A new reaction route for the synthesis of 2-methyl-5-ethylpyridine
Emanuele
Moioli
 *ab,
Leo
Schmid
b,
Peter
Wasserscheid
a and
Hannsjörg
Freund
a
*ab,
Leo
Schmid
b,
Peter
Wasserscheid
a and
Hannsjörg
Freund
a
aLehrstuhl für Chemische Reaktionstechnik, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstrasse 3, DE-91058 Erlangen, Germany. E-mail: emanuele.moioli@fau.de
bSpecialty Ingredients Research & Technology, Lonza Ltd., Lonzastrasse, CH-3930 Visp, Switzerland
First published on 29th August 2017
Abstract
In this work, a novel synthesis route to produce 2-methyl-5-ethylpyridine (MEP) from the cyclic acetaldehyde ammonia trimer (AAT) is explored. The reaction was studied in a semi-batch reactor in the presence of different promoters to adjust the pH of the reaction solution. Among various ammonium salts tested as promoters, ammonium acetate was identified as the most suitable promoter for the reaction. By using a Design of Experiments (DoE) approach, the temperature and concentration of reactants and the promoter were identified as the most important/decisive parameters influencing the course of the reaction. Additional mechanistic investigations were carried out to assess the effect of these parameters in detail and to clarify the by-product formation via oligomer formation.
Introduction
2-Methyl-5-ethylpyridine (MEP) is a largely produced pyridine base, currently used as an intermediate for the production of nicotinic acid and nicotinamide, commercially named vitamin B3 or PP. MEP is also applied in the production of 2-methyl-5-vinylpyridine, an intermediate for the production of resins.1–4 To date, the industrial production of MEP takes place via the so-called Chichibabin reaction, the condensation of aldehydes in the presence of ammonia.5 Early studies6,7 demonstrated the possibility to produce MEP by liquid-phase condensation of acetaldehyde in the presence of ammonia using ammonium acetate as a catalyst. On the other hand, it is not possible to produce MEP in high yield in the gas phase via the Chichibabin reaction, since the main products of this reaction are 2- and 4-picoline (2- and 4-methylpyridine).8–10 Nowadays, the industrial production of MEP takes place by the reaction of paraldehyde and aqueous ammonia in the presence of a catalyst. This reaction is carried out at 200–300 °C and 12–13 MPa.5 The yield to MEP of this reaction is about 70%, with the simultaneous production of small amounts of other pyridine bases (mainly 2- and 4-picoline). The use of paraldehyde allows for good reaction management, avoiding spontaneous self-oligomerisation that is typical for acetaldehyde.1,11 The reaction mechanism of MEP formation has been extensively studied, however, it is still under debate. All the proposed schemes involve the production of aldimine (CH3CHNH) from acetaldehyde and ammonia as the first step.1,12 This molecule tends to dimerise and trimerise by means of consecutive aldol reactions. At a certain chain length, the molecule undergoes cyclisation to form the skeleton of the pyridine base. The mechanism of this cyclisation step is not well described in the literature. It is, however, the key step to understand the selectivity of the reaction to MEP.In this paper, an alternative reaction route to MEP using acetaldehyde ammonia trimer (AAT) instead of paraldehyde and ammonia as feedstock is introduced. AAT is a cyclic molecule readily formed when acetaldehyde and ammonia are mixed at room temperature.13–15 This molecule can be isolated as a white solid and is soluble in water or methanol.14 AAT has various uses in organic synthesis16–18 and can be technically employed as a scavenger for sulfhydryl compounds.19 In a previous study,20 we reported the influence of pH on the formation of AAT and identified the optimal conditions for this reaction. To the best of our knowledge, the direct use of AAT in the Chichibabin reaction has never been reported in the literature as of today. In the following sections, we describe the development of this new process route in three steps: a) the fundamental demonstration of the feasibility of the reaction and analysis of promoter effects on MEP yield, b) the determination of the most influential parameters on MEP selectivity using a Design of Experiments (DoE) approach, and c) the detailed analysis of the mechanistic effect of these most influential parameters.
