Open Access Article
Open Access ArticleHigh yield production of C2–C3 olefins and para-xylene from methanol using a SiO2-coated FeOx/ZSM-5 catalyst†
Qiongfang Hua,
Xiaofan Huangbc,
Yu Cuiab,
Tengfa Luoc,
Xiaoping Tangab,
Tong Wangabc,
Weizhong Qian *a and
Fei Wei
*a and
Fei Wei a
a
aDepartment of Chemical Engineering, Tsinghua University, 100084, China. E-mail: qianwz@tsinghua.edu.cn
bCoal Chemical Industry Division, Huadian Coal Industry Group Co. Ltd., Beijing 100031, China
cShanxi Yuheng Chemical Co., Huadian Coal Industry Group Co. Ltd., Yulin, 719000, Shanxi, China
First published on 1st June 2017
Abstract
Herein, FeOx-supported ZSM-5 exhibited excellent catalytic performance in the aromatization of methanol, similar to ZnOx-supported ZSM-5 but with much longer life time. Coating of FeOx/ZSM-5 catalyst with a SiO2 shell is effective to suppress the isomerization of xylene and hydrogen transfer from propylene to propane on the external surface. As a result, FeOx/ZSM-5@SiO2 exhibited gross yield of C2–C3 olefins and para-xylene (carbon base) far higher than that of HZSM-5, ZnOx/ZSM-5, and ZnOx/ZSM-5@SiO2.
Transformation of methanol on zeolite remained a hot catalytic field in the last two decades, owing to its importance in providing C2–C3 olefins and aromatics, which are key building blocks in the chemical industry.1–7 Although numerous efforts were made in solely improving the selectivity of olefins or aromatics, there always existed both olefins and aromatics in the product,1,4–8 owing to the intrinsic dual hydrocarbon pool mechanism of conversion of methanol inside HZSM-5.9–12 Considering that both C2–C3 olefins and aromatics, especially para-xylene (PX), belong to the most valuable products in hydrocarbons, it is also a good choice to achieve high gross yield of them. While realizing this goal in a methanol-to-aromatics (MTA) reaction, it is crucial to increase the single pass yield of PX and C2–C3 olefins by maintaining high aromatization ability, as well as suppressing various reactions including dealkylation, alkylation, and isomerization of xylene (X) and the hydrogen transfer from olefins to paraffins.4,13–18 The widely investigated catalysts in the MTA process included ZnOx-, AgO- or Ga2O3-doped ZSM-5.13–19 Among these, ZnOx/ZSM-5 has received significant attention since Ag is too expensive and Ga2O3 is toxic and has very low reserve in the earth. Definitely, ZnOx interacted with ZSM-5 to create Lewis acids sites in high density on the catalyst,1,22,23 resulting in the increase of aromatization ability, compared to that of HZSM-5. However, ZnOx-doped ZSM-5 (microsized) always suffered from the limitation of rapid deactivation within 1–2 hours.13 Nanosized ZSM-5 or hierarchical structures of ZSM-5 were used to enhance the diffusion of the intermediate products outside the pores.15–21 However, the excess alkylation of xylene (X) occurred on the huge external surface of nanocrystals, resulting in very low yield of PX.15–21 In addition, another disadvantage of ZnOx for use in fluidized beds is the formation of a spinel of ZnAl2O4 when it interacts with alumina gel, as reported previously.13,18,24,25
In the present study, we proposed FeOx-doped ZSM-5 as a new platform of MTA. The acidity information was compared with that of ZnOx/ZSM-5. In addition, both FeOx/ZSM-5 and ZnOxZSM-5 were coated with SiO2 shell to supress the external acids and the associated isomerization of X. The catalytic performances, including the conversion of methanol, yield of aromatics, selectivity of PX in X, yield of PX, and effect on the hydrogen transfer of C2–C3 olefins (to paraffins) were discussed based on HZSM-5, ZnOx-based, and FeOx-based catalysts. SiO2-coated FeOx/ZSM-5 exhibited longer life time to produce C2–C3 olefins and PX in the highest gross yield among all the catalysts tested. The shorter life time of ZnOx-based catalysts is due to high density of Lewis acids as compared to those of HZSM-5 and FeOx-based catalysts. The present study not only provides the clues for the variation of C2–C3 olefins and PX influenced by the external acids but also provides a new catalyst platform for MTA reaction for the control of the product selectivity.
