbchem.kyoto-u.ac.jp
bProcess Engineering Group, Fundamental Technology Labs. Institute of Innovation, Ajinomoto Co., Inc., 1-1 Suzuki-cho, Kawasaki-ku, Kanagawa 210-8681, Japan
First published on 16th October 2017
Abstract
Generation of highly unstable functional aryllithiums followed by chemoselective reactions with difunctional electrophiles were successfully achieved using flow microreactor systems equipped with micromixers to give highly functionalized compounds without protecting functional groups. Extremely fast micromixing is responsible for high chemoselectivity.
Introduction
Protecting groups have been indispensable for synthesizing complex highly functionalized organic molecules, although the use of a protective group needs at least two additional synthetic steps, protection and deprotection. Recently, protecting group-free-synthesis has attracted a great deal of attention.1In this regard, the generation of organolithiums2 bearing unprotected functional groups has been investigated extensively (Scheme 1(a)).3 Various electrophilic functional groups can be compatible with organolithium reactions if we use flow microreactors.4 Micromixing using flow microreactors5–7 enables selective lithiation without affecting the functional group, whereas the precise residence time control enables the effective use of short-lived organolithium intermediates for the subsequent reaction with electrophiles. Reactions of such functional organolithiums with electrophiles give difunctional products.
Another approach to difunctional compounds using organolithium chemistry is the reaction of unfunctionalized organolithiums with difunctional electrophiles (Scheme 1(b)). However, it is usually difficult or impossible to achieve chemoselective reactions8 without affecting less electrophilic functionality even if one equivalent of an organolithium is used. However, recently, we reported extremely fast micromixing9 dramatically improves the chemoselectivity.10
The combination of the first and the second approaches is also effective (Scheme 1(c)). Chemoselective reactions of functional organolithiums with difunctional electrophiles should give trifunctional products.
In this paper, we report the full details of such approaches based on extremely fast micromixing using flow microreactor systems, which serve as powerful and straightforward methods for protecting group-free-synthesis of highly functionalized organic molecules.
Generation of functional aryllithiums
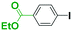
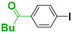
First we show the effect of mixing on the efficiency of functional aryllithium generation. Although aryllithiums bearing various electrophilic functional groups have been successfully generated using flow microreactors, we focus on the generation of 4-cyanophenyllithium by bromine–lithium exchange of 4-cyanobromobenzene with n-butyllithium (n-BuLi) as a representative (Scheme 1(a)).The reaction of 4-cyanobromobenzene with one equivalent of n-BuLi was examined using a flow microreactor system composed of two micromixers (M1 and M2) and two microtube reactors (R1 and R2) shown in Fig. 1.
 | ||
| Fig. 1 A flow microreactor system for bromine–lithium exchange of 4-cyanobromobenzene with n-BuLi followed by reaction with methanol. | ||
As shown in Table 1, the selective generation and reaction of 4-cyanophenyllithium was achieved and the desired compound was obtained in high yields at high flow rates. This indicates that the reaction selectivity depended on the flow speed because it is well known that flow speed increases with an increase with the flow rate.11 The decrease in the total flow rate caused the decrease of the yields presumably because n-BuLi attacked the cyano group of 4-cyanobromobenzene. In fact, at slower flow rates, the formation of 1-phenylpentan-1-one was observed by GC-mass analysis (see ESI† for details). The present observations indicate that extremely fast mixing is responsible for the high selectivity in the generation of functional organolithiums.
| Total flow rate in M1 (ml min−1) | Conversion (%) | Yield (%) |
|---|---|---|
| 2.5 | 100 | 36 |
| 5.0 | 100 | 61 |
| 10 | 100 | 75 |
| 20 | 100 | 85 |
Reaction of phenyllithium with difunctional electrophiles
The second approach is the reaction of unfunctionalized organolithiums with difunctional electrophiles. We chose to use phenyllithium as a representative organolithium reagent without functional groups.First, we focused on aliphatic compounds having two different electrophilic functional groups such as aldehyde, ketone, and ester carbonyl groups.