We also present a detailed comparison of the traditional MEP synthesis from paraldehyde (Fig. 1a) and the new route from AAT proposed in this work (Fig. 1b). For this purpose, for the initial experiments with AAT, the same promoters and operative conditions as those applied to the standard reaction from paraldehyde were chosen.5,20 The motivation to study this alternative reaction route and new process scheme for producing MEP stems from the observation that AAT is more reactive than paraldehyde but still easy to handle as a solid or in solution in solvents such as, e.g., methanol or acetonitrile.16 Another striking advantage is that the synthesis of MEP from AAT requires no additional ammonia or ammonia source since AAT already contains the nitrogen necessary for product formation. This offers potential to simplify the reactor set-up and the process scheme significantly. In the production scheme of MEP proposed here, ammonia is directly fed into the first reactor dedicated to AAT production. This allows the main reactor for MEP production to operate at lower temperature and pressure. This simplifies the overall process, avoiding the need to feed pure ammonia at high temperature and pressure.
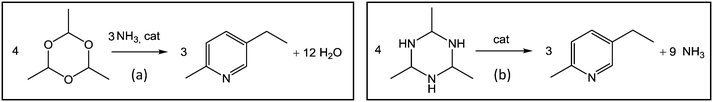 | ||
| Fig. 1 Schematic representation of the two reactions studied: (a) paraldehyde to MEP and (b) AAT to MEP. | ||
Experimental
Chemicals
All applied chemicals, acetaldehyde ammonia trimer, ammonium salts (ammonium sulphate, ammonium nitrate, ammonium chloride, ammonium phosphate, ammonium fluoride and ammonium acetate), ammonium hydroxide (25% wt), paraldehyde, methanol and acetic acid, were purchased from Sigma Aldrich in 99% purity (96% for AAT). They were used as-received.Preparation of MEP
The reaction of AAT and paraldehyde to produce MEP was carried out in a 300 mL Hastelloy autoclave. 100 mL of an aqueous solution of the promoter (ammonium salt or acid) was charged into the reactor and heated up to the reaction temperature (150–230 °C) under stirring at 1000 rpm. When the promoter solution reached the desired temperature, 100 mL of AAT or paraldehyde solution in methanol was fed using an HPLC pump at a flow rate ranging from 5 to 20 mL min−1. The pressure of the sealed reactor was not adjusted so that the reaction took place at the vapour pressure of the mixture. After addition of AAT or paraldehyde, the reaction was continued for two hours under heating and stirring. The reaction mixture was then cooled down to room temperature, the residual gas was released and the resulting solution was recovered for analysis. Throughout this study, the influencing parameters of the reaction were investigated via the same procedure as above, but varying the concentration of AAT or paraldehyde, concentration and type of the promoter and relative concentration of the two components.Analytics
Product analysis was carried out using an Agilent A6850 gas chromatograph system equipped with a FID (flame ionisation detector) and an HP-FFAP capillary column (25 m × 0.2 mm × 0.33 μm) using 3-picoline as an internal standard. This was possible since 3-picoline was not observed to be produced by the reaction. The main product of the reaction is MEP, followed by 2- and 4-picoline. Other peaks that were identified by means of GC-MS were assigned to other pyridine bases (e.g. other methyl-ethylpyridines and heavier pyridine bases). The oligomer by-products were analysed using an Agilent liquid chromatography/hybrid quadrupole time-of-flight/mass spectrometer (LC/HQ-TOF/MS).Results and discussion
Influence of heating and substrate addition
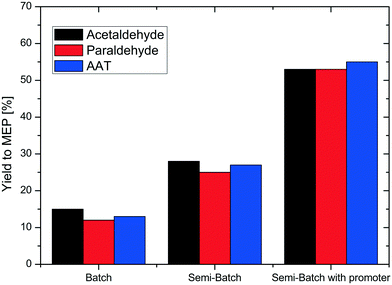
Fig. 2 shows the results of the reaction carried out in two different reactor systems, batch and semi-batch, and with three different reactants, acetaldehyde, paraldehyde or AAT. The first two sets of experiments were performed without addition of a promoter. Note that it was not possible to produce MEP in the presence of a promoter in a batch reactor as undesired oligomer formation consumed all feedstock already during the heating phase of the reactor. Without any ammonium salt promoter, the reaction in batch mode resulted in a low yield to MEP of approximately 15% independent of the use of acetaldehyde, paraldehyde or AAT as a substrate. Also without the promoter, significant oligomer formation was observed during the reactor heating phase, consuming most of the aldehyde precursor.To avoid the detrimental influence of a prolonged heating phase, we next performed the reaction in a semi-batch reactor. The solution containing the acetaldehyde, paraldehyde or AAT reactant was added over time by means of an HPLC pump to an equivalent volume of water at the reaction temperature in the autoclave (typical pressure in the reactor was 20–30 bar). In this way, improved but still low yields of MEP were obtained, not exceeding 30% for all three reactants. Consequently, the amount of the reactant lost during the heating phase can be quantified to be about 15%. An important parameter for the reaction to produce MEP is the pH of the aqueous reaction mixture. Due to the unbuffered presence of ammonia, the pH was always high in all previous reactions. Following a literature report describing the production of pyridine bases from aldehydes and paraldehyde,21 we performed our next set of experiments under semi-batch conditions, but with addition of ammonium acetate as a buffer system to adjust the pH. This indeed led to a strong increase of the MEP yield for all three substrates, obtaining up to 55% MEP in the reaction mixture. This clearly confirms the feasibility of the reaction route from AAT to MEP.
