Open Access Article
Open Access ArticleFirst-principles study of a new structure and oxidation mechanism of Pt3Zr
Yong Pan *,
Shuanglun Wang,
Linhu Jia and
Xi Zhang
*,
Shuanglun Wang,
Linhu Jia and
Xi Zhang
School of Materials Science and Engineering, Southwest Petroleum University, Chengdu 610500, China. E-mail: panyong10@mails.jlu.edu.cn; Fax: +86-028-83037406; Tel: +86-028-83037401
First published on 30th November 2017
Abstract
Zirconia (ZrO2)–metal interfaces are interesting for solid oxide fuel cells. Although the oxidation of Pt3Zr provides a new route for the formation of ZrO2–Pt interfaces, the crystal structure of Pt3Zr remains controversial and the oxidation mechanism of Pt3Zr is unclear. To solve these problems, we use first-principles calculations to explore the crystal structure of Pt3Zr. We demonstrate a stable structure of Pt3Zr based on phonon dispersion. Importantly, two new Pt3Zr structures, Ti3Pt-type (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and Fe3Al-type (Fm
m) and Fe3Al-type (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), are predicted. To study the oxidation mechanism, two possible doped models are considered. The calculated results show that the O atom prefers to occupy the tetrahedral interstitial site (TI) in comparison to the octahedral interstitial site (OI). We find that the oxidizing capacity of the Fe3Al-type cubic structure is stronger than that of other structures. In particular, we predict that Pt3Zr exhibits better oxidation capacity in comparison to other metals because of the strong localized hybridization between Zr and O.
m), are predicted. To study the oxidation mechanism, two possible doped models are considered. The calculated results show that the O atom prefers to occupy the tetrahedral interstitial site (TI) in comparison to the octahedral interstitial site (OI). We find that the oxidizing capacity of the Fe3Al-type cubic structure is stronger than that of other structures. In particular, we predict that Pt3Zr exhibits better oxidation capacity in comparison to other metals because of the strong localized hybridization between Zr and O.
1. Introduction
Zirconia (ZrO2)–metal interfaces have widely used in solid oxide fuel cells (SOFCs), heterogeneous catalysis, gas sensors, etc.1–9 Although ZrO2 shows excellent chemical and thermal stabilities, the poor electrical conductivity hinders the application because of the requirement of sensitive probing techniques.10,11 To solve this problem, an effective method is to oxidize Zr on an appropriate metal substrate. However, Zr deposited on a metal substrate should meet three conditions: oxygen reduction reaction (ORR), growth of the oxide layer and better catalytic activity of the metal substrate. In particular, the ORR clearly confirms the degree of oxidation between Zr and the metal substrate. Therefore, high ORR catalytic activity strongly depends on the d-state of the transition metal.12–14Platinum-group-metals are important catalysts because of their excellent chemical and physical properties.15–18 In particular, Pt is a fascinating catalyst because it effectively promotes the process of ORR.19–21 Recently, the oxidation behavior of Pt3Zr (0001) surface was studied by the experiment and first-principles calculations.22 They found that the weak localized hybridization between Pt and Zr is beneficial to oxidation of Zr metal. Note that the metal addition will accelerate the growth of ZrO2 film on the Pt3Zr (0001) surface.23 Therefore, structural configuration plays a crucial role in ORR and the formation of ZrO2–Pt interface. Unfortunately, the crystal structure of Pt3Zr remains controversial. Earlier work suggested that Pt3Zr is a Au3Cu-type cubic structure.24,25 On the contrary, Stalick et al. found that Pt3Zr belongs to a Ni3Ti-type hexagonal structure,26 which is different from the previous viewpoint. As a result, the nature of oxidation mechanism of Pt3Zr is unclear.
To explore the catalytic activity of Pt3Zr and improve the formation of ZrO2–Pt interface, in our works, we investigate the crystal structure and oxidation mechanism of Pt3Zr by using the first-principles calculations. According to the structural feature, we predict two possible cubic structures. Importantly, the structural stability of Pt3Zr is estimated by the formation enthalpy and phonon dispersion. To examine the oxidation mechanism and oxidation capacity of Pt3Zr, we calculate and compare the oxygen doped formation energy between O-doped Pt3Zr and many metals. In particular, we examine the possible adsorption site of Pt3Zr. Finally, we predict that Pt3Zr shows better oxidation capacity in comparison to many metals.
