Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The rheological characteristics for the mixtures of cationic surfactant and anionic–nonionic surfactants: the role of ethylene oxide moieties†
Liming Zhanga,
Wanli Kang *ab,
Derong Xua,
Haishun Fenga,
Pengyi Zhanga,
Zhe Lia,
Yao Lua and
Hairong Wu*a
*ab,
Derong Xua,
Haishun Fenga,
Pengyi Zhanga,
Zhe Lia,
Yao Lua and
Hairong Wu*a
aInstitute of Enhanced Oil Recovery, China University of Petroleum (Beijing), Beijing, 102249, P.R. China. E-mail: kangwanli@cup.edu.cn; hrwu@cup.edu.cn
bSchool of Petroleum Engineering, China University of Petroleum (East China), Qingdao 266580, P.R. China
First published on 24th February 2017
Abstract
This study systematically reports the rheological behaviour and mechanism for mixtures of cationic surfactant cetyltrimethyl ammonium bromide (CTAB) and anionic–nonionic carboxylate surfactants (NPEC-n). The effects of molar ratio, total concentration, salinity, shearing time, temperature, and ethylene oxide (EO) moieties on the microstructures of the mixtures were investigated in detail using rheometry, freeze-fracture transmission electron microscopy (FF-TEM), cryo-transmission electron microscopy (Cryo-TEM), etc. The results indicate that the conformations of the EO moieties concern the head-group areas and steric hindrance, which affect the arrangement of the surfactant molecules. The aggregates with diverse morphologies endow the solutions with different rheological behaviours. Except for the CTAB/NPEC-10 system, the CTAB/NPEC-5 system and CTAB/NPEC-7 system show viscoelastic behaviour under some conditions and their highest viscosities appear at the molar ratio of 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and 40
24 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, respectively. The transition temperature of the mixture appears at 35 °C, accompanied with a sharp decrease in the viscosity. The salt thickening and shear-resistant properties of the mixtures have also been discussed, indicating good salt-resistance and shear-resistance of the mixtures.
60, respectively. The transition temperature of the mixture appears at 35 °C, accompanied with a sharp decrease in the viscosity. The salt thickening and shear-resistant properties of the mixtures have also been discussed, indicating good salt-resistance and shear-resistance of the mixtures.
Introduction
Mixed surfactant systems have been used in a wide range of applications due to their high surface activity, low critical micelle concentration (CMC), and low interfacial tension as compared to those of single surfactant systems.1–11 Among these systems, research on cationic/anionic surfactants mixtures is ongoing for a long period of time and has yielded great achievements; the discovery and application of worm-like micelles (WLMs) is an example.12–16 WLMs make the mixed solutions present unique properties;17 therefore, they have been widely employed in many industries.18–24 However, the interactions between the surfactants (e.g. electrostatic repulsion, van der Waals attraction, and hydrogen bonding) function during the process of mixing.25–28 It has been reported that the strong interaction between cationic/anionic surfactant molecules makes the mixed solution unstable, such that the precipitation or phase separation occurs when the concentration exceeds the CMC.29 For some systems, phase separation even appears at concentrations lower than the CMC, especially in equimolar solutions.30,31 This phase behaviour impedes the practical applications of cationic/anionic systems. It was found that precipitation can be overcome by introducing ethylene oxide (EO) moieties into the hydrophobic chain of some anionic or cationic surfactant molecules.32 On the one hand, EO moieties affect the area of the head-group and packing parameter P;33 on the other hand, EO moieties lead to lower charge intensity. Both aspects can effectively reduce the electrostatic interactions between the surfactant molecules.34 Furthermore, it is convenient to introduce EO moieties into the molecules of the anionic surfactant; thus, an anionic–nonionic surfactant molecule that displays both the high interfacial activity of anionic surfactant and good salt-resistance of the non-ionic surfactant can be obtained.35The types of anionic hydrophilic head-group and alkoxy groups as