Experimentally, HZSM-5 with a Si/Al ratio of 12.5 and average size of 2 micrometers (purchased from Nankai Catal. Corp., China) was doped with a solution of Fe(NO3)3 under stirring for 5 hours. Then, the mixture was dried at 110 °C for 12 hours to evaporate water and calcined at 550 °C for 5 hours. The catalyst was denoted as FeOx/ZSM-5. Loading of FeOx, calculated by Fe, is 4.2% on ZSM-5.
Similarly, solutions of Zn(NO3)2 were impregnated on ZSM-5 powders following the same drying and calcination procedures to obtain a catalyst denoted as ZnOx/ZSM-5. Loading of ZnOx, calculated by Zn, is 3% on ZSM-5, in agreement with the optimized value reported in previous work.4,21 To create a SiO2 shell on the external surface, the catalyst powders were soaked in the solution of TEOS in cyclohexane under stirring for 5 hours.4 Then, the mixture was dried at 110 °C for 24 hours, and the powders were calcined at 550 °C for 5 hours. The catalysts with a SiO2 shell are denoted as ZnOx/ZSM-5@SiO2 and FeOx/ZSM-5@SiO2. NH3-TPD and Py-IR were used to obtain information about the acidity of different catalysts.4 Probe molecule of 2,6-di-tert-butylpyridine was used to quantitatively determine the density of external acids of different catalysts (ESI-1, Fig. S1†). To obtain the yield of C2–C3 olefins and PX from methanol, 1 g of the catalyst was placed into a quartz-made packed bed reactor to decompose methanol at 475 °C and ambient pressure. Space velocity of methanol is 0.79 g gcat−1 h−1. All gaseous products were online analysed by the GC. The yield of hydrocarbons was calculated with the carbon base.
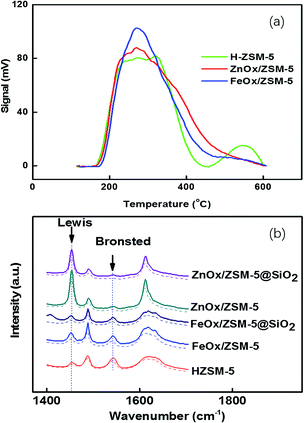
TEM characterization indicated that ZnOx species were monodispersed into the matrix of ZSM-5. Only the crystals of ZSM-5 were observed in the TEM image (ESI-2, Fig. S2†). However, for the doping of FeOx species on ZSM-5, the crystals of Fe2O3 can be clearly observed in the TEM image (ESI-1, Fig. S3†). TPR of FeOx/ZSM-5 suggested that FeOx is only partly reduced at the reaction temperature of 475 °C in the MTA reaction (ESI-3, Fig. S4†). It validated that it is FeOx, not metallic Fe, that interacts with ZSM-5. The coating method of ZnOx-based or FeOx-based ZSM-5 by SiO2 is similar to that reported in previous work,4 giving a SiO2 shell thickness around 8 nm. NH3-TPD was used to determine the strength and density of the acid sites of HZSM-5, FeOx−, and ZnOx-supported ZSM-5 (Fig. 1a). The peak at 200–250 °C is the contribution of the hydrogen bond of NH4+, assigned to the weak acid sites. Another peak of HZSM-5 at 542.9 °C was assigned to the strong acid sites. This peak (at 542 °C) disappeared after ZnOx or FeOx were doped in ZSM-5. However, peak areas between 300 °C and 500 °C increased after the doping, which were associated with the medium strong acids. Quantitatively, peak areas at 227.3, 315.6, and 542.9 °C for HZSM-5 contributed to 25.3%, 60.6%, and 14.1%, respectively (ESI-4, Table S1†). Peak areas at 224.7, 267.8, and 328.9 °C for FeOx/ZSM-5 contributed to 9.0%, 36.8%, and 54.2%, respectively. In comparison, peak areas at 221.4, 266.2, and 342.7 °C for ZnOx/ZSM-5 contributed to 11.1%, 6.5%, and 62.4%, respectively. The medium strong acids for ZnOx/ZSM-5 had a higher density with a peak temperature 14 °C higher than that of FeOx/ZSM-5. Similarly, the medium strong acids for FeOx/ZSM-5 had a peak temperature 14 °C higher than that of HZSM-5. Doping of metal oxides not only changed the peak position and acidic strength discussed above, but also significantly decreased the acid density (ESI-5, Table S2†). These differences would exhibit different effects on the catalytic performances discussed below.