2. Model and methods
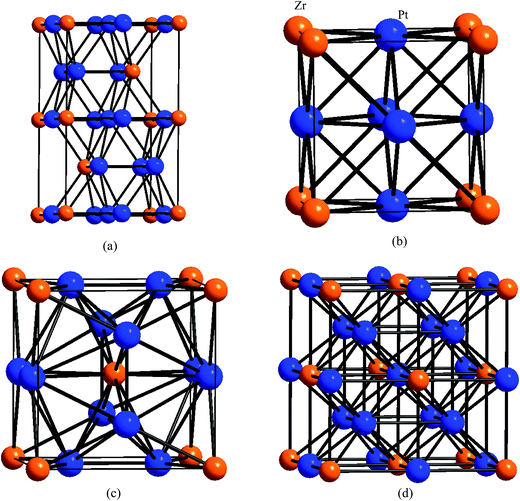
To explore the oxidation mechanism, we firstly study the crystal structure of Pt3Zr. To our knowledge, one is that Pt3Zr is a Ni3Ti-type hexagonal structure with space group of P63/mmc. The lattice parameters of hexagonal structure are a = 5.653 Å and c = 9.347 Å, respectively.24 Another is that Pt3Zr belongs to the Au3Cu-type cubic structure with space group of Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, and the lattice parameter is a = 4.051 Å.26 In comparison to hexagonal structure, we suggest that Pt3Zr with cubic structure can promote the oxidation of Pt3Zr because of the localized hybridization between oxygen and Pt3Zr. Therefore, we further design two possible structures: Ti3Pt-type with cubic structure and Fe3Al-type with cubic structure, respectively. The structural models of Pt3Zr are shown in Fig. 1.
m, and the lattice parameter is a = 4.051 Å.26 In comparison to hexagonal structure, we suggest that Pt3Zr with cubic structure can promote the oxidation of Pt3Zr because of the localized hybridization between oxygen and Pt3Zr. Therefore, we further design two possible structures: Ti3Pt-type with cubic structure and Fe3Al-type with cubic structure, respectively. The structural models of Pt3Zr are shown in Fig. 1.
In this paper, the total energy, structural information, electronic structure and chemical bonding of Pt3Zr and O-doped Pt3Zr were calculated by using the first-principles calculations, as implemented in the CASTEP code.27 The exchange–correlation-functional was calculated by using the generalized gradient approximation (GGA) within PW91 functional.28,29 To treat the interaction between electrons and the ions, the atomic configurations of O, Pt and Zr were 2s22p4, 5p65d96s1 and 4p64d25s2, respectively. Based on the convergence test, the plane-wave basis set for electron wave function with cutoff energy of 400 eV was used. The k-point grids of 10 × 10 × 5 for Ni3Ti-type structure, 12 × 12 × 12 for Au3Cu-type structure, 10 × 10 × 10 for Ti3Pt-type structure, 11 × 11 × 11 for Fe3Al-type structure, 14 × 14 × 8 for Zr and 17 × 17 × 17 for Pt were treated, respectively. To examine the dynamically stable, the phonon calculation was carried out by using the supercell method within the PHONON code.30
3. Results and discussions
3.1 Structural prediction
The structural stability of Pt3Zr is measured by the formation enthalpy and phonon dispersion, respectively. The equation of formation enthalpy (ΔH) is given by:| ΔH = EPt3Zr − 3EPt − EZr | (1) |
Table 1 lists the calculated lattice parameters, density, and formation enthalpy of Pt3Zr with four structures. It can be seen that these structures are thermodynamically stable at the ground state because the calculated formation enthalpy of these structures is smaller than zero. Our predicted two structures (Ti3Pt-type and Fe3Al-type) are also thermodynamically stable. Importantly, the calculated formation enthalpy of Ni3Ti-type structure is −8.188 eV per atom, which is smaller than that of Au3Cu-type structure. The slight difference implies that external condition is easy to result in phase transition from Ni3Ti-type structure to Au3Cu-type structure. This result is similar to the Fairbank's viewpoint.24
In addition to thermodynamically stable, the dynamically stable of Pt3Zr should examine follow. To study the dynamically stable, Fig. 2 displays the calculated phonon dispersion curves of Pt3Zr with four structures. It is clear that Pt3Zr with Ni3Ti-type structure is a dynamically unstable because the imaginary phonon frequency is observed in this structure. However, we find that there is no imaginary phonon frequency in Au3Cu-type structure, indicating that this structure is a dynamically stable at the ground state. Based on the first-principles calculations, we demonstrate the Stalick's viewpoint. Importantly, we predict two new Pt3Zr structures (Ti3Pt-type and Fe3Al-type) because no imaginary phonon frequencies are found in these structures.