well as EO number determine the performance of anionic–nonionic surfactants which include alkoxy sulfates, sulfonates and carboxylates surfactants. Anionic–nonionic surfactants composed of alkoxy sulfates have attracted significant attention. For instance, Hato et al.36–39 have studied the effect of EO number on the Kraft point for dodecyl polyoxyethylene ether sulfate, and they found that upon increasing the number of EO moieties, the surface activity is enhanced and the Kraft point is diminished. Minero et al.40 reported that the EO moieties play a role in dispersing the charges in the head-group, thus weakening the electrostatic repulsion and promoting the formation of aggregates. By studying the molecular elasticity, Masuyama et al.41 found that the introduction of EO moieties made the hydrophobic chain of the surfactant more flexible, resulting in an improved surface activity. Chen et al.42 explored the micelle formation and synergistic interactions of binary surfactant combinations, which were composed of the synthesized sodium nonylphenol polyoxyethylene ether sulfate (NPES) and various traditional surfactants, indicating that the interaction parameter varied with the composition and the synergistic interaction concurrently existed in the NPES. In contrast, research on carboxylate is relatively rare; Liu et al.43 has reported the effect of electrolytes on the interfacial tension and found that Na+ plays a smaller role in reducing the oil/water interface of polyoxyethylene ether carboxylate than that of Ca2+ and Mg2+. To date, research on anionic–nonionic surfactants is mainly focused on the surface activity and micelle formation ability of alkoxy sulfonate surfactants. However, the rheological behaviour and mechanism of mixed anionic–nonionic carboxylate/cationic surfactant have not been reported to date.
In this study, the cationic surfactant CTAB and three types of carboxylate surfactant (NPEC-n, n = 5, 7, and 10) containing different numbers of EO moieties were mixed together. The mixtures displayed various rheological behaviours on adjusting the molar ratio, total concentration, and EO number. Then, the rheological characteristics, together with the microscopic structures of the aggregates, were combined to analyse the role that EO played in the rheological properties and mechanism. The obtained results contribute to extend the application of carboxylate surfactants as well as provide theoretical guidance for this type of surfactant.
Materials and methods
Materials
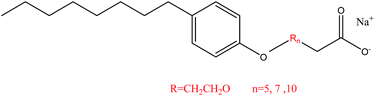
The quaternary ammonium cationic surfactant cetyltrimethyl ammonium bromide (CTAB, purity > 99%, A.R. grade) was purchased from Tianjin Fuchen Chemical Reagent Company (Tianjin, China). The anionic–nonionic surfactants NPEC-5, NPEC-7, and NPEC-10 with various numbers of EO in the hydrophobic chain were provided by Qingdao Changxing Chemical Co., Ltd. (Shandong, China), and their chemical structures are shown in Scheme 1. NaCl (>99%, A.R. grade) was provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Water used in solution preparation was pure water without distillation.Sample preparation
Samples were prepared by directly mixing CTAB and the three types of carboxylate surfactant solutions in a bottle at various molar ratios and molar concentrations. Then, a desired amount of NaCl was added to the bottle. After sealing, these samples were vortex-mixed and equilibrated at room temperature for 1 h to ensure complete dissolution and uniformity. The resulting mixtures were stored at 25 °C in a thermostatic bath for at least 24 h prior to measurement. All the measurements were performed at 25 °C unless otherwise specified.Rheological measurements
The rheological properties of the samples were measured using a rheometer (HAAKE RS600, Germany). A cone-plate sensor with a plate diameter of 50.00 mm, a cone angle of 1°, and a default gap of 0.052 mm was used. A chamber for covering the sample was used to avoid evaporation when necessary. The steady state measurement was performed at the shear rate sweep of 0.01–1000 s−1. Dynamic state rheological measurements were carried out at the constant shear stress of 0.1 Pa, which was obtained from the linear viscoelastic region of the frequency sweep measurement.Transmittance measurements