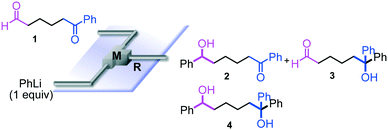
The reaction of phenyllithium with 6-oxo-6-phenylhexanal (1) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) was carried out using a flow microreactor system equipped with a micromixer (Fig. 2). A solution of 1 (0.10 M in THF, 12 ml min−1) and a solution of PhLi (0.42 M in Et2O/cyclohexane (74/26 v/v), 3.0 ml min−1) were introduced to M by syringe pumps, and the resulting mixture was passed through R (ϕ = 1000 μm, L = 100 cm). The yields of 6-hydroxy-1,6-diphenylhexan-1-one (2, mono-addition product), 6-hydroxy-6,6-diphenylhexanal (3, mono-addition product), and 1,1,6-triphenylhexane-1,6-diol (4, di-addition product) were determined by GC after aqueous work-up. As shown in Table 2, the reaction at a low flow rate (2.5 ml min−1) at −40 °C resulted in a low selectivity for 2, which was formed by selective nucleophilic attack of PhLi at the aldehyde carbonyl group. A significant amount of 4, which was derived from the nucleophilic attack of PhLi at both aldehyde and ketone carbonyl groups, was obtained. The increase in the flow rate resulted in an increase in the selectivity for 2, indicating that the selectivity for 2 increases with the mixing efficiency, because it is known that the mixing efficiency in a micromixer increases with an increase in the flow speed.11 The reaction at −78 °C also showed a similar selectivity. The highest yield of 2 (48%) was observed at the flow rate of 30 ml min−1 at −40 °C, although the selectivity is still not acceptable.
1 molar ratio) was carried out using a flow microreactor system equipped with a micromixer (Fig. 2). A solution of 1 (0.10 M in THF, 12 ml min−1) and a solution of PhLi (0.42 M in Et2O/cyclohexane (74/26 v/v), 3.0 ml min−1) were introduced to M by syringe pumps, and the resulting mixture was passed through R (ϕ = 1000 μm, L = 100 cm). The yields of 6-hydroxy-1,6-diphenylhexan-1-one (2, mono-addition product), 6-hydroxy-6,6-diphenylhexanal (3, mono-addition product), and 1,1,6-triphenylhexane-1,6-diol (4, di-addition product) were determined by GC after aqueous work-up. As shown in Table 2, the reaction at a low flow rate (2.5 ml min−1) at −40 °C resulted in a low selectivity for 2, which was formed by selective nucleophilic attack of PhLi at the aldehyde carbonyl group. A significant amount of 4, which was derived from the nucleophilic attack of PhLi at both aldehyde and ketone carbonyl groups, was obtained. The increase in the flow rate resulted in an increase in the selectivity for 2, indicating that the selectivity for 2 increases with the mixing efficiency, because it is known that the mixing efficiency in a micromixer increases with an increase in the flow speed.11 The reaction at −78 °C also showed a similar selectivity. The highest yield of 2 (48%) was observed at the flow rate of 30 ml min−1 at −40 °C, although the selectivity is still not acceptable.
| Temperature (°C) | Flow rate (ml min−1) | Conversion (%) | Yield (%) | ||
|---|---|---|---|---|---|
| 2 | 3 | 4 | |||
| −40 | 2.5 | 66 | 10 | 20 | 29 |
| −40 | 10 | 65 | 36 | 12 | 19 |
| −40 | 30 | 77 | 48 | 10 | 18 |
| −70 | 2.5 | 67 | 11 | 23 | 26 |
| −70 | 10 | 72 | 27 | 18 | 22 |
| −70 | 30 | 73 | 44 | 12 | 17 |
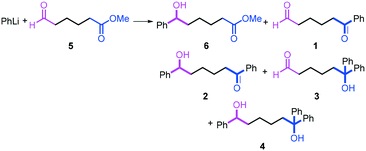
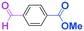
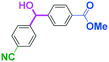
The reactions of methyl 6-oxohexanoate (5) were also examined using a flow microreactor system. The yields of methyl 6-hydroxy-6-phenylhexanoate (6, mono-addition product), 6-oxo-6-phenylhexanal (1, mono-addition product), 6-hydroxy-1,6-diphenylhexan-1-one (2, di-addition product), 6-hydroxy-6,6-diphenylhexanal (3, di-addition product), and 1,1,6-triphenylhexane-1,6-diol (4, tri-addition product) were determined by GC after aqueous work-up. As shown in Table 3, the increase in the flow rate resulted in an increase in the selectivity for 6, indicating that the increase in the mixing efficiency increases the selectivity for the attack on the aldehyde carbonyl group. The desired product 6 was obtained in 72% yield.