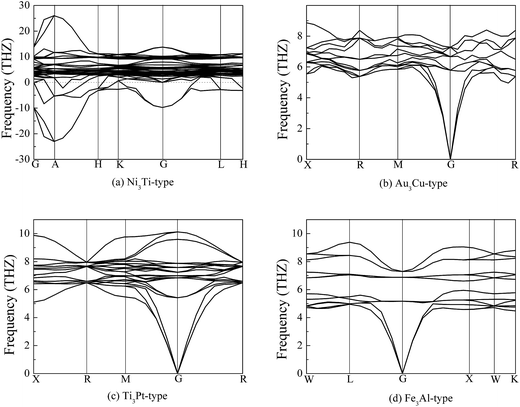 | ||
| Fig. 2 Calculated phonon dispersion curves of Pt3Zr, (a) Ni3Ti-type structure, (b) Au3Cu-type structure, (c) Ti3Pt-type structure and (d) Fe3Al-type structure, respectively. | ||
To further insight into the nature of dynamically stable, Fig. 3 shows the calculated phonon density of state (PhDOS) of Pt3Zr with four structures. It can be seen that negative frequencies are found in Ni3Ti-type structure, indicating that this structure is a mechanically unstable at the ground state. The calculated PhDOS profile reveals that the mechanically unstable of Ni3Ti-type is attributed to the vibration of Pt atom at low frequency region. However, Au3Cu-type, Ti3Pt-type and Fe3Al-type structures are mechanically stable because no negative frequencies are observed in these structures. In particular, the whole low frequency model of Au3Cu-type structure derives from the vibration of Pt atom and Zr atom. With increasing frequency, Zr's vibration plays an important role in thermodynamic properties. Therefore, it is concluded that the structural stability of Pt3Zr is markedly influenced by Pt–Zr bond.
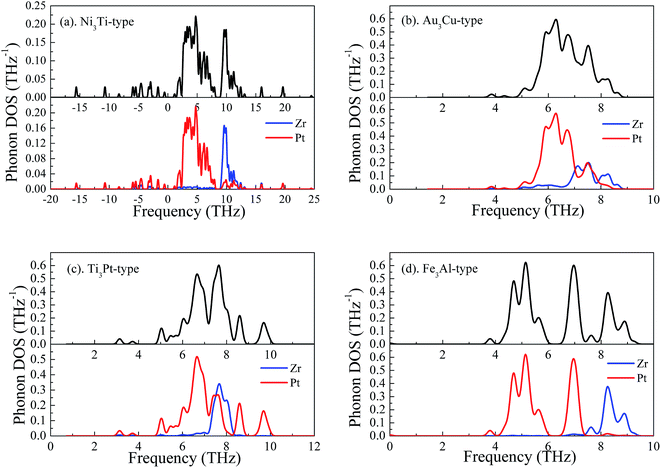 | ||
| Fig. 3 Phonon density of state of Pt3Zr, (a) Ni3Ti-type structure, (b) Au3Cu-type structure, (c) Ti3Pt-type structure and (d) Fe3Al-type structure, respectively. | ||
To further reveal the structural stability, the structural information of Pt3Zr is discussed. As listed in Table 1, the calculated lattice parameters of Ni3Ti-type structure (space group: P63/mmc, no: 194) are a = 5.742 Å and c = 9.398 Å, which are in good agreement with the experimental data.24 In this structure (see Fig. 1(a)), the alternative Pt layer and Pt–Zr layer can be viewed along the c-axis. In particular, each Zr atom is surrounded by 4 Pt atoms at Pt–Zr layer. Therefore, Pt–Zr bond (2.601 Å) can improve the structural stability of Pt3Zr with Ni3Ti-type structure. However, the cohesive force between layers is determined by the bond strength of Pt–Pt bond and part of Pt–Zr bond. In other words, Pt–Pt and Pt–Zr bonds play a key role in structural stability. The calculated bond length of Pt–Pt and Pt–Zr bonds is 2.924 Å and 2.968 Å, respectively.