The transmittance of the bicomponent surfactant solution was obtained from the scanning curves obtained using a stability analyser (TURBISCAN Lab, France), which was equipped with a pulsed near-infrared light source (λ = 880 nm). The samples were placed in a test tube and were scanned from bottom to top at different temperatures. The microscopic properties of the samples were characterized by the scanning curves at different temperatures indirectly.Freeze-fracture transmission electron microscopy (FF-TEM)
The morphology of the bicomponent surfactant was observed using a JEOL-100 CX II TEM. First, a small amount of sample was placed on a 0.1 mm thick copper disk and then covered with a second copper disk. Then, the sample was frozen by plunging into liquid propane, which was cooled using liquid nitrogen. Fracturing and replication were performed using a freeze-fracture apparatus (BalzersBAF400, Germany) at −140 °C. Pt/C was deposited at an angle of 45° to shadow the replicas and C was deposited at an angle of 90° to consolidate the replicas. Finally, the resulting replicas were observed using a JEM-100CX electron microscope.Cryo-transmission electron microscopy (Cryo-TEM)
The observation was performed using a 120 kV JEM-2010 Plus TEM instrument, which was equipped with a Gatan cryo Holder 626 and a Gatan US1000 894CCD monitor. First, the samples were stored in a controlled environment vitrification system (CEVS). Then, 5 μL of the sample was loaded onto a carbon-coated holey film, which was supported by a copper grid. The sample was immediately plunged into a −180 °C liquid ethane reservoir a few seconds later. Finally, it was transferred into liquid nitrogen (−196 °C) for storage until its observation.Results and discussion
Ratio versus EO moieties
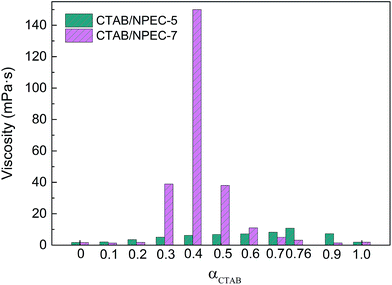
Fig. S1 in the ESI† displays the phase behaviour of the CTAB/NPEC-n solutions with CTAB at various proportions. I, II, III and IV represent the regions of the dilute solution, viscoelastic solution, phase separation, and precipitation, respectively. The viscoelastic solutions in region II were studied further.The relationships between the molar ratio and viscosity of the CTAB/NPEC-n solutions are shown in Fig. 1; αCTAB and αNPEC represent the molar percentage of CTAB and NPEC in the solutions, respectively, while αCTAB + αNPEC = 1. The results show that the viscosities of the separated anionic–nonionic and cationic surfactant solutions were small (<5 MPa s) at low concentrations. However, except for the CTAB/NPEC-10 solution, the viscosity greatly increases when mixing CTAB with the NPEC-n surfactants together at various molar ratios. Taking the system with a total concentration of 75 mmol L−1 as an example, the highest viscosities of CTAB/NPEC-5 and CTAB/NPEC-7 appear at the molar ratio of 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and 40
24 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, respectively. This indicates that the EO moieties participate in the synergistic thickening effect and the introduction of EO moieties makes a difference in the optimum mixing ratios. Fig. 2a and b display the aggregate microstructures of the CTAB/NPEC-5 and CTAB/NPEC-7 solutions in which the αCTAB values are 0.76 and 0.4, respectively. In Fig. 2a, spherical aggregates are evenly and sparsely distributed in the CTAB/NPEC-5 solution, and this phenomenon also corresponds to the low viscosity. In Fig. 2b, there are diverse aggregates, such as vesicles, rod-like micelles, etc., and they are densely packed, leading to a relatively high viscosity. These findings are in good agreement with those reported in previous studies.
60, respectively. This indicates that the EO moieties participate in the synergistic thickening effect and the introduction of EO moieties makes a difference in the optimum mixing ratios. Fig. 2a and b display the aggregate microstructures of the CTAB/NPEC-5 and CTAB/NPEC-7 solutions in which the αCTAB values are 0.76 and 0.4, respectively. In Fig. 2a, spherical aggregates are evenly and sparsely distributed in the CTAB/NPEC-5 solution, and this phenomenon also corresponds to the low viscosity. In Fig. 2b, there are diverse aggregates, such as vesicles, rod-like micelles, etc., and they are densely packed, leading to a relatively high viscosity. These findings are in good agreement with those reported in previous studies.