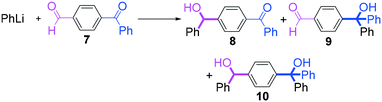
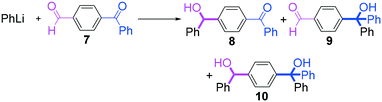
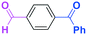
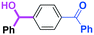
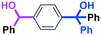
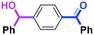
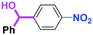
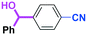
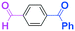
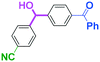
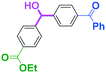
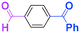
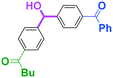
Next, we turned our attention to the reactions with aromatic compounds bearing two electrophilic functional groups. In contrast to the aliphatic case, remarkable selectivity was observed in the aromatic case, although the selectivity strongly depends on the flow rate (Table 4). The yields of (4-(hydroxy(phenyl)methyl)phenyl)(phenyl)methanone (8, mono-addition product), 4-(hydroxydiphenylmethyl)benzaldehyde (9, mono-addition product), and (4-(hydroxy(phenyl)methyl)phenyl)diphenylmethanol (10, di-addition product) were determined by GC after aqueous work-up. The yield of 8 (mono-addition product) increases and that of 10 (di-addition product) decreases with an increase in the flow rate, indicating the importance of mixing efficiency. It is interesting to note that the yield of 9 also decreases with an increase in the flow rate, although the reason is not clear at present. Notably, the selectivity decreases with an increase in the diameter of micromixer. The mixing efficiency generally decreases with an increase in the inner diameter if the flow rate is the same. In contrast, the selectivity does not vary with the reaction temperature appreciably.
| Inner diameter in M (μm) | Temperature (°C) | Flow rate (ml min−1) | Conversion (%) | Yield (%) | ||
|---|---|---|---|---|---|---|
| 8 | 9 | 10 | ||||
| 800 | −40 | 15 | 65 | 23 | 3 | 40 |
| 500 | −40 | 15 | 71 | 34 | 3 | 32 |
| 250 | −40 | 15 | 83 | 66 | 2 | 15 |
| 250 | −78 | 15 | 78 | 60 | 4 | 17 |
| 250 | −20 | 15 | 79 | 57 | 2 | 18 |
| 250 | 0 | 15 | 81 | 61 | 2 | 21 |
| 250 | 20 | 15 | 81 | 57 | 2 | 22 |
| 250 | −40 | 0.63 | 61 | 7 | 5 | 40 |
| 250 | −40 | 1.3 | 56 | 8 | 5 | 41 |
| 250 | −40 | 2.5 | 60 | 15 | 5 | 38 |
| 250 | −40 | 5.0 | 74 | 42 | 5 | 29 |
| 250 | −40 | 10 | 82 | 60 | 3 | 20 |
| 250 | −40 | 20 | 84 | 69 | 3 | 15 |
| 250 | −40 | 25 | 78 | 70 | 2 | 8 |
| 250 | −40 | 30 | 81 | 73 | 1 | 7 |
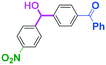
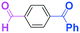
To get a deeper insight into the effect of micromixing on the selectivity, the reaction of PhLi with 4-benzoylbenzaldehyde was carried out in a conventional macrobatch reactor (a 50 ml round bottomed flask with a magnetic stirring) at various temperatures with varying the way of addition. The results are summarized in Table 5.
| Temperature (°C) | Method of addition | Conversion (%) | Yield (%) | ||
|---|---|---|---|---|---|
| 8 | 9 | 10 | |||
| −78 | Addition of PhLi to 7 | 65 | 28 | 7 | 25 |
| −40 | Addition of PhLi to 7 | 64 | 23 | 7 | 26 |
| −20 | Addition of PhLi to 7 | 64 | 20 | 7 | 30 |
| 0 | Addition of PhLi to 7 | 64 | 12 | 6 | 30 |
| 20 | Addition of PhLi to 7 | 61 | 12 | 5 | 33 |
| −78 | Addition of 7 to PhLi | 33 | 6 | 2 | 26 |
| −40 | Addition of 7 to PhLi | 37 | 5 | 1 | 27 |
| −20 | Addition of 7 to PhLi | 49 | 3 | 1 | 35 |
| 0 | Addition of 7 to PhLi | 51 | 2 | 1 | 37 |
| 20 | Addition of 7 to PhLi | 55 | 1 | 1 | 40 |
| −78 | Simultaneous addition of 7 and PhLi | 51 | 16 | 9 | 26 |
The addition of one equivalent of phenyllithium to a stirred solution of 7 for 1.0 min at −78 °C gave 8 in only 28% yield, and a significant amount of 10 (25%) was produced together with unchanged 7. Interestingly, compound 9, which is formed by the reaction of PhLi with the ketone carbonyl group without affecting the more reactive aldehyde carbonyl group, was also obtained in 7% yield. The increase in the temperature caused a decrease in the yield of desired product 8. Moreover, the reverse addition resulted in lower yields of 8, and the simultaneous addition did not give better results.
Although the selectivity should also depend on the size and shape of the reactor, the stirring speed, and the speed of the addition, the present data demonstrate the limitations of the macrobatch method for achieving this type of chemoselectivity of fast reactions.