For Au3Cu-type structure (see Fig. 1(b)), the calculated lattice parameter is a = 4.061 Å, which is in excellent agreement with the experimental data and theoretical results.25,26 Note that the symmetrical Pt–Zr bonds effectively improve it's structural stability. The calculated bond length of Pt–Zr bond is 2.871 Å, which is similar to the previous theoretical result.31 Note that the bond length of Pt–Zr bond of Au3Cu-type structure is slightly smaller than the corresponding bond for Ni3Ti-type structure, indicating that the structural feature of the former can obviously improve the structural stability in comparison to the latter. Thus, we should consider the cubic structure to oxidize Zr.
The calculated lattice parameter of Ti3Pt-type and Fe3Al-type structures is a = 5.198 Å and a = 6.513 Å, respectively. From Fig. 1, Ti3Pt-type structure is similar to Nb3Si-type structure. In comparison to Au3Cu-type structure, Pt atom in Ti3Pt-type structure occurs migration from (0.50, 0, 0.50) site to (0.25, 0, 0.50) site. As a result, the variation of atomic position changes the localized hybridization between Pt and Zr, which forms two different Pt–Zr bonds. The calculated bond length of Pt–Zr bond is 2.599 Å and 2.906 Å, respectively. In particular, the atomic configuration can provide a large number of interstitial sites to adsorb oxygen. In addition, we find that the calculated lattice parameter of Ti3Pt-type and Fe3Al-type structures is larger than that of Au3Cu-type structure.
For Fe3Al-type structure, Pt atom migrates from (0.50, 0, 0.50) site to (0.25, 0.25, 0.25) site. Each Pt atom is surrounded by 4 Zr atoms and 8 Pt atoms. The calculated bond length of Pt–Zr bond is 2.820 Å. Importantly, the network Pt–Zr bonds can improve the structural stability of Pt3Zr.
To reveal the nature of chemical bonding, Fig. 4 shows the calculated density of state (DOS) of Pt3Zr with four structures. We can see that the DOS profile of Pt3Zr is composed of Pt-5d state and Zr-4d state. The strong localized hybridization between Pt and Zr forms the Pt–Zr bond. It is worth noticing that the DOS profile of Ni3Ti-type structure is similar to Au3Cu-type structure. From Fig. 4, there is a deep valley near Fermi level (EF), which separates the bonding state and antibonding state. For Ti3Pt-type and Fe3Al-type structures, however, the Zr-PDOS profile right shifts from EF to high energy region. That is to say, Pt's migration weakens the localized hybridization between Pt and Zr. As a result, Pt–Pt bond plays an important role in structural stability, particularly for catalytic properties.