Promoter screening
To further optimize the MEP yield, solutions of several ammonium salts were tested as promoters. These solutions were prepared and no ammonia was added during the reaction. The results of the experiments are presented in Table 1. MEP is produced with over 50% yield in the presence of all ammonium salts selected. Interestingly, there is an increasing yield of MEP with increasing basicity of the ammonium salt counter-ion. The highest yield is obtained with ammonium acetate. The promoter is required to maintain the pH around 5–7 to favour the reaction towards MEP over the undesired formation of oligomers.| Catalyst | NH4HSO4 | NH4Cl | NH4NO3 | NH4H2PO4 | NH4F | NH4OAc | AcOH |
|---|---|---|---|---|---|---|---|
| MEP yield [%] | 50.3 | 53.6 | 55.8 | 56.4 | 56.8 | 58.9 | 57.5 |
In contrast to paraldehyde, AAT already contains nitrogen in its molecular structure so additional ammonia (or the ammonium ion) is in theory not required to form MEP. For this reason, a further experimental run was carried out using acetic acid instead of ammonium acetate as the promoter. The result of this run (Table 1, last column) is in line with the previous results leading to the important conclusion that the nitrogen atom found in the product originates from AAT and not from the ammonium promoter. Thus, any additive able to adjust the pH to the right value in the aqueous solution under reaction conditions is probably suitable for promoting the formation of MEP.
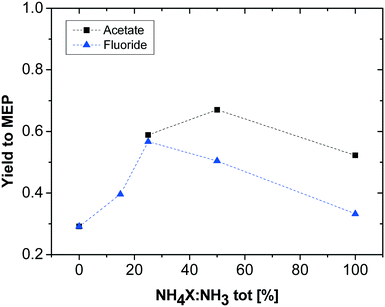
To further investigate the effect of pH on the reaction, some additional experiments with added ammonia and varying ratios of ammonia and ammonium salt were carried out. To exclude particular anion effects, the same set of experiments was performed with two different salts, ammonium acetate and ammonium fluoride. Keeping the overall molar amount of nitrogen in the promoter solution constant (and thus the ratio of AAT-based nitrogen to promoter-based nitrogen in the solution), the ratio of ammonium salts to ammonia ([NH4]X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3) was varied, from only ammonia (0% [NH4]X) to only ammonium salt (100% [NH4]X). The results of these experiments are shown in Fig. 3. As already noted during the first tests, the yield in the absence of a promoter (high pH) is low. The increase in ammonium salt content causes an increase in MEP yield until a maximum, which in turn is different for the two different ammonium salts. The different [NH4]X
NH3) was varied, from only ammonia (0% [NH4]X) to only ammonium salt (100% [NH4]X). The results of these experiments are shown in Fig. 3. As already noted during the first tests, the yield in the absence of a promoter (high pH) is low. The increase in ammonium salt content causes an increase in MEP yield until a maximum, which in turn is different for the two different ammonium salts. The different [NH4]X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 ratios for the maximum yield can be explained by the different pH values originating from the addition of the two different salts. Since the addition of ammonium fluoride results in a more strongly acidic solution than the addition of ammonium acetate, the solution with optimal pH is to be prepared with a lower quantity of fluoride salt compared to ammonium acetate. From these results, it is obvious that both too low and too high pH values are detrimental to the MEP yield, as in both cases undesired oligomerisation competes successfully with the desired MEP formation. Obviously, the maximum MEP yield is higher with addition of the acetate salt promoter compared to fluoride salt addition. Furthermore, the results reveal that the optimal pH range for maximising the MEP yield is between 8 and 9.