With the remarkable effect of micromixing on the selectivity in hand, we next examined the reactions of PhLi with various benzaldehyde having different electrophilic functional groups such as ketone carbonyl, ester carbonyl, nitro,12 and cyano groups. As shown in Table 6, high chemoselectivity was obtained by using the flow microreactor system, whereas the use of a batch reactor resulted in much lower selectivity. In all cases, the faster flow rates gave better selectivity. Furthermore, the reactions of PhLi with phenylisocyanates having a cyano or an alkoxycarbonyl group were also accomplished with high chemoselectivity, although the reactions in a batch reactor gave complex mixtures.
Reactions of functional aryllithiums with difunctional electrophiles
Next, we focused on the third case (Scheme 1(c)); the reactions of aryllithiums bearing an electrophilic functional group (FG0) with difunctional electrophiles, i.e. compounds bearing two different electrophilic functional groups (FG1 and FG2) (Scheme 2). In this case, we have to worry about not only the competition between two electrophilic functional groups on the electrophile (FG1 and FG2) but also the competition between functional groups on the electrophile (FG1 and FG2) and a functional group on the nucleophile (FG0).13,14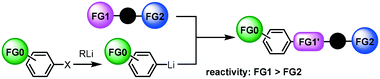 | ||
| Scheme 2 Reactions of functional aryllithiums with compounds bearing two electrophilic functional groups. | ||
An aryllithium bearing an electrophilic functional group was generated by halogen–lithium exchange of the corresponding aryl halide using a flow microreactor system consisting of a T-shaped micromixer (ϕ = 250 μm) (M1), a V-shaped micromixer (ϕ = 250 μm) (M2) and two microtube reactors (R1 and R2) (Fig. 3). The residence time (tR1) for the halogen–lithium exchange reaction was optimized individually (see ESI† for the details).
 | ||
| Fig. 3 A flow microreactor system for the reaction of compounds bearing two different electrophilic functional groups with functional aryllithium generated by halogen–lithium exchange. | ||
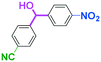
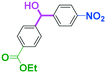
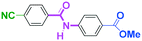
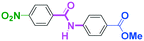
As shown in Table 7, the aldehyde carbonyl group reacted selectively without affecting other electrophilic functional groups such as ketone carbonyl, ester carbonyl, nitro, and cyano groups to give desired compounds in good yields, when one equivalent of a functionalized aryllithium was used. Also, the functional groups on the aryllithiums were not affected. It is also noteworthy that aldehyde bearing two other electrophilic functional groups such an ester carbonyl group and a nitro group (a trifunctional electrophile) also reacted chemoselectively. Competition between an isocyanate group and other electrophilic functional groups using functionalized aryllithium is also interesting. As shown in Table 7, the nucleophilic addition took place selectively on the isocyanate group without affecting other functional groups on both the electrophile and the nucleophile. The reactions seem to be useful as they allow amides having two functional groups to be easily synthesized.
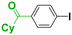
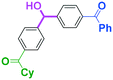
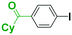
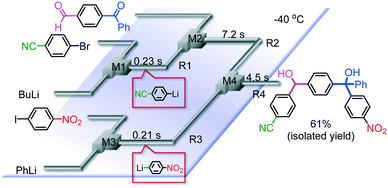
It is well known that a ketone carbonyl group reacts with organolithiums very rapidly. Therefore, aryllithiums bearing a ketone carbonyl group are very unstable and decompose very rapidly. However, with a flow microreactor system, aryllithiums bearing ketone carbonyl groups can be generated by iodine–lithium exchange reactions of the corresponding aryl iodides with mesityllithium and can be reacted with subsequently added electrophiles. Therefore, we examined the reactions of aryllithiums bearing a ketone carbonyl group with aromatic compounds having different electrophilic functional groups. A flow microreactor system consisting of three micromixers (M1, M2, and M3) and three microtube reactors (R1, R2, and R3) (Fig. 4) was used. A solution of mesityl bromide (0.090 M in THF, 5.0 mL min−1) and a solution of n-BuLi (0.22 M in hexane, 1.8 mL min−1) were introduced to M1, and the mixture was passed through R1 (ϕ = 1000 μm, L = 410 cm (200 cm at 0 °C, 10 cm at ambient temperature and 200 cm at −70 °C)). The resulting solution of mesityllithium was mixed with a solution of aryl iodide (0.10 M in THF, 3.0 mL min−1) in M2 (−70 °C), and the mixture was passed through R2 (ϕ = 250 μm, L = 1.0 cm, −70 °C). The resulting solution of an aryllithiums bearing a ketone carbonyl group was mixed with a solution of a compound bearing two different electrophilic functional groups (0.10 M in THF, 4.2 mL min−1) in M3 (−70 °C), and the mixture was passed through R3 (ϕ = 1000 μm, L = 200 cm, −70 °C).
As shown in Table 8, benzaldehyde and isocyanate bearing another electrophilic functional group such as a ketone carbonyl group, an ester carbonyl group and a cyano group reacted chemoselectively without decomposition of aryllithium bearing a ketone carbonyl group.
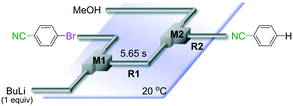
Chemoselective three-component coupling using an integrated flow microreactor system
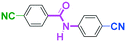
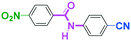
Integration of chemical reactions enhances the power and speed of organic synthesis, and recently it has been recognized that space-integration15 of reactions using flow microreactors is quite effective. Thus, we examined chemoselective three-component coupling using benzaldehyde having a ketone carbonyl group (7) in an integrated flow microreactor system consisting of four micromixers (M1, M2, M3, and M4) and three microtube reactors (R1, R2, R3, and R4) (Fig. 5). A solution of 4-bromobenzonitrile (0.10 M in THF, 5.8 mL min−1) and a solution of n-BuLi (0.42 M in hexane, 1.5 mL min−1) were introduced to M1, and the mixture was passed through R1. The resulting solution was mixed with a solution of 4-benzoylbenzaldehyde (0.10 M in THF, 5.8 mL min−1) in M2 and passed through R2. A solution of 1-iodo-4-nitrobenzene (0.10 M in THF, 6.4 mL min−1) and a solution of PhLi (0.42 M in Et2O/cyclohexane (74/26 v/v), 1.6 mL min−1) were introduced to M3 by syringe pumps and the mixture was passed through R3. Then, the two solutions produced in R2 and R3 were mixed in M4 and passed through R4. The reaction with p-cyanophenyllithium at the aldehyde carbonyl group followed by the reaction with p-nitrophenyllithium at the ketone carbonyl group was successfully achieved to obtain the desired product in 61% isolated yield. High productivity (156 mg min−1) of the present method, because of high flow rates and short residence times, is also noteworthy.Conclusions
In conclusion, we found that extremely fast micromixing enables highly chemoselective reactions of unstable functional aryllithiums with difunctional electrophiles. The present approach based on flash chemistry serves as a powerful method for protecting-group-free synthesis using organolithium compounds and opens a new possibility in the synthesis of polyfunctional organic molecules.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the Grant-in-Aid for Scientific Research (B) (no. 26288049), Scientific Research on Innovative Areas 2707 Middle molecular strategy from MEXT (no. 15H05849), Scientific Research (S) (no. 26220804), Scientific Research (S) (no. 25220913), the Asahi Glass Foundation, and Ogasawara Foundation for the promotion of science and engineering.Notes and references
- I. S. Young and P. S. Baran, Nat. Chem., 2009, 1, 193 CrossRef CAS PubMed.
- (a) J. E. Baldwin and R. M. Williams, Organolithiums: Selectivity for Synthesis, Pregamon, Amsterdam, 2002 Search PubMed; (b) N. Chinkov, H. Chechik, S. Majumdar, A. Liard and I. Marek, Synthesis, 2002, 17, 2473 Search PubMed.
- (a) P. Knochel, Handbook of Functionalized Organometallics, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) A. Boudier, L. O. Bromm, M. Lotz and P. Knochel, Angew. Chem., Int. Ed., 2000, 39, 4414 CrossRef; (c) W. E. Parham and C. K. Bradsher, Acc. Chem. Res., 1982, 15, 300 CrossRef CAS.
- (a) A. Nagaki, H. Kim and J. Yoshida, Angew. Chem., Int. Ed., 2008, 47, 7833 ( Angew. Chem. , 2008 , 120 , 7951 ) CrossRef CAS PubMed; (b) A. Nagaki, H. Kim and J. Yoshida, Angew. Chem., Int. Ed., 2009, 48, 8063 ( Angew. Chem. , 2009 , 121 , 8207 ) CrossRef CAS PubMed; (c) A. Nagaki, H. Kim, Y. Moriwaki, C. Matsuo and J. Yoshida, Chem. – Eur. J., 2010, 16, 11167 CrossRef CAS PubMed; (d) A. Nagaki, H. Kim, C. Matsuo, H. Usutani and J. Yoshida, Org. Biomol. Chem., 2010, 8, 1212 RSC; (e) H. Kim, A. Nagaki and J. Yoshida, Nat. Commun., 2011, 2, 264 CrossRef PubMed; (f) A. Nagaki, Y. Tsuchihashi, S. Haraki and J. Yoshida, Org. Biomol. Chem., 2015, 13, 7140–7145 RSC.