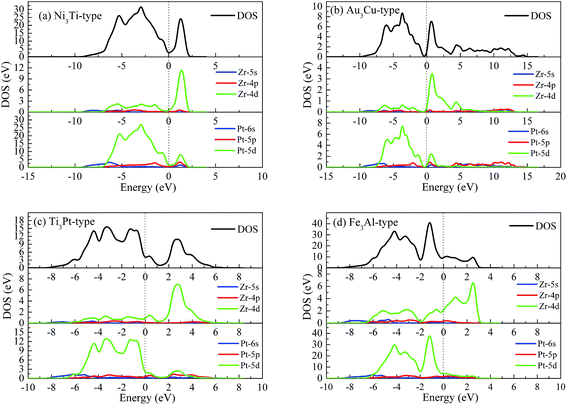 | ||
| Fig. 4 Total and partial density of state (DOS) of Pt3Zr, (a) Ni3Ti-type structure, (b) Au3Cu-type structure, (c) Ti3Pt-type structure and (d) Fe3Al-type structure, respectively. | ||
3.2 Oxidation mechanism
We suggest that the formation of ZrO2–Pt interface strongly depends on the interaction between Pt3Zr and O atom. To examine the formation of ZrO2–Pt interface, we calculate the oxygen doped formation energy of O-doped Pt3Zr. In particular, we discuss and analyze the chemical bonding of O-doped Pt3Zr to reveal the formation of ZrO2–Pt interface. It must be mentioned that the formation of ZrO2 film is related to the interstice radius of Pt3Zr, which is determined by the atomic configuration of Pt3Zr. Therefore, it is necessary to insight into the oxidation mechanism of Pt3Zr.According to the first-principles calculations, we consider the oxidation behavior of three stable Pt3Zr structures: Au3Cu-type, Ti3Pt-type and Fe3Al-type, respectively. To reveal the oxidation mechanism, we design the possible interstice models: tetrahedral interstice site (TI) and octahedral interstice site (OI), respectively. Oxygen mechanism of Pt3Zr is measured by the oxygen doped formation energy (Ead), which is given by:
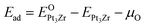 | (2) |
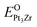 and EPt3Zr are the total energy of O-doped Pt3Zr and parent Pt3Zr at the ground state, respectively. μO is the chemical potential of O atom. Generally, the negative oxygen doped formation energy indicates the thermodynamically stable at the ground state.
and EPt3Zr are the total energy of O-doped Pt3Zr and parent Pt3Zr at the ground state, respectively. μO is the chemical potential of O atom. Generally, the negative oxygen doped formation energy indicates the thermodynamically stable at the ground state.
To explore the catalytic activity of Pt3Zr, we calculate and compare the capacity of oxygen between Pt3Zr and many metals. Firstly, we explore the oxidation mechanism of Pt3Zr. From Fig. 1, Au3Cu-type is a typical cubic structure. Therefore, we design two possible occupied sites: TI site and OI site, respectively. However, the structural feature of Ti3Pt-type structure is more complex than that of Au3Cu-type structure. According to the structural feature, we design two different OI sites and one TI site. Although the doped model of Fe3Al-type structure is similar to Au3Cu-type structure, the atomic interaction of the former is stronger than the latter. That is to say, Fe3Al-type structure is easily to adsorb a large number of oxygen.
Fig. 5 shows the calculated oxygen doped formation energy of O-doped Pt3Zr and many metals. We can conclude that TI model is more thermodynamically stable than that of OI model because the calculated oxygen doped formation energy of the former is lower than the latter. This discrepancy is attributed to the localized hybridization between O and Zr. For Pt3Zr, TI site can effectively improve the charge interaction between Zr and O, which forms the Zr–O bond. In particular, O atom prefers to occupy the TI site of Fe3Al-type structure in comparison to other structures. Therefore, we can adjust the crystal structure of Pt3Zr to promote the formation of ZrO2. In particular, the calculated oxygen doped formation energy of Fe3Al-type with TI site is about −9.029 eV, which is smaller than that of other structures. This result indicates that Pt3Zr with Fe3Al-type structure shows the better catalytic activity in comparison to other structures. This reason is attributed to the atomic configuration of Pt3Zr.