NH3 ratios for the maximum yield can be explained by the different pH values originating from the addition of the two different salts. Since the addition of ammonium fluoride results in a more strongly acidic solution than the addition of ammonium acetate, the solution with optimal pH is to be prepared with a lower quantity of fluoride salt compared to ammonium acetate. From these results, it is obvious that both too low and too high pH values are detrimental to the MEP yield, as in both cases undesired oligomerisation competes successfully with the desired MEP formation. Obviously, the maximum MEP yield is higher with addition of the acetate salt promoter compared to fluoride salt addition. Furthermore, the results reveal that the optimal pH range for maximising the MEP yield is between 8 and 9.
Determination of the most influential reaction parameters by a design of experiments approach
The following experiments were all carried out using ammonium acetate/acetic acid to promote the reaction and to adjust the pH to the right range. All experiments were performed in a semi-batch reactor. To determine the most influential parameters on MEP yield, a Design of Experiments (DoE) study was carried out. For this purpose, the initial run of the DoE was performed without a promoter to solely investigate at first the influence of temperature as well as AAT and ammonia concentrations. The extreme values of the reaction parameters were chosen in agreement with literature data referring to the process from paraldehyde.5 These values are reported in Table 2.| Factor | Letter | Low value | High value |
|---|---|---|---|
| Temperature [°C] | A | 160 | 200 |
| Ammonia [wt%] | B | 0 | 7.5 |
| AAT [wt%] | C | 2 | 10 |
The results are analysed by means of statistical methods to pinpoint the effects of the three factors on the MEP yield. The most efficient way to explain the variability of yield depending on the three factors is through a Pareto chart, which is shown in Fig. 4. Amongst the three parameters, temperature is the most relevant. An increase of temperature from the low set point to the high set point causes an average increase of yield from 15 to 20%. This is a highly significant result as the centre point of the experimental run is about 15% yield (as reported in the previous sections). Following temperature, the second most important parameter is AAT concentration. An increase of AAT concentration from 2 to 10% causes a decrease in yield from 18 to 14%. The negative influence of aldehyde concentration on yield is a well-known effect in pyridine base synthesis.22 In contrast, the effect of ammonia concentration is small. Only a slight increase of yield is obtained when ammonia is added to the reaction solution. This minor influence of ammonia is most probably due to the presence of nitrogen in excess in the molecule of AAT with respect to the stoichiometry of the reaction. The increase of nitrogen has only a limited impact on the extent of undesired side reactions. For this reason, ammonia is not considered as a very important influencing parameter in the reaction and the successive experiments were carried out without addition of ammonia to the solution.
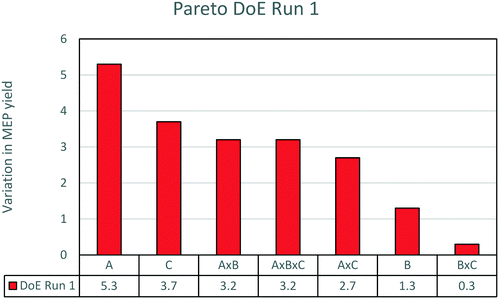 | ||
| Fig. 4 Pareto chart of the first DoE run. The effects plotted are the variation of yield due to the various factors. For the meaning of the letters, see Table 2. | ||
A second run of DoE was conducted to determine the parametric influence of temperature and AAT concentration in the presence of ammonium acetate. The boundaries of this set of experiments are reported in Table 3. The upper and lower limits for temperature and AAT addition are the same as in the previous case. The concentration of the added ammonium acetate promoter ranges from 0 to 5 wt%.
| Factor | Letter | Low value | High value |
|---|---|---|---|
| Temperature [°C] | A | 160 | 200 |
| NH4Ac [wt%] | B | 0 | 5 |
| AAT [wt%] | C | 2 | 10 |
The results of the experimental runs are displayed in Fig. 5. The influence of the promoter is the most important factor. The addition of 5% wt promoter causes an average increase in yield from 15 to 45%. This confirms that the promoter is necessary to reach a good yield to MEP. The effect of different amounts of the promoter will be discussed below. The decrease of AAT concentration between the two boundary conditions causes an increase in yield from 23 to 37%. This impact is much higher than that in the previous case without promoter addition. The influence of temperature is now the weakest of the three factors studied. The combined influences are very limited.