- Books on flow chemistry and flow microreactor synthesis: (a) W. Ehrfeld, V. Hessel and H. Löwe, Microreactors, Wiley-VCH, Weinheim, 2000 CrossRef; (b) V. Hessel, S. Hardt and H. Löwe, Chemical Micro Process Engineering, Wiely-VCH, Weinheim, 2004 Search PubMed; (c) Micro Precess Engineering, ed. V. Hessel, A. Renken, J. C. Schouten and J. Yoshida, Wiley-Blackwell, 2009 Search PubMed; (d) Microreactors in Organic Chemistry and Catalysis, ed. T. Wirth, Wiley-VCH, Weinheim, 2nd edn, 2013 Search PubMed.
- Reviews on flow chemistry and flow microreactor synthesis: (a) K. Jähnisch, V. Hessel, H. Löwe and M. Baerns, Angew. Chem., Int. Ed., 2004, 43, 406 ( Angew. Chem. , 2004 , 116 , 410 ) CrossRef PubMed; (b) G. N. Doku, W. Verboom, D. N. Reinhoudt and A. van den Berg, Tetrahedron, 2005, 61, 2733 CrossRef CAS; (c) J. Yoshida, A. Nagaki, T. Iwasaki and S. Suga, Chem. Eng. Technol., 2005, 3, 259 CrossRef; (d) P. Watts and S. J. Haswell, Chem. Soc. Rev., 2005, 34, 235 RSC; (e) K. Geyer, J. D. C. Codée and P. H. Seeberger, Chem. – Eur. J., 2006, 12, 8434 CrossRef CAS PubMed; (f) A. J. deMello, Nature, 2006, 442, 394 CrossRef CAS PubMed; (g) H. Song, D. L. Chen and R. F. Ismagilov, Angew. Chem., Int. Ed., 2006, 45, 7336 ( Angew. Chem. , 2006 , 118 , 7494 ) CrossRef CAS PubMed; (h) J. Kobayashi, Y. Mori and S. Kobayashi, Chem. – Asian J., 2006, 1, 22 CrossRef CAS PubMed; (i) M. Brivio, W. Verboom and D. N. Reinhoudt, Lab Chip, 2006, 6, 329 RSC; (j) B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300 CrossRef CAS PubMed; (k) B. Ahmed-Omer, J. C. Brandt and T. Wirth, Org. Biomol. Chem., 2007, 5, 733 RSC; (l) P. Watts and C. Wiles, Chem. Commun., 2007, 443 RSC; (m) T. Fukuyama, M. T. Rahman, M. Sato and I. Ryu, Synlett, 2008, 151 CAS; (n) R. L. Hartman and K. F. Jensen, Lab Chip, 2009, 9, 2495 RSC; (o) J. P. McMullen and K. F. Jensen, Annu. Rev. Anal. Chem., 2010, 3, 19 CrossRef CAS PubMed; (p) J. Yoshida, H. Kim and A. Nagaki, ChemSusChem, 2011, 4, 331 CrossRef CAS PubMed; (q) C. Wiles and P. Watts, Green Chem., 2012, 14, 38 RSC; (r) A. Kirschining, L. Kupracz and J. Hartwig, Chem. Lett., 2012, 41, 562 CrossRef; (s) D. T. McQuade and P. H. Seeberger, J. Org. Chem., 2013, 78, 6384 CrossRef CAS PubMed; (t) K. S. Elvira, X. C. Solvas, R. C. R. Wootton and A. J. deMello, Nat. Chem., 2013, 5, 905 CrossRef CAS PubMed; (u) J. C. Pastre, D. L. Browne and S. V. Ley, Chem. Soc. Rev., 2013, 42, 8849 RSC; (v) I. R. Baxendale, J. Chem. Technol. Biotechnol., 2013, 88, 519 CrossRef CAS; (w) T. Fukuyama, T. Totoki and I. Ryu, Green Chem., 2014, 16, 2042 RSC; (x) H. P. L. Gemoets, Y. Su, M. Shang, V. Hessel, R. Luque and T. Noël, Chem. Soc. Rev., 2016, 45, 83 RSC; (y) D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276 CrossRef PubMed; (z) M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796 CrossRef CAS PubMed.