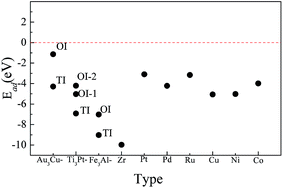 | ||
| Fig. 5 Calculated oxygen adsorption energy of O-doped Pt3Zr at tetrahedral interstice site (TI) and octahedral interstice site (OI), together with many metals. | ||
As we know, many metals such as Pt, Cu, Ni and Co etc. are important parts of solid oxide fuel cells.32–35 To estimate the catalytic activity of Pt3Zr, we compare the oxygen doped formation energy between Pt3Zr and many metals. As shown in Fig. 5, the calculated oxygen doped formation energy of metal Zr is −10.068 eV, which is smaller than that of Pt3Zr. This result indicates that metal Zr shows better oxidation capacity in comparison to Pt3Zr. This is why ZrO2 has been widely investigated over the last years. Importantly, although the oxygen doped formation energy of Pt3Zr is larger than that of metal Zr, the oxygen doped formation energy of Pt3Zr is smaller than that of other metals (see Fig. 5). The calculated electronic structure shows that the better oxidation capacity of Pt3Zr derives from the strong localized hybridization between O and metal Zr. As mentioned above, we can predict that Pt3Zr is expected to have better catalytic activity in comparison to other metals.
To reveal the nature of the oxidation mechanism, we further analyze the chemical bonding of O-doped Pt3Zr. The first-principle calculations show that the bond length of Zr–O bond for Au3Cu-type structure and Ti3Pt-type structure is 2.026 Å and 2.014 Å, respectively, which are smaller than the corresponding Zr–O bond (2.05 Å) of ZrO2.36,37 However, the calculated bond length of Zr–O bond of Fe3Al-type structure is 2.076 Å, which is close to the bond length of Zr–O bond for ZrO2. On the other hand, the calculated electronic localization density shows that there is a strong localized hybridization between Pt and O for Au3Cu-type structure. The calculated bond length of Pt–O bond is 2.117 Å. However, Pt–O antibonding state in Ti3Pt-type and Fe3Al-type structures is found. In other words, Ti3Pt-type and Fe3Al-type structures are beneficial to the formation of ZrO2. The oxidation mechanism of Pt3Zr with three structures is shown in Fig. 6. As mentioned above, we predict that Pt3Zr with Fe3Al-type structure is beneficial to the formation of ZrO2.
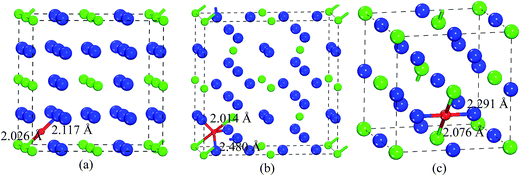 | ||
| Fig. 6 Oxygen adsorption mechanism of Pt3Zr, (a) Au3Cu-type structure, (b) Ti3Pt-type structure and (c) Fe3Al-type structure, respectively. | ||
To gain insight the nature of oxidation behavior, Fig. 7 shows the calculated DOS of O-doped Pt3Zr with three structures. We observe that the DOS profile of Pt3Zr is mainly contributed by Pt-5d state and Zr-4d state. The strong localized hybridization between Pt and Zr forms the Pt–Zr bond. However, oxygen addition can improve the charge equilibrium between Pt and Zr. As shown in Fig. 7, we can see that the charge interaction between O and Zr forms the Zr–O bond, which demonstrates the existence of Zr–O bond.
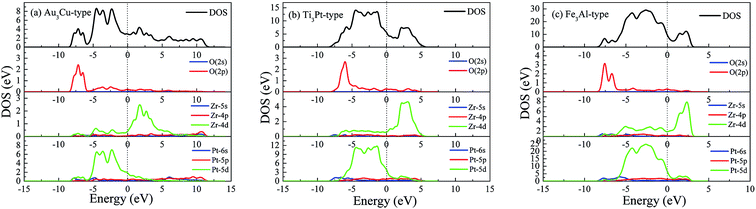 | ||
| Fig. 7 Total and partial density of state (DOS) of O-doped Pt3Zr, (a) Au3Cu-type structure, (b) Ti3Pt-type structure and (c) Fe3Al-type structure, respectively. | ||
4. Conclusions
In summary, we apply first-principles calculations to study the crystal structure and oxidation mechanism of Pt3Zr. To explore the stable structure, we calculate the formation enthalpy, phonon dispersion, lattice parameters and electronic structure of Pt3Zr. In addition, we predict two possible new Pt3Zr structures: Ti3Pt-type (space group: Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, no. 223) and Fe3Al-type (space group: Fm
m, no. 223) and Fe3Al-type (space group: Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, no. 225). To investigate the oxidation mechanism, we calculate the oxygen doped formation energy and chemical bonding of O-doped Pt3Zr. In particular, we compare the oxygen doped formation energy of Pt3Zr and many metals.