 | ||
| Fig. 1 The viscosity as a function of αCTAB for CTAB/NPEC-5 and CTAB/NPEC-7 solutions (the total concentration was 75 mmol L−1). | ||
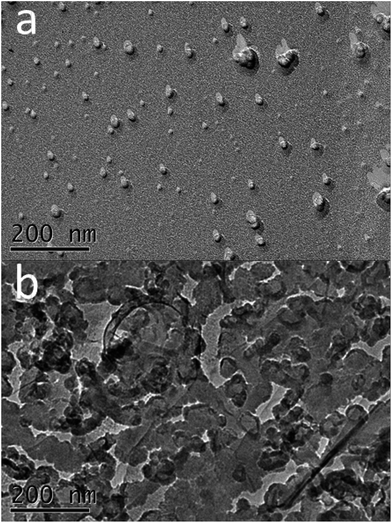 | ||
| Fig. 2 (a) The images for CTAB/NPEC-5 (αCTAB = 0.76) and (b) CTAB/NPEC-7 (αCTAB = 0.4) with a total concentration of 75 mmol L−1 at 25 °C. | ||
For systems at low concentration and with no additive composition, it is generally believed that most of the aggregates are spherical micelles if the concentration is lower than 10 times the CCMC, whereas vesicles, rod-like micelles, WLMs, and layered structure will appear if the concentration equals or exceeds 10 times the CCMC.44 At this experimental concentration (75 mmol L−1) and optimum ratio, the CTAB/NPEC-7 system was susceptible to form non-spherical aggregates including vesicles, rod-like micelles, and WLMs, whereas the CTAB/NPEC-5 system did not reach the entanglement concentration (see Fig. 10a). Further explanations have been made with the critical packing parameter P = VC/(alc), which was proposed by Israelachvili et al.45 P is used for predicting the aggregated structures in aqueous solutions by calculating the geometrical shape of the amphiphilic molecules. In this theory, VC is the volume of the hydrophobic group of surfactant, a is the area of one head-group when closely arranged at the aggregate surface, lc is the chain length of the amphiphilic molecules and the maximum length appears when the hydrophobic chain is fully extended. When the surfactant molecule is conical, P ≤ 1/3, spherical micelles are generally formed. If the molecular structure is similar to a column, 1/3 < P ≤ 1/2, asymmetric micelles are usually formed, including ellipsoid, rod-like micelles or WLMs. When 1/2 < P ≤ 1, vesicles or layered structures are likely to be formed.
The electrostatic attractions between the positively charged head-group of CTAB and the negatively charged head-group of NPEC-n contribute to the diminishing of distance among the surfactant molecules, leading to various a and P values. The CTAB/NPEC-5 system was prone to form spherical aggregates due to the large head-group at our experimental concentration. As the CTAB/NPEC-7 systems reached their entanglement concentration and maximum adsorption (see Fig. 10a), the head-group area was ∼0.49 nm2 and the P value was calculated in the following way: the head-group area of the mixture is an average value, a = (acation + aanion)/2. The total chain volume equals to the sum of two parts, VC = Vcation + Vanion. Moreover, Vcation, Vanion, and lc can be calculated by the following formula according to the carbon number on hydrophobic chain:46 V = (27.4 + 26.9n) × 10−3 nm3, lc = (0.15 + 0.1265n) nm. Therefore, P7 was calculated to be 0.68, which is in the range of 1/2 < P ≤ 1, suggesting the formation of vesicles. However, the EO moieties in the molecular chain of NPEC-7 lead to a larger head group area when the steric hindrance was considered. Thus, the practical P value was smaller than the calculated value. This result was consistent with the coexistence of various aggregates observed in Fig. 2b and the viscosity shown in Fig. 1 and 10a.