- Some selected recent examples: (a) D. Cantillo, M. Baghbanzadeh and C. O. Kappe, Angew. Chem., Int. Ed., 2012, 51, 10190 ( Angew. Chem. , 2012 , 124 , 12207 ) CrossRef CAS PubMed; (b) W. Shu and S. L. Buchwald, Angew. Chem., Int. Ed., 2012, 51, 5355 ( Angew. Chem. , 2012 , 124 , 5451 ) CrossRef CAS PubMed; (c) A. Nagaki, Y. Moriwaki and J. Yoshida, Chem. Commun., 2012, 48, 11211 RSC; (d) F. Lévesque and P. H. Seeberger, Angew. Chem., Int. Ed., 2012, 51, 1706 ( Angew. Chem. , 2012 , 124 , 1738 ) CrossRef PubMed; (e) K. C. Basavaraju, S. Sharma, R. A. Maurya and D. P. Kim, Angew. Chem., Int. Ed., 2013, 52, 6735 ( Angew. Chem. , 2013 , 125 , 6867 ) CrossRef CAS PubMed; (f) C. Brancour, T. Fukuyama, Y. Mukai, T. Skrydstrup and I. Ryu, Org. Lett., 2013, 15, 2794 CrossRef CAS PubMed; (g) J. D. Nguyen, B. Reiß, C. Dai and C. R. J. Stephenson, Chem. Commun., 2013, 49, 4352 RSC; (h) C. Battilocchio, J. M. Hawkins and S. V. Ley, Org. Lett., 2013, 15, 2278 CrossRef CAS PubMed; (i) A. S. Kleinke and T. F. Jamison, Org. Lett., 2013, 15, 710 CrossRef CAS PubMed; (j) K. Asano, Y. Uesugi and J. Yoshida, Org. Lett., 2013, 15, 2398 CrossRef CAS PubMed; (k) A. Nagaki, D. Ichinari and J. Yoshida, Chem. Commun., 2013, 49, 3242 RSC; (l) L. Guetzoyan, N. Nikbin, I. R. Baxendale and S. V. Ley, Chem. Sci., 2013, 4, 764 RSC; (m) S. Fuse, Y. Mifune and T. Takahashi, Angew. Chem., Int. Ed., 2014, 53, 851 ( Angew. Chem. , 2014 , 126 , 870 ) CrossRef CAS PubMed; (n) Z. He and T. F. Jamison, Angew. Chem., Int. Ed., 2014, 53, 3353 ( Angew. Chem. , 2014 , 126 , 3421 ) CrossRef CAS PubMed; (o) A. Nagaki, Y. Takahashi and J. Yoshida, Chem. – Eur. J., 2014, 20, 7931 CrossRef CAS PubMed; (p) M. Chen, S. Ichikawa and S. L. Buchwald, Angew. Chem., Int. Ed., 2015, 54, 263 CrossRef CAS PubMed; (q) S. Fuse, Y. Mifune, H. Nakamura and H. Tanaka, Nat. Commun., 2016, 7, 13491 CrossRef CAS PubMed; (r) A. Nagaki, Y. Takahashi and J. Yoshida, Angew. Chem., Int. Ed., 2016, 55, 5327 CrossRef CAS PubMed; (s) H. Seo, M. H. Katcher and T. F. Jamison, Nat. Chem., 2017, 9, 453 CrossRef CAS PubMed.
- (a) R. W. Hoffmann, Synthesis, 2006, 3531 CrossRef CAS; (b) N. A. Afagh and A. K. Yudin, Angew. Chem., Int. Ed., 2010, 49, 262 ( Angew. Chem. , 2010 , 122 , 270 ) CrossRef CAS PubMed.
- (a) A. Nagaki, M. Togai, S. Suga, N. Aoki, K. Mae and J. Yoshida, J. Am. Chem. Soc., 2005, 127, 11666 CrossRef CAS PubMed; (b) A. Nagaki, N. Takabayashi, Y. Tomida and J. Yoshida, Org. Lett., 2008, 10, 3937 CrossRef CAS PubMed; (c) J. Yoshida, A. Nagaki, T. Iwasaki and S. Suga, Chem. Eng. Technol., 2005, 3, 259 CrossRef; (d) A. Nagaki, D. Ichinari and J. Yoshida, Chem. Commun., 2013, 49, 3242 RSC; (e) T. Noël, Y. Su and V. Hessel, Top. Organomet. Chem., 2016, 57, 1 Search PubMed; (f) R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502 CrossRef CAS PubMed; (g) J. Yoshida, A. Nagaki, T. Iwasaki and S. Suga, Chem. Eng. Technol., 2005, 28, 259 CrossRef CAS.
- A. Nagaki, K. Imai, S. Ishiuchi and J. Yoshida, Angew. Chem., Int. Ed., 2015, 54, 1914–1918 CrossRef CAS PubMed.