m, no. 225). To investigate the oxidation mechanism, we calculate the oxygen doped formation energy and chemical bonding of O-doped Pt3Zr. In particular, we compare the oxygen doped formation energy of Pt3Zr and many metals.
The calculated results show that although Pt3Zr with hexagonal structure is more thermodynamically stable than that of cubic structure, hexagonal structure is a dynamically unstable at the ground state. The calculated oxygen doped formation energy of TI site is smaller than that of OI site. The calculated oxygen doped formation energy of Pt3Zr with Fe3Al-type structure is smaller than that of other structures. The calculated chemical bonding shows that Pt3Zr with Fe3Al-type structure is easy to form the ZrO2 because of the formation of Zr–O bond. In particular, the calculated oxygen doped formation energy of Fe3Al-type with TI site is smaller than that of other metals, indicating that Pt3Zr shows the good catalytic activity in comparison to metals.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by grants from the Sichuan Provincial Colleges’ Sate Key Laboratory of Oil and Gas Reservoir Project (X151517KCL36) and the national natural science foundation of China (No. 51267007). We acknowledge the help from Lady Yun Zheng.References
- H. Y. T. Chen, S. Tosoni and G. Pacchioni, ACS Catal., 2015, 5, 5486–5495 CrossRef CAS.
- L. M. Toscani, A. Raievich, M. C. Aantini, D. G. Lamas and S. A. Larrondo, J. Phys. Chem. C, 2016, 120, 24165–24175 CAS.
- A. P. Kulkarni, S. Giddey and S. P. S. Badwal, J. Phys. Chem. C, 2016, 120, 15675–15683 CAS.
- N. S. Yuzbasi, A. M. Kierzkowska, Q. Imtiaz, P. M. Abdala, A. Kurlov, J. L. M. Rupp and C. R. Muller, J. Phys. Chem. C, 2016, 120, 18977–18985 CAS.
- P. Li, X. Chen, X. Wang, J. Shao, G. Lin, H. Yang, Q. Yang and H. Chen, Energy Fuels, 2017, 31, 3979–3986 CrossRef CAS.
- A. Ganesan, M. Narayanasamy, K. Shunmugavel and I. J. Chinnappa, Int. J. Hydrogen Energy, 2016, 41, 8963–8977 CrossRef CAS.
- F. Menegazzo, M. Signoretto, D. Marchese, F. Pinna and M. Manzoli, J. Catal., 2015, 326, 1–8 CrossRef CAS.
- E. Ciftyurek, C. D. Mcmillen, K. Sabolsky and E. M. Sabolsky, Sens. Actuators, B, 2015, 207, 206–215 CrossRef CAS.
- J. Ftouni, A. M. Murillo, A. Goryachev, J. P. Hofmann, E. J. M. Hensen, L. Lu, C. J. Kiely, P. C. A. Bruijnincx and B. M. Weckhuysen, ACS Catal., 2016, 6, 5462–5472 CrossRef CAS.
- F. Giannici, G. Canu, M. Gambino, A. Longo, M. Salome, M. Viviani and A. Martorana, Chem. Mater., 2015, 27, 2763–2766 CrossRef CAS.
- M. Kuzminska, T. V. Kovalchuk, R. Backov and R. M. Gaigneaux, J. Catal., 2014, 320, 1–8 CrossRef CAS.
- S. Grieshammer, J. Phys. Chem. C, 2017, 121, 15078–15084 CAS.
- H. H. Li, S. Y. Ma, Q. Q. Fu, X. J. Liu, L. Wu and S. H. Yu, J. Am. Ceram. Soc., 2015, 137, 7862–7868 CAS.
