Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, solution and solid-state fluorescence of 2-diethylaminocinchomeronic dinitrile derivatives†
O. V. Ershov *a,
M. Yu. Ievlev
*a,
M. Yu. Ievlev a,
M. Yu. Belikov
a,
M. Yu. Belikov a,
A. I. Naidenovaa,
V. N. Maksimovaa and
V. A. Tafeenkob
a,
A. I. Naidenovaa,
V. N. Maksimovaa and
V. A. Tafeenkob
aUlyanov Chuvash State University, Moskovsky pr., 15, Cheboksary, Russia. E-mail: oleg.ershov@mail.ru
bLomonosov Moscow State University, Leninskie Gory 1, Moscow, Russia
First published on 12th July 2017
Abstract
A facile approach to the synthesis of novel 2-diethylaminocinchomeronic dinitriles, which are found to be fluorescent both in the solution and in a solid states, was developed. Absorption, fluorescence and solvatochromic properties as well as a crystal structure of the synthesized compounds were investigated. It was found that the 2-diethylaminocinchomeronic dinitrile derivatives with methoxy groups in the aryl moiety possess the most intensive emission in nonpolar solvents with fluorescence quantum yields up to 0.59.
Introduction
Luminescent dyes are widely used in various fields of industry and science. There are many examples of optical devices, chemo- and biosensors, and nanomaterials which are based on luminescent substances.1 Because of the theoretical and practical interest, compounds of the cyano pyridine series should be mentioned separately among the diverse fluorescent organic molecules.2–8 They are commonly used in light-emitting diodes (OLED),2 photovoltaics,3 nonlinear optical (NLO) materials,4 liquid crystalline,5 dyes,6 sensors for the detection of metal ions7 and fluorescent security markers.8 Such a diverse practical use of fluorophores causes an interest for the targeted synthesis of new fluorescent structures with desired properties.It was reported earlier, that the cyano group is able to significantly increase fluorescence intensity.9 Because of its structural simplicity, compactness, strong electron-withdrawing properties and high polarizability, the cyano group has been frequently utilized as a functional unit in the design of advanced optical materials.10 Various «push–pull» chromophores wherein cyano group or a polynitrile moiety is an acceptor are widely presented in the modern scientific literature. A number of reports describes that including of cyano group in the molecule could increase such characteristics of the substances as solubility, thermal stability, sublimability, optical and electrochemical properties, and so on, making them interesting as advanced functional materials for novel opto-electronic devices.11 Amino (or alkylamino) group, in such a kind donor–acceptor structures, is often used as a donor moiety. It was noted that amino substituted pyridines possess a higher fluorescence quantum yield in compare to unsubstituted pyridines, and 2-aminopyridines has the most one.12 Therefore, the combination of a cyano group and an amino group in one molecule is a promising approach to structures with promising optical properties.
The known compounds with such a donor–acceptor moiety are 2-aminopyridine-3-carbonitriles possessing fluorescent and other practically useful properties.7,8,13 It was also mentioned that diethylamino group leads to the highest fluorescence quantum yield.14
We have reported previously, that pyridines containing two cyano groups as acceptors – 2-oxo-1,2-dihydropyridine-3,4-dicarbonitriles and 2-chlorocinchomeronic dinitriles possess fluorescence properties in solutions (an intensive blue emission) and in solid state (an emission in the blue, green and yellow regions of the spectrum).15 Moreover, the other authors reported that various porphyrazines could be synthesized from these cinchomeronic dinitriles.16 Taking into account the importance of diethylamino group as well as the described prospects of using the cinchomeronic dinitrile moiety we decided to construct structures with improved fluorescent properties. Therefore, we had synthesized a series of 2-diethylaminocinchomeronic dinitriles combining key fragments in the structure, and studied fluorescence properties of these compounds both in solutions and in a solid state.
Results and discussions
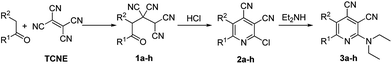
2-Diethylaminocinchomeronic dinitrile derivatives 3 were synthesized by the method shown in Table 1. Starting 4-oxoalkane-1,1,2,2-tetracarbonitriles 1a–c were prepared by interaction of TCNE and carbonyl compounds in 1,4-dioxane in the presence of hydrochloric acid17 as well as compounds 1d–h were obtained according to the «solvent-free» protocol.18 Compounds 1 were further transformed to corresponding 2-chlorcinchomeronic dinitriles 2 by an interaction with hydrochloric acid for the synthesis of compounds 2a–d,g or with generated in situ dry hydrogen chloride to obtain compounds 2e,f,h.15bTargeted 2-diethylaminocinchomeronic dinitrile derivatives 3 were synthesized as a result of an interaction between 2-chlorocinchomeronic dinitriles 2 and diethylamine. The reactions were carried out in an excess of diethylamine. It was observed, that the interaction carried out in the organic solvents (alcohols, dioxane, THF were tested) took much more time. For example, the reaction of 2-chloro-5,6-dimethylpyridine-3,4-dicarbonitrile 3a with diethylamine in THF takes 30 h.16a Moreover, we have found that final products are not required a further purification after the reaction carried out in the excess of diethylamine without heating. An addition of metal carbonates as a catalyst did not increase a yield of compounds 3 but led to the formation of byproducts obtaining as a result of the addition of diethylamine to cyano group.19 According to the developed facile protocol a series of 2-diethylaminocinchomeronic dinitrile derivatives 3 were synthesized containing alkyl, cycloalkyl and aryl substituents, including the moieties with electron-donating methoxy groups (Table 1). Compounds 3b–h are new and were not described in the literature.
2-Diethylaminocinchomeronic dinitrile derivatives 3 are crystalline powders from green to yellow-green color, they were found to be fluorescent both in the solution and in the solid state. There are very few dyes that exhibit intensive fluorescence both in the solid state and in solutions because generally the molecular aggregation in the solid state causes fluorescence quenching (ACQ). It causes an increased interest to study the compounds 3.
Spectroscopic properties in solution
The structure of the synthesized pyridines 3 containing conjugated donor and acceptor moieties prompted us to explore their photophysical properties. The absorption and emission spectra of compound 3f in various polar and nonpolar solvents are shown in Fig. 1, the obtained spectral characteristics are summarized in Table 2. A position of absorption maxima (342–346 nm and 409–415 nm) does not change significantly in various solvents.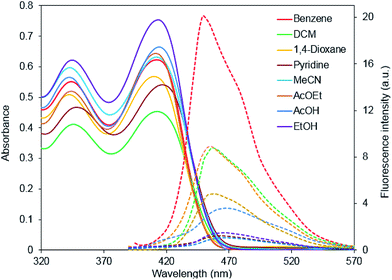 | ||
| Fig. 1 Absorption (solid) and emission (doted) spectra of 3f in various solvents, excitation wavelength is 375 nm. | ||
| Solvent | λabs;max, nm | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) εmax εmax |
λflu;max, nm | Stokes shift, cm−1 (nm) | ΦF |
|---|---|---|---|---|---|
| Benzene | 343 | 4.04 | 449 | 2000 (37) | 0.59 |
| 412 | 4.10 | ||||
| DCM | 346 | 3.92 | 456 | 2342 (44) | 0.33 |
| 412 | 3.96 | ||||
| Dioxane | 343 | 4.01 | 456 | 2520 (47) | 0.29 |
| 409 | 4.06 | ||||
| Pyridine | 349 | 3.97 | 465 | 2475 (48) | 0.06 |
| 417 | 4.04 | ||||
| MeCN | 343 | 4.08 | 468 | 2845 (55) | 0.11 |
| 413 | 4.09 | ||||
| AcOEt | 342 | 4.02 | 459 | 2544 (48) | 0.14 |
| 411 | 4.11 | ||||
| AcOH | 343 | 4.05 | 468 | 2729 (53) | 0.04 |
| 415 | 4.12 | ||||
| EtOH | 346 | 4.09 | 466 | 2637 (51) | 0.05 |
| 415 | 4.18 |
The compound 3f exhibits fluorescence in various solvents, the most intensive emission was noted in benzene, an emission band undergoes a slight red-shift and significantly loses its intensity with an increase of the polarity of the solvent. The relative fluorescence quantum yield (ΦF) was also calculated (see Table 2). It decreased when the solvent polarity increased, probably, because of the charge transfer phenomena. The intermolecular charge transfer (ICT)4c phenomena occurs, when an electron is transferred from the amino group to the acceptor moiety upon light absorption, producing an (ICT) excited state, which is more sensitive to the solvent polarity. A change in the excited state leads to the change in the lifetime and hence the results are a decrease of radiative emission.