- W. Ehrfeld, K. Golbig, V. Hessel, H. Löwe and T. Richter, Ind. Eng. Chem. Res., 1999, 38, 1075 CrossRef CAS.
- It has been reported that aryllithiums react with nitroarenes to give diarylamines: T. Yang and B. P. Cho, Tetrahedron Lett., 2003, 44, 7549 CrossRef CAS.
- (a) V. Peron, E. Porhiel, V. Ferrand and H. L. Bozec, J. Org. Chem., 1997, 539, 201 CrossRef CAS; (b) A. L. K. S. Shun, E. T. Chernick, S. Eisler and R. R. Tykwinski, J. Org. Chem., 2003, 68, 1339 CrossRef PubMed; (c) C. W. Chen and T. Y. Luh, J. Org. Chem., 2008, 73, 8357 CrossRef CAS PubMed; (d) K. D. Safa, J. V. Mardipour and Y. M. Oskoei, J. Organomet. Chem., 2011, 696, 802 CrossRef CAS; (e) K. D. Safa, T. Shokri, H. Abbasi and R. T. Mofrad, J. Heterocycl. Chem., 2014, 51, 80 CrossRef CAS.
- (a) V. H. Rawal, J. A. Rao and M. P. Cava, Tetrahedron Lett., 1985, 26, 4275 CrossRef CAS; (b) V. B. Schmidt and D. Seebach, Angew. Chem., Int. Ed. Engl., 1991, 30, 1321 ( Angew. Chem. , 1991 , 103 , 1383 ) CrossRef; (c) K. Soai, H. Hori and M. J. Kawahata, J. Chem. Soc., Chem. Commun., 1992, 106 RSC; (d) D. Seebach, A. K. Beck, B. Schmidt and Y. M. Wang, Tetrahedron, 1994, 50, 4363 CrossRef CAS; (e) M. Yasuda, T. Fujibayashi, I. Shibata, A. Baba, H. Matsuda and M. Sonoda, Chem. Lett., 1995, 24, 167 CrossRef; (f) K. Soai, Y. Inoue, T. Takahashi and T. Shibata, Tetrahedron, 1996, 52, 13355 CrossRef CAS; (g) T. C. Chan, C. P. Lau and T. H. Chan, Tetrahedron Lett., 2004, 45, 4189 CrossRef CAS; (h) A. M. Piggott and P. Karuso, Tetrahedron Lett., 2005, 46, 8241 CrossRef CAS; (i) J. X. Wang, K. Wang, L. Zhao, H. Li and Y. Fu, Adv. Synth. Catal., 2006, 348, 1262 CrossRef CAS; (j) S. Chassaing, M. K. Stotz, G. Isorez and R. Brouillard, Eur. J. Org. Chem., 2007, 2438 CrossRef CAS; (k) K. Yoshida, H. Takahashi and T. Imamoto, Chem. – Eur. J., 2008, 14, 8246 CrossRef CAS PubMed; (l) H. Zheng, Q. Zhang, J. Chen, M. Liu, S. Chen, J. Ding, H. Wu and W. Su, J. Org. Chem., 2009, 74, 943 CrossRef CAS PubMed; (m) N. Ahlsten, A. Bartoszewicz, S. Agrawal and B. M. Matute, Synthesis, 2011, 2600 CAS; (n) M. Mineno, Y. Sawai, K. Kanno, N. Sawada and H. Mizufune, J. Org. Chem., 2013, 78, 5843 CrossRef CAS PubMed; (o) W. Zeng, M. Ishida, S. Lee, Y. M. Sung, Z. Zeng, Y. Ni, C. Chi, D. Kim and J. Wu, Chem. – Eur. J., 2013, 19, 16814 CrossRef CAS PubMed; (p) Z. Sun, S. Lee, K. H. Park, X. Zhu, W. Zhang, B. Zheng, P. Hu, Z. Zeng, S. Das, Y. Li, C. Chi, R. W. Li, K. W. Huang, J. Ding, D. Kim and J. Wu, J. Am. Chem. Soc., 2013, 135, 18229 CrossRef CAS PubMed.
- (a) S. Suga, D. Yamada and J. Yoshida, Chem. Lett., 2010, 39, 404 CrossRef CAS; (b) A. Nagaki, A. Kenmoku, Y. Moriwaki, A. Hayashi and J. Yoshida, Angew. Chem., Int. Ed., 2010, 49, 7543 ( Angew. Chem. , 2010 , 122 , 7705 ) CrossRef CAS PubMed; (c) J. Yoshida, K. Saito, T. Nokami and A. Nagaki, Synlett, 2011, 1189 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7re00142h |
| This journal is © The Royal Society of Chemistry 2017 |