Rheological behaviour of the CTAB/NPEC-5 mixtures
To study the rheological properties of the mixed systems at different concentrations, static shear and oscillation measurements were conducted. Taking the CTAB/NPEC-5 solutions as an example, the molar ratios were fixed at 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 (αCTAB = 0.76). As shown in Fig. 3, the solutions behave as Newtonian fluids without viscoelasticity when the total concentration was less than 100 mmol L−1 and as non-Newtonian fluids when the total concentrations were higher than 100 mmol L−1. The viscosity increases upon an increase in the total concentration. This could be interpreted by the following statement. At lower concentrations, the CTAB and NPEC-5 molecules align at the surface. When the concentration is above the CMC, the surfactant molecules assemble into various aggregates such as rod-like micelles, WLMs, etc. presenting a high viscoelastic property.47
24 (αCTAB = 0.76). As shown in Fig. 3, the solutions behave as Newtonian fluids without viscoelasticity when the total concentration was less than 100 mmol L−1 and as non-Newtonian fluids when the total concentrations were higher than 100 mmol L−1. The viscosity increases upon an increase in the total concentration. This could be interpreted by the following statement. At lower concentrations, the CTAB and NPEC-5 molecules align at the surface. When the concentration is above the CMC, the surfactant molecules assemble into various aggregates such as rod-like micelles, WLMs, etc. presenting a high viscoelastic property.47
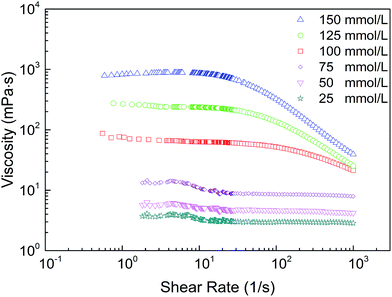
Furthermore, the viscosity as a function of shear rate (γ) for the CTAB/NPEC-5 mixtures at various total concentrations is displayed in Fig. 3. When the concentration was lower than 100 mmol L−1, the viscosity of the solution was rather low and independent of the shear rate. In contrast, when the concentration was above 100 mmol L−1, a Newtonian plateau appears in each solution at low γ and shear-thinning behaviour occurs after the critical shear rate (γc). These are typical properties for WLMs when subjected to a high flow-rate.48 The WLMs undergo a quick and repeated destruction-recovery process in the plateau region until the shear rate reaches γc.49,50
The zero-shear viscosity of the samples was obtained by extrapolating the Newtonian plateau to γ = 0. It was found that η0 increases upon increasing the total concentration (see Fig. 10a). The high η0 value was attributed to the formation of entangled WLMs. When the shear rate was above γc, the WLMs do not have enough time to deform in response to the shear stress, which leads to the destruction of the network structure. Accordingly, the viscoelastic solution completely converses into a water-like fluid, exhibiting a shear-thinning behaviour.51,52
The Maxwell model, which describes the dynamic rheological properties of viscoelastic materials, is an important criterion for characterizing WLMs. The equations are shown below:53–56
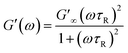 | (1) |
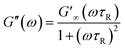 | (2) |
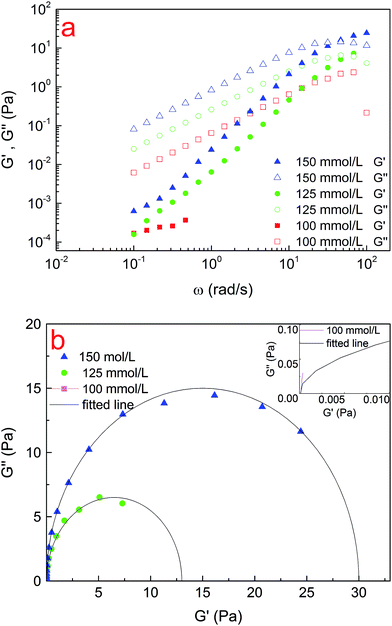
Oscillatory measurements were conducted to describe the rheological behaviour of the solutions. Fig. 4a shows the variation of the modulus as a function of oscillatory angular frequency ω for the CTAB/NPEC-5 mixed systems at various total concentrations. At low frequency, the system showed a liquid-like property (G′′ > G′), whereas at high frequency, a solid-like property was observed (G′′ < G′). When the total concentration was higher than 100 mmol L−1, the elastic modulus and the viscous modulus intersected at an exclusive point, i.e. ωc, rendering a single relaxation time, which is another characteristic of WLMs;58 the higher the total concentration, the higher the relaxation time and viscoelasticity.
To investigate how well the rheological data fit the Maxwell model, a Cole–Cole curve (G′′ versus G′) is usually applied. As presented in Fig. 4b, the solid points refer to the experimental data, whereas the solid line is the fitted data of the samples. When the concentration exceeds 100 mmol L−1, the data can fit well with the semicircle at low frequency. However, it deviates from the Cole–Cole curves at high frequency, which denotes an appearance of breathing or Rouse relaxation moles at short time scales.59 In addition, when the total concentration increases above the entangled concentration, the surfactant molecules more closely self-assemble and thus the aggregates tend to rapidly grow into WLMs, as verified in Fig. 2a and 5. Moreover, the spherical aggregates transform into long entangled WLMs.