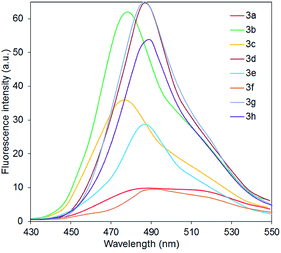
The other compounds 3 possess similar spectral characteristics which are presented in Table 3. Fig. 2 shows absorption and emission spectra of 2-diethylaminocinchomeronic dinitriles 3 in benzene. Solutions of all the studied compounds 3 are characterized with an absorption maximum in the violet region of the spectrum (399–416 nm), corresponding, probably, to the intramolecular charge transfer (ICT) according to TD-DFT calculations. The intensity of this band increases with the growth of the number of donor fragments in the molecule. Moreover, in the spectra of the compounds 3e,f,h containing MeO– groups a new shortwave absorption band is appeared which is corresponds to n–π* electronic transition in the aryl substituent.
| Compound | λabs;max, nm | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) εmax εmax |
λflu;max, nm | Stokes shift, cm−1 (nm) | ΦF |
|---|---|---|---|---|---|
| 3a | 399 | 3.44 | 453 | 2988 (54) | 0.46 |
| 3b | 399 | 3.63 | 450 | 2840 (51) | 0.45 |
| 3c | 403 | 3.52 | 453 | 2739 (50) | 0.53 |
| 3d | 410 | 3.77 | 457 | 2508 (47) | 0.20 |
| 3e | 339, 410 | 3.73, 3.89 | 449 | 2119 (39) | 0.41 |
| 3f | 344, 412 | 4.04, 4.10 | 449 | 2000 (37) | 0.59 |
| 3g | 416 | 3.93 | 460 | 2299 (44) | 0.11 |
| 3h | 319, 416 | 4.12, 4.07 | 457 | 2157 (41) | 0.35 |
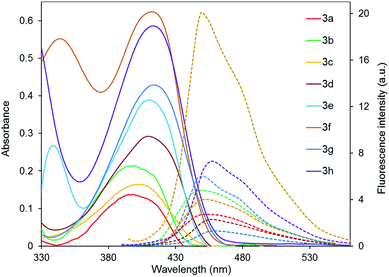 | ||
| Fig. 2 Absorption (solid) and emission (doted) spectra of compounds 3 in benzene, excitation wavelength is 375 nm. | ||
It was observed that the structure of aminopyridines 3 has a little influence on the positions of the absorption and emission maxima. Compounds 3d–h with aryl substituent are characterized with a slight (up to 15 nm) bathochromic shift of the absorption band and a more intensive fluorescence.
A relative fluorescence quantum yield of the dimethoxy derivative 3f is the highest (ΦF 0.59) and comparable to the used standard (7-hydroxy-4-methylcoumarine in 0.1 M phosphate buffer with pH = 10, ΦF 0.63, excitation wavelength is 375 nm). Compounds 3d and 3g possess the lowest emission intensity probably due to the absence of the donating moieties in the aryl substituent. The full characteristics of the luminescence are given in the Table 3.
Solid-state fluorescence of synthesized compounds
Microcrystalline compounds 3a–f are bright green or yellow-green. Upon illumination by an UV lamp, compounds 3b–e,g,h are brightly emissive in the blue-green region of the spectrum while compounds 3a,f are less emissive. The solid-state fluorescence spectra were recorded at room temperature and showed a main band around 480 nm and a shoulder around 510 nm. The emission bands appeared in a narrow region (478–491 nm) (see Fig. 3, Table 4). Nevertheless, a substituent in the aryl moiety possesses a significant influence on the emission intensity. Methoxy group insertion led to the decreasing of solid-state fluorescence intensity for compound 3h by about 20% in compare to unsubstituted compound 3g. In compare to unsubstituted compound 3d methoxy derivative 3e has a 55% less intensive emission, and dimethoxy derivative 3f has an 85% less one. It should be noted that an observed regularity is opposite to the fluorescence in solution.| 3 | λex; max, nm | λem; max, nm | R.I.a | HOMOb, eV | LUMOb, eV | GAPb, eV |
|---|---|---|---|---|---|---|
| a Excited by 351 nm, R.I. – relative intensity of emission is given in compare to 3h.b Calculated in the basis TDDFT(B3LYP)/6-31+G(d,p). | ||||||
| 3a | 444 | 489 | 0.18 | −6.2 | −2.29 | 3.91 |
| 3b | 446 | 478 | 1.16 | −6.15 | −2.24 | 3.91 |
| 3c | 451 | 481 | 0.71 | −6.14 | −2.29 | 3.85 |
| 3d | 455 | 486 | 1.21 | −6.36 | −2.57 | 3.79 |
| 3e | 450 | 486 | 0.54 | −6.18 | −2.42 | 3.76 |
| 3f | 460 | 491 | 0.18 | −6.03 | −2.41 | 3.62 |
| 3g | 453 | 487 | 1.23 | −6.20 | −2.44 | 3.76 |
| 3h | 459 | 488 | 1.00 | −6.09 | −2.36 | 3.73 |
Also, an interesting phenomenon is the wide region of the excitation of solid-state fluorescence which is located in area of 320–470 nm. The highest emission intensity is observed for compounds 3 upon excitation by irradiation of the blue region of the spectrum with wavelengths of 450–460 nm, which is clearly shown in Fig. 4.
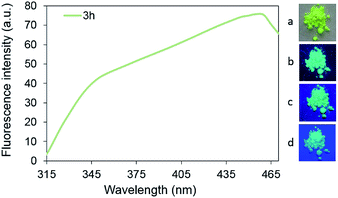 | ||
| Fig. 4 Solid-state excitation spectrum of compound 3h and photos under daylight (a), UV (b: 365 nm) and visible (c: 400 nm, d: 455 nm) irradiation. | ||
The quantum-chemical calculations in the basis TDDFT(B3LYP)/6-31+G(d,p) (see Fig. 5) showed that electron density of the highest occupied molecular orbital (HOMO) is localized on the diethylamino moiety and the lowest unoccupied molecular orbital (LUMO) involved cyano group. It indirectly confirms that absorption of light could lead to the intramolecular charge transfer from the donor moiety to acceptor. The energy levels of Frontier orbitals and gap values are also given in Table 4.
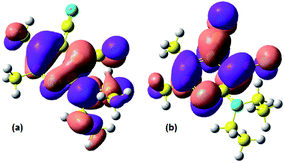 | ||
| Fig. 5 (a) HOMO and (b) LUMO for compound 3a, computed with TDDFT(B3LYP)/6-31+G(d,p). The pink (purple) lobes indicate a positive (negative) isocontour value. | ||
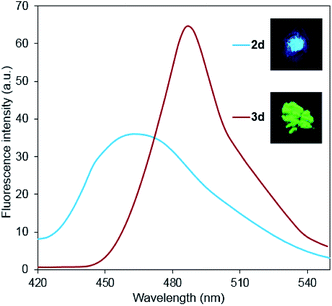
Continuing our earlier studies,15b it is important to note that the fluorescence intensity increases significantly, and its band shifts to the long-wavelength region upon transformation of 2-chloropyridines 2 to 2-diethylaminopyridine derivatives 3 (Fig. 6). It demonstrates the promise of this work, as well as the subsequent studies of spectral properties of other 3,4-dicyano-substituted aminopyridines.
Crystal structures characterization
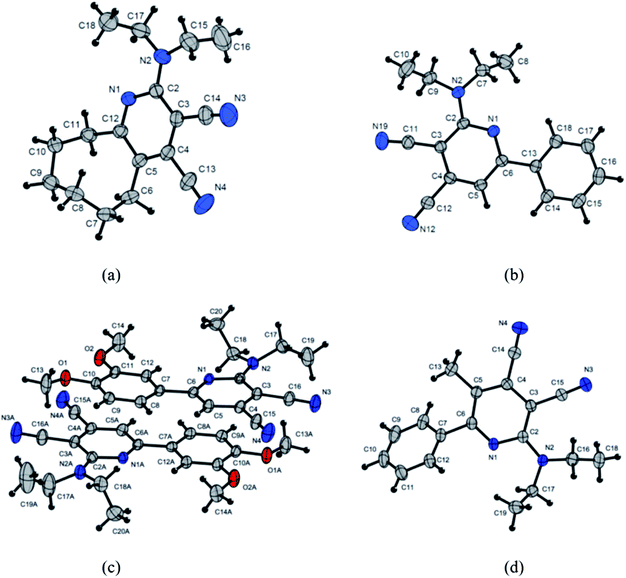
The obtained single crystal X-ray crystallographic data of the synthesized compounds can be used to gain a better understanding of the relationship between the optical properties and the molecular conformation and packing mode. Single crystals of 3c, 3d, 3f and 3g suitable for X-ray structural analysis were prepared by slow evaporation of acetonitrile solution, at room temperature (Fig. 7).The pyridine ring of the 3g (Fig. 7d) is overloaded with substituents, which leads to a substantial deformation of the heterocycle. The deviation of the atoms C3, C2, N1 from the plane through C4⋯C5⋯C6 – are 0.05, −0.20, −0.12 Å, respectively. The angle along the C6⋯C3⋯N3 line of pyridine cycle and cyano group at the third position is 171.1, while for another cyano group at the forth position, the angle along the N1⋯C4⋯N4 line is 177.8. The heterocycle forms a dihedral angle −130.5 with a phenyl ring. Despite the significant steric interactions, the amino group (nitrogen atom N2) actively interacts with the π-heterocycle system. The bond length C2–N2 (1.351 Å) coincides with the value of the delocalized bond N1 C2 (1.354 Å). The nitrogen atom N2 is located outside (0.1 Å) of plane running through atoms C2, C17, C18. This means, that the nitrogen atom, despite its conjugation with the π-system, retains, to some extent, the tetrahedral (sp3) configuration.
Interestingly, there are two independent molecules in the structure of 3f (Fig. 7c). The two molecules possess the different conformations with the different torsion angles. The greatest difference is observed in twisted conformation in which the torsion angles between pyridine ring and ethyl moieties. Torsion angles C3⋯C2⋯N2⋯C17 and C3⋯C2⋯N2⋯C18 are −34.7 and 167.5 respectively, while angles C3A⋯C2A⋯N2A⋯C17A and C3A⋯C2A⋯N2A⋯C17A are – 25.9 and 171.6.
Pyridine ring in the molecule 3f as well as in the compound 3g is significantly deformed. The deviations from the plane running through the atoms C4⋯C5⋯C6 (C4A⋯C5A⋯C6A) have C2 −0.12 and C2A −0.07 Å. The angle C6⋯C3⋯N3 (C6A⋯C3A⋯N3) of the line along the pyridine cycle and the cyano group at the third position is 171.7 (173.7), while for the cyano group at the forth position the angle N1⋯C4⋯N4 (N1A⋯C4A⋯N4A) is 178.9 (177.6).
Amino groups of N2, N2A are conjugated to pyridine rings with parameters close to 3g N2⋯C2 is 1.356 Å and N2A⋯C2A is 1.354 Å. In the crystal, molecules form dimers, as a result of the π–π interaction. The shortest distance C12⋯C6A is 3.364 Å.
Phenyl ring in structure of 3f lie in the same plane with the pyridine rings in contrast to the molecule 3g. This difference can be explained by the influence of the methyl group at the fifth position of the pyridine ring, which creates steric hindrances for the planar arrangement of the rings in the case of 3g. In the crystal of compound 3f, wherein the hydrogen atom in the fifth position, the repulsion between the atoms H5⋯H8 (H5⋯H8a) is compensated by the interaction of the lone electron pair of the nitrogen atom, N1 (N1A), and the hydrogen atoms, H12 (H12A), respectively, for each of the independent molecules.
The structure of the molecule 3d (Fig. 7b) in the crystal is similar to the structure of the 3f molecule. Small differences are observed in the packing of molecules in a crystal. Molecules form stacks in which the values of the distances between neighboring molecules are substantially similar, but greater than 3.5 Å, that is, more than the sum of van der Waals radii of carbon–carbon.
Pyridine cycles in molecule 3d are planar, in contrast to molecules 3f and 3g. Nevertheless, the cyano group in the third position deviates from the plane of the pyridine ring, and the angle along the line C6⋯C3⋯N19 is 173.1, while the cyano group in the fourth position does not deviate, and the angle N1⋯C4⋯N12 is 178.3.
Conformational features of the 8-membered cycle of the molecule 3c can be seen in Fig. 7a. As in the structures presented earlier, the amino group (ethyl moieties) prevents from the π–π interacting adjacent molecules in the stack. The angle C12⋯C3⋯N3 along the pyridine ring and the cyano group at the third position is 173.0, while with the cyano group at the fourth position the angle N1⋯C4⋯N4 is to 179.4.
To summarize the obtained data for the compounds studied, the deviation of angles for cyano groups in the third position of pyridine cycle for all the crystals obtained is observed in crystalline form and is in the range of 171–173°. It is probably the result of the influence of the adjacent diethylamine fragment, but on the other hand it also can indicate a significant contribution of the intermolecular charge-transfer (ICT) structure. The latter is supported by the fact that exactly the acceptor cyano group in the third position is in effective conjugation with the donor diethylamine fragment.
Moreover, the obtained data for crystal 3f shows a difference in the torsion angles for the diethylamino group in two independent molecules of the dimer (Fig. 7c). A different conjugation degree of the amino group with the π-heterocycle system is the consequence of the above. This is probably the reason for the appearance of a “shoulder” in the emission spectra in the solid state and in solutions for all compounds. It is especially noticeable for the solid state emission of compounds 3a and 3f (Fig. 3).
Conclusions
In conclusion, we have developed the facile method for the synthesis of 2-diethylaminocinchomeronic dinitrile derivatives and have studied fluorescence properties thereof in the solutions and in the solid state. High fluorescence quantum yield was observed for the solutions of pyridine derivatives with electron donating groups in nonpolar solvents. Moreover, an intensive solid-state emission in the blue-green region of the spectrum was observed for alkyl substituted 2-diethylaminocinchomeronic dinitriles, as well as compounds with unsubstituted aryl moieties. This work is a continuation of the series of papers aimed to the study of the correlation of the structural framing of cyano-substituted pyridines and their derivatives with their photoluminescent properties.15Acknowledgements
The study was performed within the framework of the baseline of the State Assignment for scientific activity of the Ministry of Education and Science of Russia No. 4.6283.2017/8.9. The XRD study was carried out using the equipment purchased from the funds of the Program of development of Moscow University and within the framework of the Agreement on collaboration between the Chemical Department of the Lomonsov Moscow State University and the Chemical-Pharmaceutical Department of the I. N. Ulyanov Chuvash State UniversityNotes and references
- (a) S. Mukherjee and P. Thilagar, Dyes Pigm., 2014, 110, 2 CrossRef CAS; (b) W. Guan, W. Zhou, J. Lu and C. Lu, Chem. Soc. Rev., 2015, 44, 6981 RSC; (c) J. Li, C. Yin and F. Huo, Dyes Pigm., 2016, 131, 100 CrossRef CAS; (d) D. Jaque and F. Vetrone, Nanoscale, 2012, 4, 4301 RSC; (e) M. Saleem and K. H. Lee, RSC Adv., 2015, 5, 72150 RSC.
- (a) W. Liu, Z. Chen, C.-J. Zheng, X.-K. Li, K. Wang, F. Li, Y.-P. Dong, X.-M. Ou and X.-H. Zhang, J. Mater. Chem. C, 2015, 3, 8817 RSC; (b) W. Li, J. Li, D. Liu, D. Li and F. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 21497 CrossRef CAS PubMed; (c) J. You, S.-L. Lai, W. Liu, T.-W. Ng, P. Wang and C.-S. Lee, J. Mater. Chem., 2012, 22, 8922 RSC; (d) N. Li, P. Wang, S.-L. Lai, W. Liu, C.-S. Lee, S.-T. Lee and Z. Liu, Adv. Mater., 2010, 22, 527 CrossRef CAS PubMed.
- (a) J. You, M.-F. Lo, W. Liu, T.-W. Ng, S.-L. Lai, P. Wang and C.-S. Lee, J. Mater. Chem., 2012, 22, 5107 RSC; (b) Y. Ooyama, S. Inoue, T. Nagano, K. Kushimoto, J. Ohshita, I. Imae, K. Komaguchi and Y. Harima, Angew. Chem., Int. Ed., 2011, 50, 7429 CrossRef CAS PubMed; (c) E. V. Verbitskiy, P. A. Slepukhin, Y. O. Subbotina, M. S. Valova, A. V. Schepochkin, E. M. Cheprakova, G. L. Rusinov and V. N. Charushin, Chem. Heterocycl. Compd., 2014, 50, 814 CrossRef CAS; (d) T. N. Ahipa, K. M. Anoop and R. K. Pai, New J. Chem., 2015, 39, 8439 RSC.
- (a) M. R. S. A. Janjua, W. Guan, L. Yan, Z.-M. Su, A. Karim and J. Akbar, Eur. J. Inorg. Chem., 2010, 2010, 3466 CrossRef; (b) V. K. Indirapriyadharshini, P. Ramamurthy, V. Raghukumar and V. T. Ramakrishnan, Spectrochim. Acta, Part A, 2002, 58, 1535 CrossRef CAS; (c) Yo. Li, T. Liu, H. Liu, M.-Z. Tian and Yu. Li, Acc. Chem. Res., 2014, 47, 1186 CrossRef CAS PubMed.
- (a) T. N. Ahipa and A. V. Adhikari, Photochem. Photobiol. Sci., 2014, 13, 1496 RSC; (b) T. N. Ahipa, V. Kumar and A. V. Adhikari, Liq. Cryst., 2013, 40, 31 CrossRef CAS.
- M. D. Bowman, M. M. Jacobson and H. E. Blackwell, Org. Lett., 2006, 8, 1645 CrossRef CAS PubMed.
- (a) R. R. Koner, S. Sinha, S. Kumar, C. K. Nandi and S. Ghosh, Tetrahedron Lett., 2012, 53, 2302 CrossRef CAS; (b) J. Yan, J. Li, P. Hao, F. Qiu, M. Liu, Q. Zhang and D. Shi, Dyes Pigm., 2015, 116, 97 CrossRef.
- A. Basta, M. Missori, A. S. Girgis, M. De Spirito, M. Papi and H. El-Saieda, RSC Adv., 2014, 4, 59614 RSC.
- (a) J. Kuthan, P. Nesvadba, M. Popl and J. Fahnrich, Collect. Czech. Chem. Commun., 1979, 44, 2409 CrossRef CAS; (b) O. V. Ershov, S. V. Fedoseev, M. Y. Ievlev and M. Y. Belikov, Dyes Pigm., 2016, 134, 459 CrossRef CAS.
- Y. Hong, J. W. Y. Lama and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- (a) S. Kato and F. Diederich, Chem. Commun., 2010, 46, 1994 RSC; (b) V. Jeux, O. Segut, D. Demeter, O. Aleveque, P. Leriche and J. Roncali, ChemPlusChem, 2015, 80, 697 CrossRef CAS.
- A. Weisstuch and A. C. Testa, Phys. Chem., 1968, 72, 1982 CrossRef CAS.
- (a) A. M. Asiri, S. A. Khan and S. H. Al-Thaqafya, J. Fluoresc., 2015, 25, 1203 CrossRef CAS PubMed; (b) S. M. Afzal, A. M. Asiri, M. A. N. Razvi, A. H. Bakry, S. A. Khan and M. E. M. Zayed, J. Fluoresc., 2016, 26, 559 CrossRef CAS PubMed; (c) H.-Y. Wang, J.-J. Shi, C. Wang, X.-X. Zhang, Y. Wan and H. Wu, Dyes Pigm., 2012, 95, 268 CrossRef CAS; (d) S. A. Khan and A. M. Asiri, J. Mol. Liq., 2016, 221, 381 CrossRef CAS; (e) T. Landmesser, A. Linden and H. J. Hansen, Helv. Chim. Acta, 2008, 91, 265 CrossRef CAS.
- (a) S. Nandi, M. M. Islam, M. Saha, S. Mitra, S. Khatua and A. K. Pal, Synth. Commun., 2016, 46, 1461 CrossRef CAS; (b) J. D. Cheon, T. Mutai and K. Araki, Org. Biomol. Chem., 2007, 5, 2762 RSC.
- (a) O. V. Ershov, S. V. Fedoseev, M. Y. Belikov and M. Y. Ievlev, RSC Adv., 2015, 5, 34191 RSC; (b) O. V. Ershov, M. Y. Ievlev, M. Y. Belikov, K. V. Lipin, A. I. Naydenova and V. A. Tafeenko, RSC Adv., 2016, 6, 82227 RSC.
- (a) L. Vachova, M. Machacek, R. Kučera, J. Demuth, P. Cermak, K. Kopecky, M. Miletin, A. Jedlickova, T. Simunek, V. Novakova and P. Zimcik, Org. Biomol. Chem., 2015, 13, 5608 RSC; (b) T. C. Tempesti, M. G. Alvarez and E. N. Durantini, Dyes Pigm., 2011, 91, 6 CrossRef CAS.
- V. P. Sheverdov, O. V. Ershov, A. V. Eremkin, O. E. Nasakin, I. N. Bardasov and V. A. Tafeenko, Russ. J. Org. Chem., 2005, 41, 1757 CrossRef CAS.
- O. V. Ershov, M. Y. Ievlev, M. Y. Belikov and O. E. Nasakin, Russ. J. Org. Chem., 2016, 52, 1353 CrossRef CAS.
- V. N. Maksimova, O. V. Ershov, K. V. Lipin, A. V. Eremkin and O. E. Nasakin, Russ. J. Org. Chem., 2012, 48, 426 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1545662–1545665. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra06217f |
| This journal is © The Royal Society of Chemistry 2017 |