Py-IR suggested that peaks of the Brönsted acids of HZSM-5, centred at 1540 cm−1, almost disappeared for ZnOx/ZSM-5 (Fig. 1b), but most of them were retained after doping of FeOx. Coating of the catalyst by the SiO2 shell resulted in the decrease of both the Brönsted acids and Lewis acids, whether for ZnOx/ZSM-5 or for FeOx/ZSM-5. Compared to the ZnOx-based catalysts, FeOx-based catalysts had higher density of Brönsted acids but much lower density of Lewis acids.
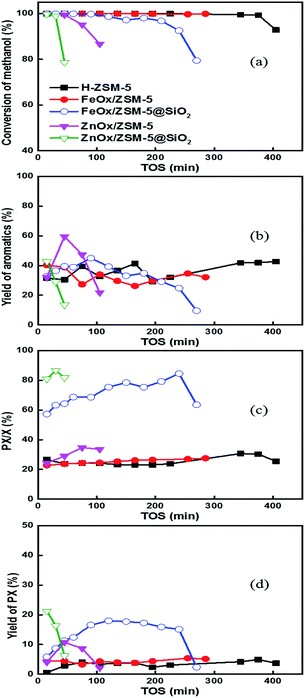
Catalytic performances of different catalysts were evaluated using the MTA reaction. Conversion of methanol with HZSM-5 was always close to 100% within 420 minutes (Fig. 2a). The doping of metal oxides or coating with the SiO2 shell on ZSM-5 resulted in the decrease of both surface area and pore volume (ESI-5, Table S2†), which influenced the diffusion of the product molecules and therefore shortened the life time of the catalyst. In detail, doping of FeOx did not change this trend, and the conversion of methanol was close to 100% within 300 min. Conversion of methanol with FeOx/ZSM-5@SiO2 decreased to 97% at 120 min but could remain above 90% within 240 min, due to the decreased strength of external acids by coating (Fig. 1b). In contrast, the conversion of methanol with ZnOx/ZSM-5 did not remain constant at 60 min. Even worse, the deactivation of ZnOx/ZSM-5@SiO2 catalyst was accelerated, due to the decrease of surface area and pore volume after coating with the SiO2 shell (ESI-5, Table S2†).
Previous results suggested that ZnOx interacted with ZSM-5 to form stable acidic sites, compared to FeOx or Ga2O3, and it may be preparation method dependent.22 Similar trends were observed with the yield of aromatics with different catalysts (Fig. 2b). HZSM-5, FeOx/ZSM-5, and FeOx/ZSM-5@SiO2 all exhibited longer life times for producing aromatics as compared to the ZnOx-based catalyst. In detail, FeOx-based catalyst exhibited higher yield of aromatics in the initial stage of the reaction as compared to HZMS-5. In addition, yields of aromatics with three catalysts (HZSM-5, FeOx/ZSM-5, and FeOx/ZSM-5@SiO2) were very high in the initial stage. In sharp contrast, there is an obvious induction period for ZnOx/ZSM-5. Yield of aromatics drastically increased from 15 to 60 minutes, reached the highest value at 60 minutes, but quickly dropped within 60–120 minutes. Yield of aromatics with ZnOx/ZSM-5@SiO2 drastically dropped from 15 to 30 minutes, showing a trend of rapid deactivation.
The coating effect with the SiO2 shell on the selectivity of PX in X was studied, and the results are shown in Fig. 2c. For HZSM-5 and FeOx/ZSM-5, this value is always 23–25% at very long times, close to the equilibrium ratio of PX in X with the catalyst with strong acids.4,13,26–28 However, the value slightly increased with the reaction time using ZnOx/ZSM-5, due to the coke deposition. In sharp contrast, coating of FeOx/ZSM-5 with SiO2 increased the selectivity of PX in X from 25% to 60% in the initial stage of the reaction, and the value sustainably increased to 80%, due to the gradual coke deposition with the reaction time.4 Only when the pores of the catalyst were seriously blocked, the value decreased at 240–270 minutes. Using ZnOx/ZSM-5@SiO2, the selectivity of PX in X was directly increased to 80% from the pristine 25% and further increased to 90% at 60 minutes. Based on the selectivity of PX in X and the yield of X, we were able to calculate the yield of PX (carbon base, Fig. 2d). The yield of PX with FeOx/ZSM-5@SiO2 was around 3 times of those with HZSM-5 and FeOx/ZSM-5. More strikingly, the gross yield of PX with FeOx/ZSM-5@SiO2, in single pass conversion, is 4–5 times that of ZnOx/ZSM-5@SiO2, owing to the longer life time of the former. The value also ranked as the highest value ever reported, validating that the SiO2-coated FeOx/ZSM-5 is a very promising catalyst for the production of PX in high yield. External acids of ZnOx/ZSM-5 are approximately 3.27% of the total but decreased to 2.44% of the total after it was coated with the SiO2 shell (ESI-5, Table S2†). In comparison, external acids of FeOx/ZSM-5 are approximately 5.85% of the total but decreased to 1.81% after it was coated with the SiO2 shell, qualitatively in agreement with the previous statement.4,26–28 Density of external acids is 0.024 mmol g−1 and 0.013 mmol g−1 for ZnOx/ZSM-5@SiO2 and FeOx/ZSM-5@SiO2, respectively. The low value is key to the high selectivity of PX in X via the suppression of isomerization of X. In addition, coating the catalyst with the SiO2 shell resulted in the significant decrease of both surface area and pore volume (ESI-5, Table S2†). Pores of the amorphous layer of SiO2, studied with the adsorption of PX,4 were in the range of 0.51–0.57 nm. ZSM-5 exhibited shape selectivity of ortho-xylene (OX), but not on PX, significantly dominating their decomposition behaviour on an external surface or in the internal pores of the zeolite.4
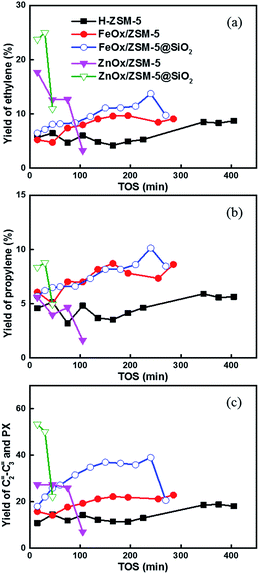
Furthermore, we compared the yield of olefins obtained using different catalysts (Fig. 3). The yield of ethylene with FeOx/ZSM-5 is 3–5% higher than that obtained with HZSM-5. This value can be further increased by coating FeOx/ZSM-5 with SiO2. In comparison, the yield of ethylene with ZnOx-based catalyst is much higher, but the trend could not remain stable for a long time (Fig. 3a). Interestingly, FeOx-based catalyst gave higher yield of propylene, compared to HZSM-5 and ZnOx-based catalysts (Fig. 3b). The difference in obtaining different yields of different olefins would be attributed to the dual hydrocarbon pool mechanism with different catalysts.9–12 Actually, propylene is mostly produced from the olefin pool, nearly independently of the aromatics pool. Ethylene is mostly produced from the aromatics pool, which should exhibit similar change trends with the aromatic products.12 The conclusion is supported by the change trends of aromatics and ethylene in Fig. 2b and 3a, respectively. For the yield of C2–C3 olefins and PX together (Fig. 3c), FeOx/ZSM-5@SiO2 exhibited highest gross yield, which is approximately 2 times that obtained using FeOx/ZSM-5, 2.5 times that obtained using HZSM-5, and 3–4 times that obtained using ZnOx/ZSM-5 and ZnOx/ZSM-5@SiO2.
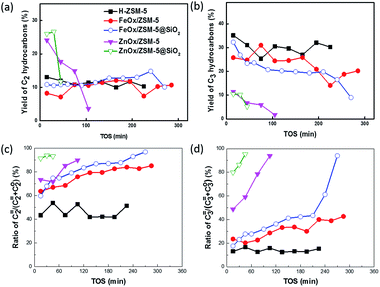
In addition, the yields of C2 or C3 hydrocarbons with different catalysts were also compared (Fig. 4a and b). ZnOx-based catalysts exhibited high yield of C2 hydrocarbons but much lower yield of C3 hydrocarbons, compared to HZSM-5 or FeOx-based catalysts. The yields of C2 and C3 hydrocarbons with FeOx catalysts were similar to those obtained with HZSM-5. These results suggested that the aromatic pool and olefin pool can be tuned with catalysts with different acids. FeOx/ZSM-5 had densities of Lewis acids and Brönsted acids similar to those of HZSM-5. They gave similar distribution of C2 and C3 hydrocarbons. In comparison, a high density of Lewis acids on ZnOx-based catalysts enhanced the aromatic pool, considering that C2 hydrocarbons were mostly produced from aromatics.12 The yield of C3 hydrocarbons with ZnOx/ZSM-5 is only 1/3 of that obtained with HZM-5, suggesting that the olefin pool was significantly suppressed with ZnOx-based catalysts. In addition, the ratios of ethylene to C2 hydrocarbons (ethylene and ethane) and propylene to C3 hydrocarbons (propylene and propane) were calculated (Fig. 4c and d). The modification of ZSM-5 with ZnOx and FeOx increased the ratio of ethylene/(ethylene and ethane), compared to HZSM-5. It validated the suppression of hydrogen transfer of olefins, and the enhancement of the aromatization could be simultaneously realized. Quantitatively, coating of FeOx/ZSM-5 with SiO2 resulted in the ratio of ethylene to C2 hydrocarbons increasing by 10–15%. Moreover, selectivity of PX in X is increased by 55–60%. Reasonably, the isomerization of X mainly occurred at the external surface of the catalyst and not inside the pores, due to the shape selectivity of ZSM-5.5 However, the hydrogen transfer reaction of ethylene mainly occurred inside the pores, considering that the inner surface area of microsized ZSM-5 is far larger than that of the external surface. However, the hydrogen transfer of propylene would be effectively suppressed using the catalysts with the SiO2 shell. Since propylene had a diffusion rate slower than that of ethylene, the contact between them with the external surface of the catalyst would exhibit a significant effect on their selectivity, compared to that of the smaller molecule.29
In addition, the relation between the acidity information and the catalytic performance of the catalysts also suggested that Lewis acids in large amounts with ZnOx/ZSM-5 resulted in the increased yield of aromatics and ethylene, but shortened the lifetime of the catalyst. FeOx/ZSM-5 with slightly higher amount of Brönsted acids and less amount of Lewis acids exhibited higher yield of aromatics and longer lifetime. The difference may be attributed to the interaction of ions of Zn2+ and ions of Fe+, Fe2+, and Fe3+ with the ZSM-5. Further investigation is needed to develop an understanding of the different acids with different catalysts. Moreover, the syngeneic effect, by coating FeOx/ZSM-5 with the SiO2 shell, gave a better catalyst than ZnOx-based catalyst, in terms of producing a higher gross yield of C2–C3 olefins and PX for long times (Fig. 3c).
In summary, we evaluated that the FeOx-doped ZMS-5 exhibited better performance in terms of stability in high temperature reaction. Coating the catalyst with the SiO2 shell effectively suppresses the isomerization of X and the hydrogen transfer of propylene on an external surface of the catalyst.
FeOx/ZSM-5@SiO2 exhibited longer life time to produce C2–C3 olefins and PX in far higher gross yield, compared to other catalysts. It was also validated that the catalyst with high density of Lewis acids was easily deactivated as compared to the catalyst with both Brönsted acids and Lewis acids with medium strength. These results were useful for the further control of the product selectivity in methanol-to-aromatics process.
Acknowledgements
The authors thank the support of NSFC program (21376135, 91434122 and 51236004).Notes and references
- M. Stöcker, Microporous Mesoporous Mater., 1999, 29, 3–48 CrossRef.
- S. T. Xu, A. M. Zheng, Y. X. Wei, J. R. Chen, J. Z. Li, Y. Y. Chu, M. Z. Zhang, Q. Y. Wang, Y. Zhou, J. B. Wang, F. Deng and Z. M. Liu, Angew. Chem., Int. Ed., 2013, 125, 11778–11782 CrossRef.
- Y. X. Li, M. Y. Zhang, D. Z. Wang, F. Wei and Y. Wang, J. Catal., 2014, 311, 281–287 CrossRef CAS.
- J. G. Zhang, W. Z. Qian, C. Y. Kong and F. Wei, ACS Catal., 2015, 5, 2982–2988 CrossRef CAS.
- Y. Bhawe, M. Moliner-Marin, J. D. Lunn, Y. Liu, A. Malek and M. Davis, ACS Catal., 2012, 2, 2490–2495 CrossRef CAS.
- J. Zhou, J. W. Teng, L. P. Ren, Y. D. Wang, Z. C. Liu, W. Liu, W. M. Yang and Z. K. Xie, J. Catal., 2016, 340, 166–176 CrossRef CAS.
- J. F. Haw, J. B. Nicholas, W. G. Song, F. Deng, Z. K. Wang, T. Xu and C. S. Heneghan, J. Am. Chem. Soc., 2000, 122, 4763–4775 CrossRef CAS.
- D. L. Cai, Q. Wang, Z. Jia, Y. H. Ma, Y. Cui, U. Muhammad, Y. Wang, W. Z. Qian and F. Wei, Catal. Sci. Technol., 2016, 6, 1297–1301 CAS.
- S. Ilias and A. Bhan, J. Catal., 2012, 290, 186–192 CrossRef CAS.
- U. Olsbye, S. Svelle, M. Bjorgen, P. Beato, T. V. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810–5831 CrossRef CAS PubMed.
- S. Svelle, F. Joensen, J. Nerlov, U. Olsbye, K. P. Lillerud, S. Kolboe and M. Bjørgen, J. Am. Chem. Soc., 2006, 128, 14770–14771 CrossRef CAS PubMed.
- R. Khare and A. Bhan, J. Catal., 2015, 329, 218–228 CrossRef CAS.
- T. Wang, X. P. Tang, X. F. Huang, W. Z. Qian, Y. Cui, X. Y. Hui, W. Yang and F. Wei, Catal. Today, 2014, 233, 8–13 CrossRef CAS.
- D. Freeman, R. P. K. Well and G. J. Hutchings, J. Catal., 2002, 205, 358–365 CrossRef CAS.
- N. Wang, W. Z. Qian, K. Shen, C. Su and F. Wei, Chem. Commun., 2016, 52, 2011–2014 RSC.
- J. A. Lopez-Sanchez, M. Conte, P. Landon, W. Zhou, J. K. Bartley, S. H. Taylor, A. F. Carley, C. J. Kiely, K. Khalid and G. J. Hutchings, Catal. Lett., 2012, 142, 1049–1056 CrossRef CAS.
- Y. Inoue, K. Nakashiro and Y. Ono, Microporous Mater., 1995, 4, 379–383 CrossRef CAS.
- Y. H. Ma, N. Wang, W. Z. Qian, Y. Wang, J. M. Zhang and F. Wei, RSC Adv., 2016, 6, 81198–81202 RSC.
- K. Shen, W. Z. Qian, N. Wang, C. Su and F. Wei, J. Am. Chem. Soc., 2013, 135, 15322–15325 CrossRef CAS PubMed.
- K. Shen, W. Z. Qian, N. Wang, J. Zhang and F. Wei, J. Mater. Chem. A, 2013, 1, 3272–3275 CAS.
- K. Shen, W. Z. Qian, N. Wang, C. Su and F. Wei, J. Mater. Chem. A, 2014, 2, 19797–19808 CAS.
- El-M. El-Malki, R. A. van Santen and W. M. H. Sachtler, J. Phys. Chem. B, 1999, 103, 4611–4622 CrossRef CAS.
- S. M. T. Almutairi, B. Mezari, P. C. M. M. Magusin, E. A. Pidko and E. J. M. Hensen, ACS Catal., 2012, 2, 71–83 CrossRef CAS.
- R. Roesky, J. Weiguny, H. Bestgen and U. Dingerdissen, Appl. Catal., A, 1999, 176, 213–220 CrossRef CAS.
- S. Pin, M. A. Newton, F. D. Acapito, M. Zema, S. C. Tarantino, G. Spinolo, R. A. De Souza, M. Martin and P. Ghigna, J. Phys. Chem. C, 2012, 116, 980–986 CAS.
- J. H. Ahn, R. Kolvenbach, S. S. Al-Khattaf, A. Jentysa and J. A. Lercher, Chem. Commun., 2013, 49, 10584–10586 RSC.
- A. Ghorbanpour, A. Gumidyala, L. C. Grabow, S. P. Crossley and J. D. Rimer, ACS Nano, 2015, 9, 4006–4016 CrossRef CAS PubMed.
- T. Otto, S. I. Zones and E. Iglesia, J. Catal., 2016, 339, 195–208 CrossRef CAS.
- F. L. Bleken, S. Chavan, U. Olsbye, M. Boltz, F. Ocampo and B. Louis, Appl. Catal., A, 2012, 447, 178–185 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images of catalysts, TPR pattern of FeOx/ZSM-5, NH3-TPD data of catalysts. See DOI: 10.1039/c7ra04111j |
| This journal is © The Royal Society of Chemistry 2017 |