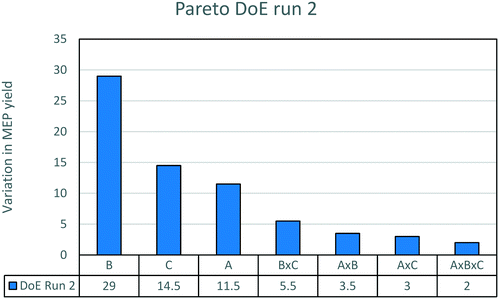 | ||
| Fig. 5 Pareto chart of the second DoE run. The effects plotted are the variation of yield due to the various factors. For the meaning of the letters, see Table 3. | ||
In a next set of experiments, we studied the feed flow rate of AAT. For this purpose, experiments with feeding rates ranging from 1 to 20 mL min−1 were carried out. The results are shown in Fig. 6. In this range, the yield is almost constant, so the feed flow rate is set to 10 mL min−1 for the following experiments.
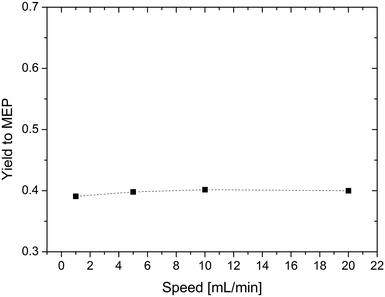 | ||
| Fig. 6 Effect of the feeding rate on the yield of MEP. Reaction conditions: T = 200 °C, concentration of reactant = 5% wt, and AAT/promoter ratio = 1.0 mol/mol. | ||
Mechanistic study of the reaction
For the following mechanistic study of the reaction, all experiments were carried out with both paraldehyde and AAT. This allows studying the two reactions in direct comparison to find out whether MEP production from AAT is a suitable alternative to the current reaction route from paraldehyde.The first parameter that we evaluated in detail is temperature. The experimental results are displayed in Fig. 7. The yield to MEP increases linearly in the range between 150–200 °C from 0.3 to 0.6. This is due to the increasing rate of MEP formation with temperature compared to oligomerisation. Note that for temperatures above 200 °C, the MEP yield decreases again due to other thermally induced side reactions. The temperature dependence of the reactions from AAT and from paraldehyde is comparable. Additionally, the type and quantity of side product formation are independent of the substrate. The high similarity of both reactions is probably due to the formation of a common intermediate that is produced from either of the two different starting materials.
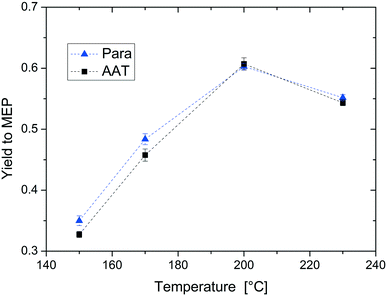 | ||
| Fig. 7 Effect of temperature on the yield of MEP. Reaction conditions: concentration of reactant = 5% wt, AAT/promoter ratio = 1.0 mol/mol, and feed flow rate = 10 mL min−1. | ||
The second parameter investigated during this mechanistic study is the concentration of the substrate (AAT or paraldehyde). The initial concentration of the reactant was varied from a low value (2% wt) to the solubility limit of AAT in methanol (ca. 20% wt). The results are shown in Fig. 8. The increase in concentration has a strong negative influence on yield. Obviously, too high concentration of the substrate pushes the balance between MEP formation and oligomerisation in favour of the latter. The GC-MS analyses of the product solutions show the presence of many different side products, all of them with a molecular weight higher than MEP. Again, the two different substrates behave very similarly under all conditions under investigation.
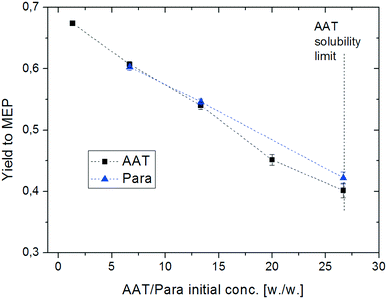 | ||
| Fig. 8 Effect of reactant concentration on the yield of MEP. Reaction conditions: T = 200 °C, AAT/promoter ratio = 1.0 mol/mol, and feed flow rate = 10 mL min−1. | ||
In a next set of experiments, we investigated in more detail the effect of different amounts of the added promoter. Three different scenarios have been studied: the reaction of paraldehyde in the presence of ammonium acetate, the corresponding reaction of AAT in the presence of ammonium acetate and the reaction of AAT in the presence of acetic acid. The corresponding results are displayed in Fig. 9.
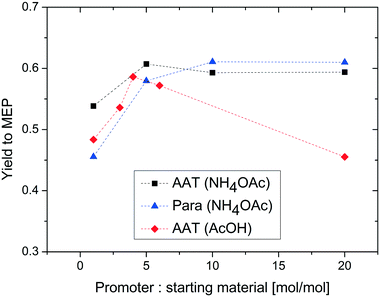 | ||
| Fig. 9 Effect of the reactant/promoter ratio on the yield to MEP. Reaction conditions: T = 200 °C, concentration of reactant = 5% wt, and feed flow rate = 10 mL min−1. | ||
To make the results comparable, the yield is plotted as a function of promoter-to-AAT or paraldehyde molar ratio. The three series of experiments show different results. The reaction of AAT in the presence of ammonium acetate leads to a high yield of MEP for the entire range considered. At lower promoter concentrations, there is a slight increase in yield, from 55 to 60%. This value remains constant when the concentration of the promoter is further increased.
The results obtained with paraldehyde are quite different. The yield at lower promoter concentrations is lower (ca. 45%) and an increasing trend is observed until the yield reaches a maximum of ca. 60% in large excess of the promoter. The yield in large excess of the promoter is comparable for AAT and paraldehyde, confirming the previous observations. The different trend at lower concentrations of the promoter is due to the stoichiometric effect of ammonia in the two reactions. While AAT does not require ammonia to yield MEP, paraldehyde needs ammonia to form the precursor for the pyridine ring. Consequently, the reaction of AAT to MEP is carried out in excess of nitrogen independent of promoter concentration, thanks to the presence of nitrogen in the molecule. In contrast, the assumption of a limited influence of ammonia is not valid for the reaction using paraldehyde as the substrate. This reaction requires the presence of much more ammonia to yield the highest amount of MEP.
To further assess the possibility to carry out the reaction of AAT to MEP in the absence of ammonia, the effect of acetic acid as a promoter was also studied. Interestingly, the results of these experiments show a different trend compared to those of the reactions using ammonium acetate. Here, the MEP yield shows a maximum at a stoichiometric ratio slightly lower than that in the case of ammonium acetate. This behaviour is due to a pH effect. For ammonium acetate, the increase of concentration has only a limited effect on the pH of the solution while the change in the concentration of acetic acid results in a stronger reduction of the pH. Note that the use of acetic acid as the promoter can yield the same results as those for ammonium acetate, but this promoter leads to stronger shifts in pH resulting in a smaller window of optimal yields. The use of acetic acid as the promoter allows carrying out the reaction with no additional ammonia source.
Oligomer characterisation
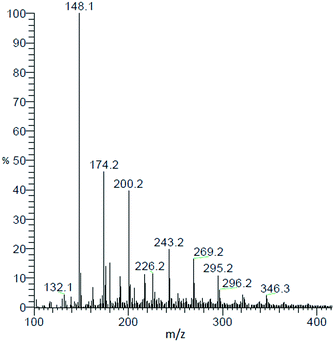
In the results described so far, a relevant fraction of the product mixture is composed of oligomeric by-products. Characterisation of this fraction is necessary to fully understand the course of the reaction and to draw the right mechanistic conclusions. LC-MS analysis allows for a qualitative description of the by-product formation. A representative MS spectrum of the reaction mixture obtained from the reaction of AAT and ammonium acetate is shown in Fig. 10. Only molecular weights higher than 121 (MW of MEP) are considered. The distribution of by-products follows a regular scheme, resulting from the consecutive addition of aldimine to the growing oligomer chain followed by the release of ammonia. The structures formed have the general formula CnHn+3N (MEP would correspond to n = 8). The intensity of the peaks continuously decreases from MW = 147 to MW = 225. After this latter value, the structure of the products changes to the formula CnHn+6N2 meaning that the molecules contain one more molecule of ammonia compared to the previous case.The elemental C, H and N content in the molecules is summarized in Table 4. This analysis is not sufficient to fully characterise the oligomer, but it supports the interpretation that all heavier products result from the same type of chain growth mechanism with the various species being produced by termination of the oligomerisation at different stages.
| m/z for various products | C | H | N |
|---|---|---|---|
| 147 | 10 | 13 | 1 |
| 173 | 12 | 15 | 1 |
| 199 | 14 | 17 | 1 |
| 225 | 16 | 19 | 1 |
| 242 | 16 | 22 | 2 |
| 268 | 18 | 24 | 2 |
| 294 | 20 | 26 | 2 |
| 346 | 24 | 30 | 2 |
Mechanistic conclusions
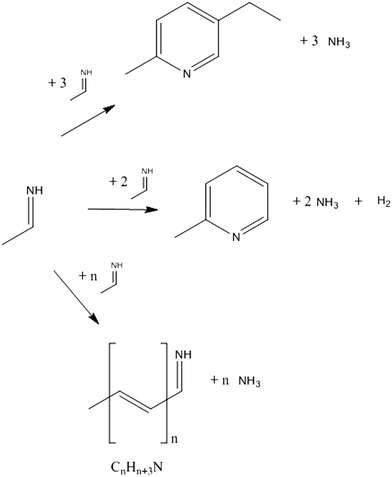
The proposed simplified reaction scheme for MEP syntheses from AAT and paraldehyde is shown in Fig. 11. Both AAT and paraldehyde decompose thermally. AAT directly forms aldimine. Paraldehyde decomposes to acetaldehyde in the presence of an acid catalyst, but decomposes to aldimine if the promoter is an ammonium salt. If the ammonium salt is not present in excess, paraldehyde reacts partially to aldimine and partially to acetaldehyde, which can further react in aldol condensation. The two decomposition reactions are equilibrium reactions. The intermediate formed can undergo an aldol-type reaction to form longer chain intermediates. The release of ammonia produces unsaturated imines, which can undergo further reactions. When four monomeric species consecutively react, it is possible that internal condensation to form the pyridine ring takes place. The special configuration of MEP (methyl and ethyl groups in the 1,4 relative position, respectively), together with the simplicity of the reaction (no dehydrogenation required), makes MEP the predominant product of the reaction, with only low production of other pyridine bases. The pathway to 2- and 4-picoline involves a high-energy dehydrogenation step, which makes MEP production the most favourable. This mechanistic advantage is represented in Fig. 12. If the ring formation does not take place producing MEP, the chain can further grow to form higher molecular weight species. The internal ring closure is in competition with the growth of the chain. This is the crucial step to determine selectivity. The polymeric structures are not well identified; they can be linear, branched or contain several ring entities. The longest chains are formed by the consecutive reaction of 8–10 monomers. Since these oligomers have an average molecular weight higher than that of MEP, an increase in reactant concentration causes an increase in formation of side products. Higher substrate concentrations lead to an increase in intermediate concentration promoting the reaction towards oligomers. This reaction proceeds already at low temperature, while the formation of pyridine bases requires temperatures higher than 170 °C. The temperature does not influence the reaction mechanism, but only the relative relevance of the two parallel reactions. For this reason, while the oligomer/MEP ratio changes with temperature, no different products are found when changing the temperature.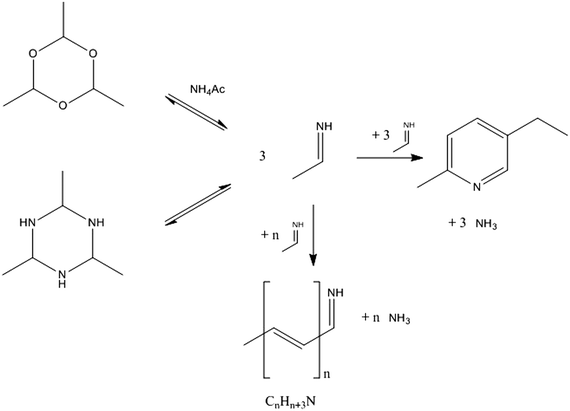 | ||
| Fig. 11 Proposed simplified reaction scheme for the formation of MEP and oligomer from AAT and paraldehyde. | ||
Conclusion
A novel reaction route for the synthesis of 2-methyl-5-ethylpyridine (MEP) is presented in this study. The route is based on/proceeds via a liquid-phase transformation of acetaldehyde ammonia trimer (AAT) in the presence of an acid promoter. The promoter was found to be necessary to obtain MEP in high yield. Ammonium acetate and acetic acid were identified as the most suitable promoters. The most relevant parameters for maximising the MEP yield are temperature and concentration of the reactant and the promoter. Under the reaction conditions of an isothermal and perfectly mixed reactor, the MEP yield starting from AAT or paraldehyde was found to be comparable. However, the use of AAT allows for carrying out the reaction without addition of ammonia or ammonium acetate. The absence of ammonia allows for applying a significantly lower operating pressure in the production of MEP and makes the downstream processing easier. Moreover, MEP production from AAT is more robust, e.g., the effect of varying ammonia and promoter concentrations is relatively limited.From the obtained results, we conclude that both AAT and paraldehyde form aldimine, which is the key intermediate in the reaction towards MEP. AAT quantitatively forms the intermediate, while paraldehyde decomposition depends on the quantity and type of the promoter used for the reaction. The reaction of aldimine to MEP is favoured by temperatures between 190 and 210 °C, while increasing substrate concentration favours the competing oligomer formation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Seventh Framework Program for research, technological development and demonstration under grant agreement no. 607114. The authors acknowledge the contribution of Lonza Analytics team for analytical measurements and especially S. Lobsiger and V. Ruppen.References
- J. I. Grayson and R. Dinkel, Helv. Chim. Acta, 1984, 67, 2100 CrossRef CAS
.
- R. S. Sagitullin, G. P. Shkil', I. I. Nosonova and A. A. Ferber, Chem. of Het. Comp., 1996, 32, 2 Search PubMed
.
- R. Kuhn and G. Wendt, Ber. Dtsch. Chem. Ges. B, 1938, 71, 1118 CrossRef
.
- P. Karrer and H. Heller, Helv. Chim. Acta, 1939, 22, 1292 CrossRef CAS
.
-
S. Schimizu, et al., Pyridine and Pyridine Derivatives, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012 Search PubMed
.
- A. Hanzsch, Justus Liebigs Ann. Chem., 1882, 215, 72.4 Search PubMed
.
- A. E. Chichibabin, Zh. Russ. Fiz.-Khim. O-va., 1905, 37, 1229 Search PubMed
.
- A. Nenz and M. Pieroni, Hydrocarbon Process., 1968, 47(11), 139–144 CAS
.
- R. L. Frank, F. J. Pilgrim and E. F. Riener, Org. Synth., 1963, 4, 451 Search PubMed
.
- T. Shoji and N. Abe, Petrotech, 1984, 7, 195 CAS
.
- M. I. Farberov, V. V. Antonova, B. F. Ustavshchikov and N. A. Titova, Khim. Geterotsikl. Soedin., 1975, 12, 1587–1592 Search PubMed
.
- D. C. Frost, B. MacDonald, C. A. McDowell and N. P. C. Westwood, J. Electron Spectrosc. Relat. Phenom., 1978, 14, 379–390 CrossRef CAS
.
- A. Nielsen, R. Atkins, D. Moore, R. Scott, D. Mallory and J. LaBerge, J. Org. Chem., 1973, 38, 3288–3295 CrossRef CAS
.
- W. Hull, B. Sykes and B. Babior, J. Org. Chem., 1973, 38, 2931–2939 CrossRef CAS
.
- H. Puchtler and S. N. Meloan, Histochemistry, 1981, 72, 321–332 CAS
.
- V. Vinogradoff, F. Duvernay, M. Farabet, G. Danger, P. Theulé, F. Borget, J. C. Guillemin and T. Chiavassa, J. Phys. Chem. A, 2012, 116, 2225–2233 CrossRef CAS PubMed
.
-
G. Gobron and R. Proust, US Pat., 3594420, 1971 Search PubMed
.
-
J. MacLachlan, P. Morken, W. Schmiegel and K. Takahashi, US Pat., 6281296, 2001 Search PubMed
.
-
M. Callaway and G. T. Rivers, US Pat., 5958352, 1999 Search PubMed
.
- E. Moioli, L. Schmid, P. Wasserscheid and H. Freund, React. Chem. Eng., 2017, 2, 382–389 CAS
.
-
C. Rosas, J. Wantuck and A. Kaufman, US Pat., 3846435A, 1972 Search PubMed
.
- X. Zhang, C.-W. Luo, C. Huang, B.-H. Chen, D.-G. Huang, J.-G. Pan and Z.-S. Chao, Chem. Eng. J., 2014, 253, 544–553 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2017 |