- Y. Sha, T. H. Yu, B. V. Merinov and W. A. Goddard, ACS Catal., 2014, 4, 1189–1197 CrossRef CAS.
- L. A. Avakyan, N. A. Kolpacheva, E. V. Paramonova, J. Singh, U. Hartfelder, J. A. V. Bokhoven and L. A. Bugaev, J. Phys. Chem. C, 2016, 120, 28057–28066 CAS.
- H. Ostrom, H. Oberg, H. Xin, J. Larue, M. Beye, M. D. Angela and A. Nilsson, Science, 2015, 347, 978–982 CrossRef CAS PubMed.
- K. Ding, A. Gulec, A. M. Johnson, N. M. Schweitzer, G. D. Stucky, L. D. Marks and P. C. Stair, Science, 2015, 350, 189–192 CrossRef CAS PubMed.
- Y. Pan, C. Jin and P. Mao, J. Electron. Mater., 2017, 46, 6639–6645 CrossRef CAS.
- H. Sohn and U. Ozkan, Energy Fuels, 2016, 30, 5309–5322 CrossRef CAS.
- J. Durst, M. L. Haro, L. Dubau, M. Chatenet, Y. S. Olivier, L. Guetaz, P. B. Guillemaud and F. Maillard, J. Phys. Chem. Lett., 2014, 5, 434–439 CrossRef CAS PubMed.
- C. Zhang, S. Y. Hwang, A. Trout and Z. Peng, J. Am. Chem. Soc., 2014, 136, 7805–7808 CrossRef CAS PubMed.
- M. Antlanger, W. M. Schmölzer, J. Pavelec, F. Mittendorfer and J. Redinger, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 035451 CrossRef.
- J. J. Choi, W. M. Schmolzer, I. Valenti, P. Luches, F. Mittendorfer, J. Redinger, U. Diebold and M. Schmid, J. Phys. Chem. C, 2016, 120, 9920–9932 CAS.
- G. B. Fairbank, C. J. Humphreys, A. Kelly and C. N. Jones, Intermetallics, 2000, 8, 1091–1100 CrossRef CAS.
- Y. Pan, Y. Lin, X. Wang, S. Chen, L. Wang, C. Tong and Z. Cao, J. Alloys Compd., 2015, 643, 49–55 CrossRef CAS.
- J. K. Stalick and R. M. Waterstrat, J. Alloys Compd., 2007, 430, 123–131 CrossRef CAS.
- M. D. Segall, P. J. D. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717–2744 CrossRef CAS.
- J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 13244–13249 CrossRef.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- S. Baroni, S. d. Gironcoli, A. D. Corso and P. Giannozzi, Rev. Mod. Phys., 2001, 73, 515 CrossRef CAS.
- Y. Pan, W. Guan, M. Wen, J. Zhang, C. Wang and Z. Tan, J. Alloys Compd., 2014, 585, 549–554 CrossRef CAS.
- Z. Zheng, C. Sun, R. Dai, S. Wang, X. Wu, X. An, Z. Wu and C. Xie, Energy Fuels, 2017, 31, 3091–3100 CrossRef CAS.
- M. Deutsch, F. Horvath, C. Knoll, D. Lager, C. G. Mayer, P. Weinberger and F. Winter, Energy Fuels, 2017, 31, 2324–2334 CrossRef CAS.
- K. Nakao, T. Ishimoto and M. Koyama, J. Phys. Chem. C, 2016, 120, 16641–16648 CAS.
- X. Yang, Y. Wang, M. Li, B. Sun, Y. Li and Y. Wang, Energy Fuels, 2016, 30, 2198–2203 CrossRef CAS.
- W. Piskorz, J. Grybos, F. Zasada, S. Cristol, J. F. Paul, A. Adamski and Z. Sojka, J. Phys. Chem. C, 2011, 115, 24274–24286 CAS.
- M. Taddei, F. Costantino, V. Manuali and R. Vivani, Inorg. Chem., 2011, 50, 10835–10843 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |