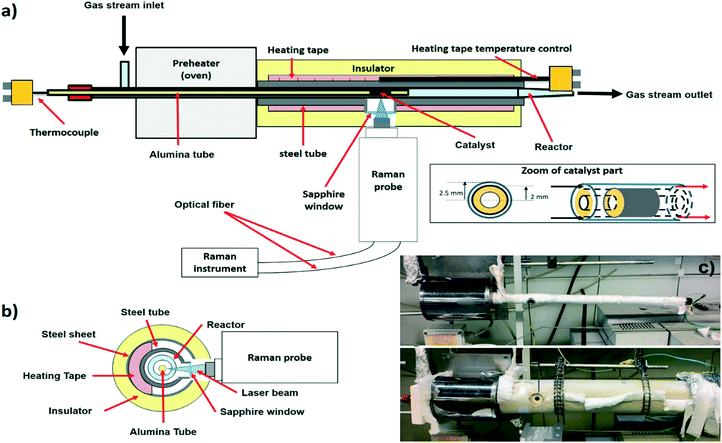
Design and testing of an operando-Raman annular reactor for kinetic studies in heterogeneous catalysis†
Ali
Maghsoumi
 a,
Andrea
Ravanelli
a,
Federico
Consonni
a,
Fabio
Nanni
a,
Andrea
Lucotti
a,
Andrea
Ravanelli
a,
Federico
Consonni
a,
Fabio
Nanni
a,
Andrea
Lucotti
 b,
Matteo
Tommasini
b,
Matteo
Tommasini
 b,
Alessandro
Donazzi
b,
Alessandro
Donazzi
 a and
Matteo
Maestri
a and
Matteo
Maestri
 *a
*a
aLaboratory of Catalysis and Catalytic Processes, Dipartimento di Energia, Politecnico di Milano, via La Masa 34, 20156, Milano, Italy. E-mail: matteo.maestri@polimi.it
bDipartimento di Chimica, Materiali e Ingegneria Chimica, Politecnico di Milano, Piazza Leonardo da Vinci 32, 20133, Milano, Italy
First published on 15th November 2017
Abstract
In this work, we present a novel experimental tool that integrates in situ Raman spectroscopy and an annular reactor for the operando-Raman kinetic analysis of heterogeneous catalytic reactions. The proposed configuration can monitor via Raman spectroscopy the catalytic surface under kinetically limited reaction conditions, with reliable product analysis, thus retaining the main features of both Raman spectroscopy and kinetic investigation in an annular reactor. We report a thorough description of the key constraints in developing online Raman spectroscopic tools for kinetic investigations. These constraints are considered in the design, assembly and testing of the experimental method by minimizing the mutual invasiveness of the Raman spectroscopy and of the annular reactor configurations. Show-cases of dry reforming and partial oxidation of CH4 on Rh catalysts are used to establish proof of concept of the method, demonstrating the acquisition of time-resolved Raman spectroscopic data under kinetically relevant conditions. Experiments both on clean and coked Rh surfaces reveal that well-structured graphitic deposits are likely to form during DR. During CPO, instead, the presence of O2 and H2O limits the formation of organized graphitic-like carbonaceous species. On a more general basis, this reactor allows a detailed structural characterization of a catalyst material during the reaction and at conditions of temperature, pressure and composition relevant to catalysis. Therefore, it is an important breakthrough for the simultaneous collection of spectroscopic and kinetically relevant data for the investigation of the structure–activity relationship in heterogeneous catalysis.
1. Introduction
In a gas–solid catalytic reaction, the active sites can adapt dynamically to the variations of the local chemical potential, and modifications involving the particle structure (both bulk and surface) can occur. Such a “living” character of the catalyst within the reaction environment has a major impact on the observed functionality.1 A significant breakthrough in the quantitative understanding of catalytic reaction mechanisms would be achieved by establishing methodologies which allow simultaneous investigation of the kinetics and of the state of the catalyst surface in terms of phases, morphologies or adsorbed intermediates. This in turn asks for coupling suitable spectroscopic operando techniques with kinetically controlled reactors.2 On model systems, spectroscopic studies substantially contribute in providing fundamental insights into the mechanisms of heterogeneous catalytic reactions,3,4 and several configurations are available for spectroscopic reactors.5 However, these studies usually imply that the reaction is performed under conditions that are far from those relevant to a simultaneous kinetic analysis. Typically, these cells are optimized for collecting spectroscopic information, but the fluid dynamic patterns either are poorly defined or lead to strong interactions between transport and kinetic effects. Additionally, parameters relevant for the quantification of the kinetic rates (e.g., catalyst load and temperature, gas hourly space velocity (GHSV), reactant conversion and product selectivity) cannot be accurately determined.5 Weckhuysen and co-workers pioneered the integration of several spectroscopic techniques with kinetically-informative catalytic reactors. Careful design features were applied to combine XRD, UV-vis and Raman probes with packed bed reactors mounting undiluted catalytic powders (e.g. Co/TiO2, CrOx/Al2O3) for various industrially relevant processes (Fischer–Tropsch, alkane dehydrogenation).6–11 Time-resolved and space-resolved variations of the surface composition, oxidation state, carbon coverage and phase could be revealed, and the relationship between these dynamic phenomena and the gas-phase composition was addressed, by sampling with a GC or a mass-spectrometer. In the case of Raman spectroscopy, experimental and numerical methods were demonstrated, which allow quantifying of the amount of species accumulated on the surface, either by comparison with the signal of a catalytically-inactive internal standard phase (boron nitrate), or by analysis of the diffuse reflectance of the spectra. As a matter of fact, the combination of operando characterization methods and catalytic reactors has been mostly applied for the detailed investigation of degradation, contamination and conditioning effects, and benefits arise from the local resolution achieved. Despite this important breakthrough, the application of operando spectroscopic reactors devoted to quantitative mechanistic analysis is still limited and requires additional design solutions. For instance, in the case of highly exo- or endo-thermic reactions, the control of radial and axial temperature gradients becomes critical, preventing significant kinetic data collection: the catalyst must be diluted with an inert material, causing an almost complete loss of relevant spectral signals. Capillary micro reactors are adopted for combined X-ray and Raman experiments:12–14 although this configuration approximates the ideal plug flow, giving access to intrinsic reaction rates, inaccuracies arise when estimating the very small catalyst load and its temperature. Also in this case, the extraction of relevant kinetic data is hindered. By reversing the problem viewpoint, Geske et al.15 adapted a Raman probe to the reactor design, and realized a novel spatially-resolved operando Raman technique. This technique allows collection of profiles of temperature, gas-phase composition and catalyst oxidation state along the axis of an adiabatic packed bed reactor. The key-role of axially-resolved measurements clearly emerges, but the requisites of the reactor and the catalyst support (spheres) forbid isolation of chemical-kinetic effects from mass transfer and diffusive limitations. On a more general basis, several additional literature examples suggest that an appropriate cell design is a requisite to collect kinetically informative data.2,16,17In order to guarantee kinetically-informative data and to achieve a successful spectro–kinetic coupling, the reactor design must comply with some specifications: (i) no catalyst bypass should be present, i.e., undesired dead volume or preferential paths of the gas flow must be avoided; (ii) optimal temperature control over the catalyst zone should be realized, in order to guarantee near isothermal operation; (iii) pressure drop should be absent or almost negligible, in order to achieve high GHSV; and (iv) the onset of mass or energy transfer and diffusional limitations should be avoided, since they hinder an exact elaboration of kinetic data.3,5 Considering these points, the annular reactor appears as an ideal choice, since it is a well-established experimental configuration which overcomes the kinetic limitations of both traditional packed bed reactors (high pressure drops, low GHSV, limited control of temperature gradient) and capillary reactors (limited reliability on signal quantification).18–21
The aim of this work is the presentation of the design and testing of a novel tool of investigation, which combines the annular reactor with in situ Raman spectroscopy: in this way, the set-up allows monitoring of the catalytic surface under kinetically limited reaction conditions, with reliable product analysis. Among the number of characterization techniques that provide a detailed understanding of the relationship between the structure and the activity of a catalyst, Raman spectroscopy stands out as very informative and rather advantageous. In fact, being a vibrational-spectroscopy method, this technique provides highly detailed molecular information in all phases and over a wide range of temperatures and pressures.22 It also allows the identification of organic and sulfur compounds, and can provide pieces of evidence about the structure of both the C-aggregates and of the S-compounds, as well as about their interactions with the catalyst during a deactivation event.23 Additionally, Raman spectroscopy appears to be well suited for in situ measurements, since it is little invasive and it does not require complex constructive constrains (indeed, the gas phase gives negligible scattering and the scattering of glass and quartz is rather weak). Thanks to these features, together with the possibility of bringing the probe as close as possible to the catalyst surface (offered by modern fiber optics), Raman spectroscopy is commonly identified as one of the most powerful techniques for surface characterization of working catalysts.17,24
We report the detailed presentation of the design, assembly and testing of the operando-Raman annular reactor. Kinetic, optical and material constraints must be fulfilled to design this new configuration and reach the required targets. In section 2, these constraints are outlined and the experimental setup is described. Particular attention is given to detailing the focusing procedure (section 3.1). Also, the local and general heating effect of the laser has been considered and analysed. Next, in sections 3.2 to 3.5, a proof of concept of the novel set-up is provided using dry reforming (DR) and catalytic partial oxidation (CPO) of CH4 on a 4 wt% Rh/α-Al2O3 catalyst as case-studies. These case-studies are used to establish a proof of concept of the method, demonstrating the acquisition of time-resolved Raman spectroscopic data under kinetically relevant conditions.
2. Design of the Raman-annular reactor
Several different constraints must be taken into account to obtain reliable kinetic information and Raman spectra. The set-up of the operando Raman annular reactor is reported in Fig. 1. All of the parts of the set-up and the related constraints are described in the following sections.2.1. Annular reactor
The reactor consists of a hollow alumina tube (35 cm long, 4 mm external diameter, inset of Fig. 1a) gas-tight sealed at one end. The catalyst is coated on the outer surface of the alumina tube as a thin layer (20 mm long), located near the tip of the tube (20 mm off) to enhance the pre-heating of the incoming gas. The coated alumina support is coaxially inserted into a quartz tube (5 mm internal diameter, inset of Fig. 1a). The gas stream flows along the annular duct, between the alumina tube and the internal wall of the quartz. The regular geometry of the reactor guarantees a well-defined, parallel laminar flow pattern with negligible pressure drops. In this way, tests with very high GHSV (1 up to 5 × 106 Nl kgcat−1 h−1) can be performed to investigate the kinetics of fast reactions, like total oxidations and partial oxidations. External mass transfer limitations are avoided due to the small annular gap (0.5 mm channel height), while internal diffusive limitations are negligible thanks both to the thin catalyst layer (10–30 μm) and to the low surface area of the support (α-Al2O3 in the present work). The extent of the axial temperature gradients is moderated by efficient heat dissipation via radiation and conduction along the alumina tube. Further reduction of the temperature gradients is achieved by dilution of the feed stream with an inert gas, allowing nearly isothermal profiles. Given that thermal equilibrium is established in the cross section, the reactor design permits the rigorous measurement of the catalyst superficial temperature, sliding a K-type thermocouple inside the hollow alumina tube. Thanks to this configuration, the gas stream is in contact longitudinally with the catalytic wall and very high space velocities can be obtained without building any pressure drop along the catalytic bed. As a consequence, it is possible to operate in a kinetically-informative regime, even when fast and strongly exo- or endo-thermic reactions are performed. For these reasons, the annular reactor overtakes the limits of the traditional (not ultra-diluted) packed bed configurations, wherein hot spots and strongly non-uniform temperature profiles occur at high conversions and the onset of large pressure gradients prevents the use of high flow rates.The annular reactor configuration must be adequately adapted to meet the Raman requirements (section 2.2). Fig. 1c shows a picture of the set-up. Usually, the annular reactor is inserted in an oven, such that the catalyst is located in correspondence of the central isothermal zone.19–21 The transmission of the Raman signal scattered by the catalyst surface requires the opening of a window in proximity to the catalyst. Such a window can strongly affect the behavior of the oven, especially with respect to the kinetic need of realizing an isothermal zone in correspondence of the catalyst. Thus, a classical oven configuration could not be used and a special oven was realized to tackle this issue. The quartz reactor is coaxially inserted in a stainless-steel tube (AISI 310S, 15 mm external diameter, 1.5 mm thick), which is wrapped by a temperature-controlled heating tape (Isopad IT-H, 150 W m−1 power, 900 °C maximum operating temperature). The hole is drilled on the steel tube and a sapphire window (12 mm diameter, 4 mm thick) is tightened to limit the heat dissipations and to protect the Raman objective from overheating. Upstream of the steel tube, a tubular oven (150 mm long, 1000 °C maximum temperature) is used as a pre-heater. A cylindrical shell of a ceramic microporous insulator (Microtherm MPS) is clamped around the steel tube to minimize thermal dissipations. A K-type thermocouple is connected to the control loop of the heating tape (Eurotherm 2132) and placed in correspondence of the catalyst. Isothermal conditions are maintained by appropriate selection of the oven and heating tape set points. The absence of homogeneous reactions both upstream (in the oven) and downstream (in the heating tape) has been carefully evaluated for CH4 DR and CPO conditions, which are used in this paper as show-cases (ESI† Fig. S1). Four lines (equipped with pressure-reducers, check-valves, filters, mass flow meters) feed the gaseous species from high-pressure tanks to the annular reactor. For the quantitative analysis of the gas composition, the reactor is connected to a gas chromatograph (6890, Hewlett Packard) equipped with a Porapaq column and a molecular sieves column.
2.2. Raman instrument
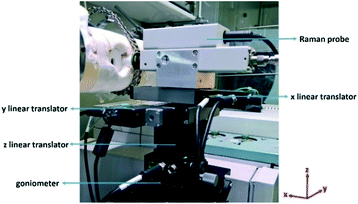
A JASCO NRS-4100 Raman spectrometer equipped with an external probe has been used. The Raman spectrometer is coupled with a laser providing a 488 nm excitation (Sapphire SF CDRH USB laser, single-frequency optically pumped semiconductor laser). This laser wavelength allows measuring of Raman spectra at high temperature (800 °C), for which the very strong black-body radiation background in the NIR/red range hinders the detection of the Raman signal.25 Additional information is provided in the ESI† Fig. S2.The external Raman probe is essential to collect the radiation as close as possible to the catalyst surface, by focusing on the catalyst layer through the sapphire window (section 2.1 and Fig. 1). The external probe is connected to the main instrument by two optical fibers, one for shining the laser and another one for collecting the Raman light. Based on this operational mode, the catalyst is analysed in a back-scattering geometry. The external probe is equipped with a 20× objective (Olympus SLMPLN20×) with the longest commercially available working distance (WD), which is 2.5 cm. The intensity of the Raman spectrum will be higher by using a shorter working distance. However, a minimum limiting distance exists due to the fact that the closer the probe is to the radiation source, the higher the overheating is. Both the objective and the Raman probe are indeed designed to work at room temperature. Long-time exposure to temperatures higher than 100–150 °C can irreversibly damage the objective (due to the presence of coatings and glues in the lens structure) and the Raman probe (due to the presence of several electronic and optical devices). Thanks to a unique combination of mechanical and optical properties,26 the sapphire window protects the probe from convective overheating and allows transferring of the Raman signal. Additionally, the objective is protected by radiative overheating with a cooling system, consisting of a N2 flow fluxed directly over the objective. Given the importance of a correct focusing (section 3.1), the external probe must be moved with a high precision and must be maintained in a fixed position. The supporting system is shown in Fig. 2. The probe is moved along the x, y and z directions by using three micrometrical linear translators (two Zaber X-LHM050A for x and y, one Zaber X-VSR20A for z). The linear translators are digitally controlled with a software. A goniometer (GOH-65A100-M6-OPTOSIGMA) allows tilting of the angular direction of the probe, in order to correct non-perfectly axial positions with respect to the catalyst. By using these four degrees of freedom, the laser beam is focused on the catalyst surface as a circular spot of approximately 2 μm diameter (the so-called focusing point). The laser power is always of the order of a few mW to prevent local laser-induced effects on the catalyst structure and on the reaction.
2.3. Catalyst preparation
4 wt% Rh/α-Al2O3 was used in the experiments. The α-Al2O3 support is typically chosen for its thermal stability and low surface area, which prevent the inclusion of Rh particles at the highest temperatures. The α-Al2O3 support was prepared by calcining commercial γ-Al2O3 powders (96–98%, Puralox SBA-200, Sasol) in air at 1100 °C for 10 h. The phase composition was verified by XRD, the superficial area (8.1 m2 g−1) by BET analysis, and the pore size distribution by Hg porosimetry. The pore diameter size distribution was centered around 216 nm, and the pore volume amounted to 0.21 ml g−1. The catalyst was prepared by dry impregnation of the α-Al2O3 support with a commercial Rh(NO3)3 solution (9 wt% Rh, Chempur). Two impregnation steps with intermediate drying were necessary to achieve the target 4 wt% Rh load. After impregnation, the powders were dried at 110 °C for 3 h. The dried powders were then deposited in the form of thin layers (10–50 μm) on the alumina tubular supports by a dip-coating procedure, described in detail elsewhere.19,27 The main steps are briefly summarized as follows: a slurry was prepared by adding the catalyst powders to an acidic solution (HNO3/powder = 1.7 mmol g−1, H2O/powder = 1.7 g g−1); the slurry was ball-milled for 24 h; the alumina tubular supports were coated by dipping into the slurry and extracting at constant velocity; and the coated tube was flash-dried at 280 °C for 10 min, obtaining a well-adhered catalyst layer. The Rh loading of the catalyst was verified by atomic adsorption analysis (AAS) of the flash-dried powders.3. Testing of the Raman-annular reactor
Next, three case studies are used to establish a proof of concept of the method presented in section 2, in order to demonstrate the acquisition of time-resolved Raman spectroscopic data under kinetically relevant conditions. In particular, first we present the procedure to correctly focus the laser beam on the catalyst surface. This procedure is detailed in section 3.1. Then, we present tests to show the capabilities and the potentialities of the operando Raman annular reactor. As a first show-case, we report the analysis of induced dynamic structural changes of the catalyst surface (section 3.2). To this aim, the catalyst surface was purposely partially covered with carbon by cracking pure methane, and then cleaned by a supply of air: this test allows proving of the capability of the system to follow rapid dynamics at the catalyst surface. As the second and third show-cases, the catalyst surface was monitored during CH4 CPO experiments and CH4 dry reforming (DR) experiments by using both a clean catalyst and a catalyst partially covered by coke (sections 3.3 and 3.4). These tests allowed verification of the reliability of the annular reactor and showed the sensitivity of the Raman spectroscopy in detecting the formation of carbonaceous species during the reaction.3.1. Focusing procedure
The proper angular alignment of the Raman probe is a key constraint (Fig. 3a). Indeed, if the laser beam is tilted with respect to the catalyst surface, a fraction of the back-scattered signal might get lost (Fig. 3b). To avoid this loss, a systematic focusing procedure was established. First, the camera is fixed at 0° with reference to the ground by using the goniometer. Then, the camera is brought in axis with the reactor by moving the probe vertically (z direction in Fig. 2). Finally, the focus on the catalyst surface is achieved by shifting the probe back or forth, along the x direction. With this set-up, it is also possible to move the probe along the axis of the catalyst (y direction) and record Raman spectra at different longitudinal points.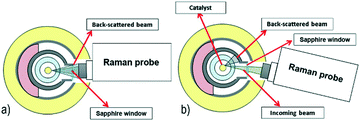 | ||
| Fig. 3 Sketch of the laser alignment in the Raman operando experimental setup. a) Correct alignment. b) Undesired tilted alignment. | ||
Two methods are used to prove that the camera is correctly focused. The first proof is provided by checking the consistency of the distance between fixed and known focusing points, as detailed below. The camera allows observation of the laser focusing point on various layers, while the probe is moved towards the catalyst surface (which is the last focusing point). The Raman spectra of each of these layers were recorded and are shown in Fig. 4: four focusing points can be distinguished before the catalyst surface, namely, the outer and the inner walls of the sapphire window and of the quartz reactor (named 1 to 4). The distance between the focusing point on the catalyst surface and the focusing point of the inner wall of the quartz reactor was independently verified (based on the position of the x linear translator at focusing points) and amounts to 650 μm. This value corresponds to the geometrical distance given by the reactor set-up, which is 500 μm (Fig. 1A), plus a correction of 150 μm, related to the refractive index of the sapphire window and of the quartz reactor. If the distance measured by the camera is not 650 μm, a wrong focus is achieved and the correct position must be reached by moving the probe along the vertical coordinate. Additionally, the contribution of the four focusing layers must not be neglected when interpreting the spectra of the catalyst surface. As a general result, the contribution of the background is limited for Raman signals with wavenumbers higher than 1500 cm−1, while care must be adopted in the interpretation of signals with wavenumbers lower than 1500 cm−1. The second proof is provided by comparing the spectrum of the blank alumina tube recorded outside the reactor, i.e. ex situ, with the one recorded inside the reactor, i.e. in situ. If a complete match of both Raman peak wavenumbers and relative intensities is achieved, the focusing is complete. Additional details and information are reported in Fig. S3 of the ESI.†
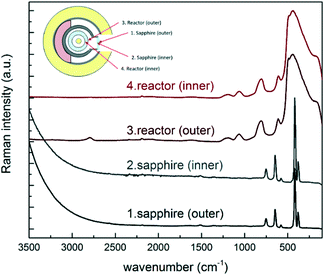 | ||
| Fig. 4 Raman spectra of the outer and inner surfaces of the reactor and of the sapphire window. The spectra have been normalized and offset for better display. | ||
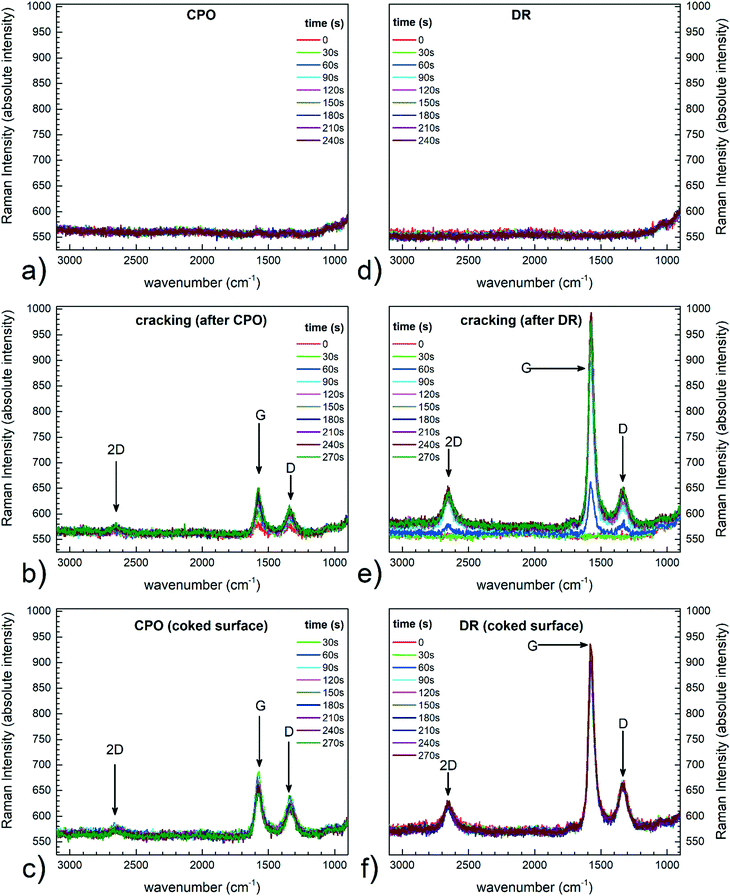
3.2. Methane cracking and oxidation
In order to observe clear and intense variations in the Raman spectra with time on stream, a reaction leading to strong carbon deposition, such as methane cracking, was performed. Raman spectroscopy is indeed a very informative technique for the characterization of carbonaceous species, including coke deposits on catalysts. The Raman spectrum of carbonaceous species features characteristic signals (named G and D), whose relative intensities, peak position and width carry useful information on the nanostructures.28,29 The reactor was heated up to 500 °C under a nitrogen flow. Once steady-state temperature conditions were reached, a 100 Ncc min−1 methane flow was supplied to the reactor, and time-resolved Raman spectra were recorded. Each spectrum was recorded by 1 accumulation of 30 seconds of laser excitation. As reported in Fig. 5a, Raman spectra were recorded at different times after switching to methane. The Raman spectrum of the catalyst recorded at time t = 0 before methane admission is also reported for comparison. The two intense peaks that steadily grew at around 1590 and 1350 cm−1 are the G and the D peaks, respectively. The intensity of these peaks increased until a steady-state level was reached after 180 seconds. After the Raman peaks of the deposits reached a stationary intensity level, the feed was switched to 100 Ncc min−1 of air, with the aim of capturing their progressive removal via oxidation. The recorded time-resolved Raman spectra are shown in Fig. 5b. A progressive decrease in G and D peak intensities was observed. After 120 seconds under air flow, no residual peaks related to carbon deposition could be detected. The results confirmed that the system was able to record Raman spectra at high temperature, under in situ conditions.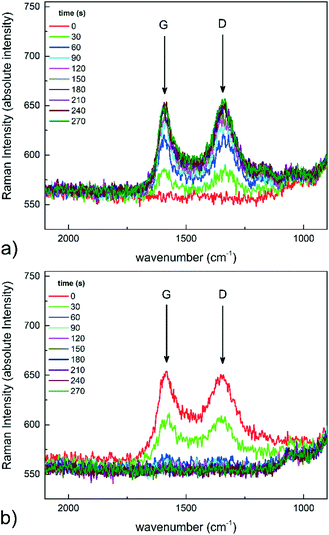 | ||
| Fig. 5 Time-resolved Raman spectra: a) methane cracking at 500 °C, and b) oxidation in air at 500 °C. | ||
3.3. CH4 CPO operando-Raman experiments
In order to check whether the system worked properly as an annular reactor, standard CH4 CPO tests were carried out first without recording Raman spectra. Details of the experiment and the molar fractions of reactants and products at the exit of the reactor have been reported in the ESI† section 4. By comparison with previous results,19,20 it was possible to confirm that the new set-up worked properly as an annular reactor. The catalyst surface during CH4 CPO at 650 °C was then analysed with Raman spectroscopy, while simultaneously measuring the outlet gas composition with the chromatograph. It is worth noticing that the Raman analyses were targeted to a wide region of the spectrum, but relevant signals appeared only in the range pertinent to carbon species. In this latter case, the catalyst surface was scanned to trace spots where carbon could be found, due to the inhomogeneous nature of the deposits. Initially, a fully clean surface was found, which was taken as a reference. Then, the catalyst was partially covered by carbon deposition via CH4 cracking; finally, the coked catalyst was exposed again to CH4 CPO conditions at 650 °C, to verify the occurrence of any possible cleaning effect related to the mixture and to assess any effect on the activity of the catalyst material. The conversions and the molar fractions measured at steady state are reported in Table 1. In the tests, the temperature was ramped from 25 °C to 650 °C under a N2 flow. Then, at 650 °C, the CH4 CPO mixture was admitted to the reactor (CH4 = 8.8%, O2/CH4 = 0.52, N2 to balance, GHSV of 4 × 105 Nl Kgcat−1 h−1) and Raman spectra were recorded every 30 seconds. As reported in Fig. 6a, a clean conditioned catalyst was first analysed: as expected,30 no signal associated to carbon deposition was detected. This result was expected, given that CH4 CPO can be performed on Rh-based catalysts with almost no coke deposition under more concentrated conditions, even up to CH4/air mixtures. Subsequently, O2 was removed from the CPO mixture and carbon deposition began due to CH4 cracking. The G and D signals (Fig. 6b) associated to the carbonaceous species immediately appeared, followed by a peak related to the overtone of the D peak (2D, 2800 cm−1). The intensity of these signals increased with time on stream and reached a steady level after 4 minutes. Further cracking did neither result in an increase of the signal intensity, nor in the appearance of other peaks. This in turn suggested that no variation of the carbonaceous species occurred, for instance due to organization of the structures, and that the exposure to the cracking atmosphere was short enough to avoid excessive coverage of the surface. After CH4 cracking, the O2 flow was restored to the one of the CH4 CPO experiments (Fig. 6c). A minor effect on the Raman signals of the carbonaceous species was observed (the G, D and 2D peaks remained almost unaltered), suggesting that there was very limited removal of the deposits. The presence of carbon on the surface led to a decrease of the catalyst performance in terms of CH4 conversion (from 75% to 70%) and syngas production (Table 1): this decrease indicated that coking caused the observed loss in the catalyst activity, but the exposure to the cracking atmosphere was not long enough to cause an irreversible damage. More importantly, although the partial oxidation mixture contained O2, no cleaning effect was detected locally, aside from a limited decrease of the G peak intensity. Hence, the O2 amount was not enough to skip the preferential reaction with CH4 and selectively remove coke. Prolonged exposure to the CPO mixture did not modify the picture.| CPO (%) (clean catalyst) | CPO (%) (coked catalyst) | CPO (%) (equilibrium) | |
|---|---|---|---|
| CH4 conversion | 75.4 | 70.4 | 89.9 |
| H2 molar fraction | 10.0 | 9.1 | 13.4 |
| CO molar fraction | 4.6 | 4.1 | 6.4 |
Noteworthily, although the absolute intensity of the Raman signals cannot be taken as a straightforward indication of the amount of C species, the focusing procedure allowed maintaining the same measuring distance in all the experiments, meaning that it was reasonable to assume identical optical conditions, and therefore that the surface did not experience significant carbon removal during the second CPO runs. The absolute intensity of all the Raman spectra can be fairly taken into account, thanks to the stability of the instrument background (Fig. 5 and 6a). The stable background is related to the good control of the optical setup and focusing. Several factors can influence the optical properties of the surface and the associated Raman signals: most relevantly to the CPO case, the optical properties of the surface may vary due to chemical modifications (i.e. it gets covered by sp2 carbon), or due to morphological changes over the nm range (e.g. loss of particle dispersion). Considering that the catalyst was fully conditioned,31 the morphology of the nanoparticles did not change much, thus allowing association of the changes exclusively to C formation. Additionally, as also supported by the moderate loss of CH4 conversion experienced after the cracking period (Table 1), the extent of coking was moderate and did not lead to a significant modification of the overall scattering background,8 which is instead expected to vary when the surface becomes blacker and less prone to scattering. As proved by the results of Fig. 7, in the CPO experiments, the relative changes in the ID/IG ratio maintained, on average, constant with the time on stream around 0.6, and did not change much during and after CH4 cracking. This result confirms that the signal from the surface was little influenced by optical issues (reflectance) as well as coking, and also shows that no relevant changes occurred in the chemical structure of the carbon species. Possibly, the CPO atmosphere helped in cleaning the coked surface, thanks to the O2 content and H2O formed during the reaction, which allowed limiting the formation of organized graphitic-like carbonaceous species, and which avoided further accumulation.
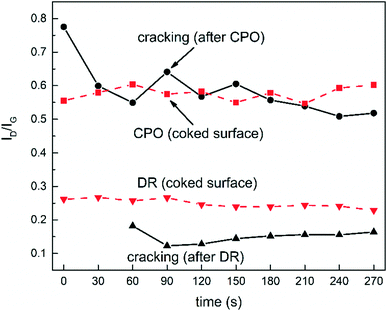 | ||
| Fig. 7 Variation of ID/IG over time for the Raman spectra of Fig. 6. | ||
3.4. CH4 dry reforming operando-Raman experiments
The dry reforming (DR) of methane was performed at 650 °C, following the same procedure outlined for the CPO tests. First, the fully conditioned and clean catalyst was analysed with Raman spectroscopy during DR; then, the catalyst was partially deactivated by carbon deposition; finally, the coked catalyst was re-exposed to the DR atmosphere and monitored. The aim of the test was to verify the occurrence of either cleaning or coking effects due to the presence of CO2 in the reacting mixture. The temperature was ramped from 25 °C to 650 °C in the presence of a N2 flow. The CH4 DR tests were performed with diluted feed streams (CH4 = 9.2%, CO2/CH4 = 1.12, N2 to balance, 4 × 105 Nl Kgcat−1 h−1 GHSV), and the Raman spectra were recorded every 30 seconds. The steady state conversions and molar fractions measured by GC are reported in Table 2. As in the experiments with CPO, the starting situation was a clean surface, with no traces of deposited carbon: this confirmed that dry reforming is feasible on Rh/α-Al2O3 catalysts with no deactivation due to coking (Fig. 6d). The CH4 cracking mixture was obtained by removal of CO2. Immediately after the removal, carbon deposition began and the G, D and 2D signals appeared. The intensity of these signals increased and reached the steady level approximately in 4 minutes, hence taking the same time of the CPO tests (Fig. 6e). Once the signal did not show any further variation, CO2 was re-admitted in the mixture and the catalyst surface was again monitored with Raman (Fig. 6f). No beneficial effect was observed, with the signals unaltered with the time on stream. Coking severely reduced the catalytic activity, almost halving the reactant conversions and the syngas production (Table 2). A comparison between the Raman signals obtained upon re-admission of the mixture (Fig. 6cvs. f) reveals that clearer and more intense peaks were observed during DR. Given that the focusing procedure was the same, this indication possibly suggested that the dry reforming atmosphere had a role in producing more ordered forms of carbon deposits with different compositional features. Also in this case, the analysis of the ratio between the intensity of the G and D peaks allowed further insight, due to the limited modifications of the background signal. Fig. 7 shows that the ID/IG ratio of DR experiments was less scattered than in CPO, and that the evolution with time on stream was clearly defined. During the cracking period, the ratio showed an initial decrease and then stabilized at 0.1; after CH4 cracking, upon re-exposure to the dry reforming atmosphere, the ratio maintained constant at 0.25. A higher ID/IG ratio means that more amorphous graphitic carbon was present, suggesting that the dry reforming atmosphere had a role in modifying the deposits by attacking or degrading the graphitic structure. Comparatively, however, the ID/IG ratio of DR experiments was lower than the ID/IG in CPO, meaning that well-structured graphitic deposits were formed in DR. The 2D peak was also significantly more visible in DR than in CPO, in line with the more ordered character of the carbonaceous species. These pieces of evidence possibly suggest that CO2 still played a role in cleaning the surface, although less efficiently than O2, leaving a higher fraction of less reactive graphitic structures. The poor, although stable, performance observed after CH4 cracking is coherent with this picture: after dropping, the CH4 conversion could not be restored in DR, while the exposure to the CPO atmosphere allowed limiting the conversion loss. Finally, it is interesting to note that, although CH4 cracking was performed following identical procedures, the ID/IG ratios maintained on two distinct levels (≥0.5 before and after CPO, ≤0.25 before and after DR): this difference possibly indicates that the initial DR and CPO exposure (that is, the history of the catalyst) led to surfaces with different characteristics, which in turn influenced the formation of the carbonaceous species.| DR (%) (clean catalyst) | DR (%) (coked catalyst) | DR (%) (equilibrium) | |
|---|---|---|---|
| CH4 conversion | 60.4 | 31.1 | 84.8 |
| CO2 conversion | 54.3 | 29.2 | 84.4 |
| H2 molar fraction | 8.8 | 4.3 | 12.5 |
| CO molar fraction | 9.9 | 5.2 | 13.9 |
Aside from the specific consequences of coking, on a general basis, these show-cases demonstrate the possibility of the acquisition of time-resolved Raman spectroscopic data under kinetically relevant conditions, thus relating structural changes with activity measurements. This information is expected to play a significant role in enabling fundamental understanding of mechanisms of catalyst materials under reacting conditions.32
4. Conclusions
An operando Raman annular reactor is presented in this work. This set-up combines the annular reactor configuration with in situ Raman spectroscopy: in this way, information on the status of the catalyst surface can be obtained, while collecting data under kinetically relevant conditions. The full potential of the annular reactor is exploited: on the one hand, it allows quantitative measurement of the product and reactant amounts, overtaking the limits of capillary microreactors. On the other hand, it is ideal for coupling with a Raman spectrometer, since it allows direct exposure of the catalytic surface to the laser excitation source. The Raman measurements are carried out in a back-scattering geometry, by means of a movable probe equipped with a high-precision positioning system, with an appropriate objective, and with a dual optical fiber line-up to supply the laser and collect the radiation. The dedicated design of the reactor furnace minimizes the heat dissipation and permits gathering of the largest amount of Raman scattered radiation. Specific procedures are developed in order to correctly focus the laser on the catalyst surface, to guarantee the reproducibility of the Raman spectra and to discriminate the contributions associated to the catalyst from other backgrounds.Full details on the realization of the measurements are provided and the results of three case studies are reported and discussed, which prove the concept of the operando Raman annular reactor. Methane cracking tests followed by oxidation in air demonstrate the capability of the system to follow rapid accumulation and consumption of carbon species, and provide the basis for further investigation of degradation mechanisms. The Raman analysis of the catalyst surface confirms that CH4 CPO and DR on Rh/α-Al2O3 do represent coke-clean situations, wherein the surface shows neither relevant traces of carbonaceous species nor a tendency to accumulate carbon with time on stream under reaction conditions. As well, no carbon removal is achieved upon exposure of a partially fouled Rh catalyst to both CPO and DR mixtures, confirming that steady state measurements are readily achieved. Overall, the operando Raman annular reactor demonstrates a valuable experimental tool to bridge the gap between kinetic studies and operando Raman studies, as well as to investigate dynamic effects, such as catalyst degradation, regeneration or modification.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from MIUR, Roma (Italy) – SIR 2014 (Scientific Independence of Young Researchers – Project THEOREMA Grant No. RBSI14TG3E) is gratefully acknowledged. We also acknowledge useful technical discussions on the construction of the rig with Mr. Enrico Aliprandi (Politecnico di Milano, Italy).References
- R. Schlögl, Heterogeneous Catalysis, Angew. Chem., Int. Ed., 2015, 54(11), 3465–3520 CrossRef PubMed.
- M. V. Martínez-Huerta, G. Deo, J. L. G. Fierro and M. A. Bañares, Operando Raman-GC Study on the Structure−Activity Relationships in V5+/CeO2 Catalysts for Ethane Oxidative Dehydrogenation: The Formation of CeVO4, J. Phys. Chem. C, 2008, 112(30), 11441–11447 Search PubMed.
- U. Bentrup, Combining in situ characterization methods in one set-up: looking with more eyes into the intricate chemistry of the synthesis and working of heterogeneous catalysts, Chem. Soc. Rev., 2010, 39(12), 4718–4730 RSC.
- E. Stavitski and B. M. Weckhuysen, Infrared and Raman imaging of heterogeneous catalysts, Chem. Soc. Rev., 2010, 39(12), 4615–4625 RSC.
- F. C. Meunier, The design and testing of kinetically-appropriate operando spectroscopic cells for investigating heterogeneous catalytic reactions, Chem. Soc. Rev., 2010, 39(12), 4602–4614 RSC.
- K. H. Cats and B. M. Weckhuysen, Combined Operando X-ray Diffraction/Raman Spectroscopy of Catalytic Solids in the Laboratory: The Co/TiO2 Fischer–Tropsch Synthesis Catalyst Showcase, ChemCatChem, 2016, 8(8), 1531–1542 CrossRef CAS PubMed.
- S. J. Tinnemans, J. G. Mesu, K. Kervinen, T. Visser, T. A. Nijhuis and A. M. Beale, et al., Combining operando techniques in one spectroscopic-reaction cell: New opportunities for elucidating the active site and related reaction mechanism in catalysis, Catal. Today, 2006, 113(1–2), 3–15 CrossRef CAS.
- S. J. Tinnemans, M. H. F. Kox, T. A. Nijhuis, T. Visser and B. M. Weckhuysen, Real time quantitative Raman spectroscopy of supported metal oxide catalysts without the need of an internal standard, Phys. Chem. Chem. Phys., 2005, 7(1), 211–216 RSC.
- T. A. Nijhuis, S. J. Tinnemans, T. Visser and B. M. Weckhuysen, Operando spectroscopic investigation of supported metal oxide catalysts by combined time-resolved UV-VIS/Raman/on-line mass spectrometry, Phys. Chem. Chem. Phys., 2003, 5(20), 4361–4365 RSC.
- J. J. H. B. Sattler, A. M. Beale and B. M. Weckhuysen, Operando Raman spectroscopy study on the deactivation of Pt/Al2O3 and Pt–Sn/Al2O3 propane dehydrogenation catalysts, Phys. Chem. Chem. Phys., 2013, 15(29), 12095–12103 RSC.
- J. J. H. B. Sattler, A. M. Mens and B. M. Weckhuysen, Real-Time Quantitative Operando Raman Spectroscopy of a CrOx/Al2O3 Propane Dehydrogenation Catalyst in a Pilot-Scale Reactor, ChemCatChem, 2014, 6(11), 3139–3145 CrossRef CAS.
- J.-D. Grunwaldt and B. S. Clausen, Combining XRD and EXAFS with On-line Catalytic Studies for in situ Characterization of Catalysts, Top. Catal., 2002, 18(1), 37–43 CrossRef CAS.
- J.-D. Grunwaldt, S. Hannemann, C. G. Schroer and A. Baiker, 2D-Mapping of the Catalyst Structure Inside a Catalytic Microreactor at Work: Partial Oxidation of Methane over Rh/Al2O3, J. Phys. Chem. B, 2006, 110(17), 8674–8680 CrossRef CAS PubMed.
- M. Rønning, N. E. Tsakoumis, A. Voronov, R. E. Johnsen, P. Norby and W. van Beek, et al., Combined XRD and XANES studies of a Re-promoted Co/γ-Al2O3 catalyst at Fischer–Tropsch synthesis conditions, Catal. Today, 2010, 155(3–4), 289–295 CrossRef.
- M. Geske, O. Korup and R. Horn, Resolving kinetics and dynamics of a catalytic reaction inside a fixed bed reactor by combined kinetic and spectroscopic profiling, Catal. Sci. Technol., 2013, 3(1), 169–175 CAS.
- M. A. Bañares, Operando methodology: combination of in situ spectroscopy and simultaneous activity measurements under catalytic reaction conditions, Catal. Today, 2005, 100(1–2), 71–77 CrossRef.
- G. Mestl, In situ Raman spectroscopy — a valuable tool to understand operating catalysts, J. Mol. Catal. A: Chem., 2000, 158(1), 45–65 CrossRef CAS.
- A. Beretta, P. Baiardi, D. Prina and P. Forzatti, Analysis of a catalytic annular reactor for very short contact times, Chem. Eng. Sci., 1999, 54(6), 765–773 CrossRef CAS.
- A. Donazzi, A. Beretta, G. Groppi and P. Forzatti, Catalytic partial oxidation of methane over a 4% Rh/α-Al2O3 catalyst Part II: Role of CO2 reforming, J. Catal., 2008, 255(2), 259–268 CrossRef CAS.
- A. Donazzi, A. Beretta, G. Groppi and P. Forzatti, Catalytic partial oxidation of methane over a 4% Rh/α-Al2O3 catalyst: Part I: Kinetic study in annular reactor, J. Catal., 2008, 255(2), 241–258 CrossRef CAS.
- G. Groppi, W. Ibashi, E. Tronconi and P. Forzatti, Structured reactors for kinetic measurements in catalytic combustion, Chem. Eng. J., 2001, 82(1–3), 57–71 CrossRef CAS.
- M. Horch, J. Schoknecht, M. A. Mroginski, O. Lenz, P. Hildebrandt and I. Zebger, Resonance Raman Spectroscopy on [NiFe] Hydrogenase Provides Structural Insights into Catalytic Intermediates and Reactions, J. Am. Chem. Soc., 2014, 136(28), 9870–9873 CrossRef CAS PubMed.
- O. Korup, C. F. Goldsmith, G. Weinberg, M. Geske, T. Kandemir and R. Schlögl, et al., Catalytic partial oxidation of methane on platinum investigated by spatial reactor profiles, spatially resolved spectroscopy, and microkinetic modeling, J. Catal., 2013, 297, 1–16 CrossRef CAS.
- M. A. Bañares and G. Mestl, Structural Characterization of Operating Catalysts by Raman Spectroscopy, Advances in Catalysis, Academic Press, 2009, chapter 2, vol. 52, pp. 43–128 Search PubMed.
- O. Korup, R. Schlögl and R. Horn, Carbon formation in catalytic partial oxidation of methane on platinum: model studies on a polycrystalline Pt foil, Catal. Today, 2012, 181(1), 177–183 CrossRef CAS.
- O. V. Fat'yanov, R. L. Webb and Y. M. Gupta, Optical transmission through inelastically deformed shocked sapphire: stress and crystal orientation effects, J. Appl. Phys., 2005, 97(12), 123529 CrossRef.
- T. Bruno, A. Beretta, G. Groppi, M. Roderi and P. Forzatti, A study of methane partial oxidation in annular reactor: activity of Rh/α-Al2O3 and Rh/ZrO2 catalysts, Catal. Today, 2005, 99(1–2), 89–98 CrossRef CAS.
- A. Maghsoumi, L. Brambilla, C. Castiglioni, K. Mullen and M. Tommasini, Overtone and combination features of G and D peaks in resonance Raman spectroscopy of the C78H26 polycyclic aromatic hydrocarbon, J. Raman Spectrosc., 2015, 46(9), 757–764 CrossRef CAS.
- A. C. Ferrari and D. M. Basko, Raman spectroscopy as a versatile tool for studying the properties of graphene, Nat, NANO, 2013, 8(4), 235–246 CAS.
- A. Donazzi, D. Pagani, A. Lucotti, M. Tommasini, A. Beretta and G. Groppi, et al., Annular reactor testing and Raman surface characterization in the CPO of methane and propylene, Appl. Catal., A, 2014, 474(Supplement C), 149–158 CrossRef CAS.
- A. Beretta, T. Bruno, G. Groppi, I. Tavazzi and P. Forzatti, Conditioning of Rh/α-Al2O3 catalysts for H2 production via CH4 partial oxidation at high space velocity, Appl. Catal., B, 2007, 70(1), 515–524 CrossRef CAS.
- M. Maestri, Escaping the trap of complication and complexity in multiscale microkinetic modelling of heterogeneous catalytic processes, Chem. Commun., 2017, 53(74), 10244–10254 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Assessment of homogeneous reactions; black-body radiation; focusing procedure; annular reactor performances; XRD, BET and Hg-porosimetry analysis of α-Al2O3. See DOI: 10.1039/c7re00092h |
| This journal is © The Royal Society of Chemistry 2017 |