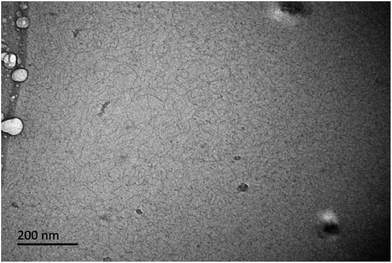 | ||
| Fig. 5 A Cryo-TEM image obtained for CTAB/NPEC-5 without salt at the total concentration of 150 mmol L−1. | ||
The effect of NaCl on the rheological behaviour of the CTAB/NPEC-5 mixtures
The repulsion between head groups with like charges will hinder the micellization. Upon the addition of NaCl, the charge repulsion was screened. This process resulted in a smaller distance between these micelles,60 leading to various rheological behaviours in the mixed solutions.61 The viscosity, viscoelasticity, and shear-resistance of CTAB/NPEC-5 with NaCl were investigated and the effect of NaCl was analyzed in detail. Fig. S2 in the ESI† shows the viscosity as a function of shear rate at various NaCl concentrations for the CTAB/NPEC-5 systems with a fixed molar ratio of 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, total concentration of 150 mmol L−1, and at the temperature of 25 °C. All the samples exhibited a Newtonian plateau at low γ and shear-thinning behaviour when the shear rates were above γc. This phenomenon can be explained by Rheo-SANS (Rheo-Small Angle Neutron Scattering) measurements.62,63 When the shear rate reaches γc, the WLMs are inclined to arrange themselves along the direction of shearing, i.e. no entanglement among the WLMs, and thus, a reduction in the viscosity occurs. Moreover, the viscosity increases at first and then drops, as shown in Fig. 6. The transition of viscosity occurred when the NaCl concentration was 2565 mmol L−1. This may be attributed to the transformation from linear WLMs to branched WLMs. Phase separation appeared at 3420 mmol L−1 NaCl due to the formation of aggregates with various sizes. The electrostatic attraction between the positive charges of the anionic–nonionic surfactants and negative charges of the cationic surfactants facilitated the formation of aggregates via self-assembly. In addition, there were electrostatic repulsions between the molecules with like charges. When the counter-ions were added to the solution, the electrostatic repulsion between the head-groups with like charges were screened. The reduction of the repulsion led to a decreased area of the head-group and an increased P value. This phenomenon suggests that the micelles will quickly grow along one dimension. Thus, it leads to a sharp increase in the viscosity before the NaCl concentration reaches 2565 mmol L−1. Once the branching behaviour of the entangled WLMs occurs, the viscosity will gradually decrease (see Fig. 6).
24, total concentration of 150 mmol L−1, and at the temperature of 25 °C. All the samples exhibited a Newtonian plateau at low γ and shear-thinning behaviour when the shear rates were above γc. This phenomenon can be explained by Rheo-SANS (Rheo-Small Angle Neutron Scattering) measurements.62,63 When the shear rate reaches γc, the WLMs are inclined to arrange themselves along the direction of shearing, i.e. no entanglement among the WLMs, and thus, a reduction in the viscosity occurs. Moreover, the viscosity increases at first and then drops, as shown in Fig. 6. The transition of viscosity occurred when the NaCl concentration was 2565 mmol L−1. This may be attributed to the transformation from linear WLMs to branched WLMs. Phase separation appeared at 3420 mmol L−1 NaCl due to the formation of aggregates with various sizes. The electrostatic attraction between the positive charges of the anionic–nonionic surfactants and negative charges of the cationic surfactants facilitated the formation of aggregates via self-assembly. In addition, there were electrostatic repulsions between the molecules with like charges. When the counter-ions were added to the solution, the electrostatic repulsion between the head-groups with like charges were screened. The reduction of the repulsion led to a decreased area of the head-group and an increased P value. This phenomenon suggests that the micelles will quickly grow along one dimension. Thus, it leads to a sharp increase in the viscosity before the NaCl concentration reaches 2565 mmol L−1. Once the branching behaviour of the entangled WLMs occurs, the viscosity will gradually decrease (see Fig. 6).
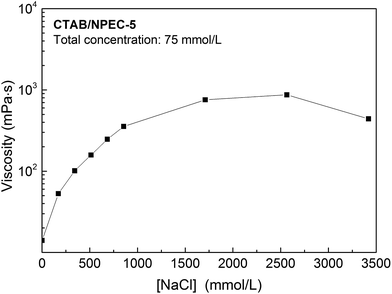 | ||
| Fig. 6 The viscosity as a function of the molar concentration of NaCl for CTAB/NPEC-5 (total concentration is 75 mmol L−1 and the shear rate is 2.5 s−1). | ||
The effects of NaCl concentration on the dynamic oscillation measurements of CTAB/NPEC-5 are illustrated in Fig. S3a (see the ESI†). Except for the mixed solutions with 171 mmol L−1 NaCl, the elastic modulus and the viscous modulus meet at one point, indicating the presence of a single relaxation time and the formation of WLMs. Fig. S3b in the ESI† depicts the Cole–Cole plots of the samples with different NaCl concentrations. When the NaCl concentration was higher than 171 mmol L−1, it can be seen that the experimental data can fit well with the theoretical value at low frequency, which is consistent with the previous finding related to the formation of WLMs.
τR and G′∞ as a function of NaCl concentration are presented in Fig. S4 (ESI,† see line a and line b). As is known, τR and G′∞ are usually applied to characterize the viscosity and elasticity of the systems, respectively. As seen in Fig. S4 (see the ESI†), G′∞ monotonically increases with the NaCl concentration. This indicates that more entangled WLMs intertwined with each other, i.e. the degree of the entanglement increases. In contrast with G′∞, when the NaCl concentration was lower than 2565 mmol L−1, the τR value increases, leading to an extension of the WLMs in one dimension and an increase in viscosity. The tendency of the change in τR was consistent with that of viscosity; both of them decreased when the NaCl concentration reached 2565 mmol L−1, indicating that the relaxation process was relevant to the length of WLMs. Taking τR and G′∞ into consideration, it is clear that the viscosity decreases due to the branching of the micelles.
These results are in agreement with that reported by Pei et al.49 In spite of branching, the micelles can still entangle with each other and form a network; hence, the elasticity increases. Fig. 7a and b show the microstructures of CTAB/NPEC-5 with 171 mmol L−1 and 855 mmol L−1 NaCl. At a relatively low NaCl concentration, vesicles and rod-like micelles appear; at a relatively high NaCl concentration, WLMs are observed, making the solution a viscoelastic fluid. In conclusion, NaCl promotes the growth of aggregates to some extent.
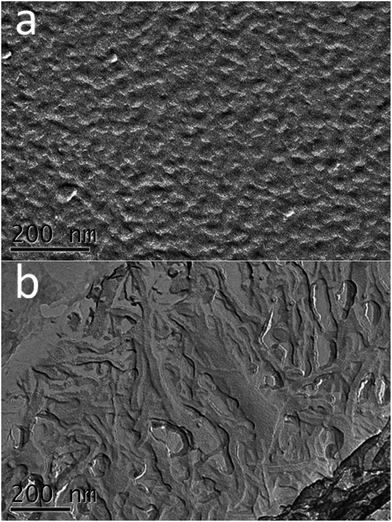 | ||
Fig. 7 Images of CTAB/NPEC-5 (75 mmol L−1, 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24) with 171 mmol L−1 (a) and 855 mmol L−1 (b) NaCl at 25 °C. 24) with 171 mmol L−1 (a) and 855 mmol L−1 (b) NaCl at 25 °C. | ||
Shearing resistance of the CTAB/NPEC-5 mixtures with salt
The addition of NaCl to a CTAB/NPEC-5 solution promotes the conversion of the aggregates, which indirectly affects the rheological behaviour and shear-resistance. Fig. 8 shows the viscosity as a function of shearing time. The viscosity of CTAB/NPEC-5 with salt remains almost constant under a shear rate of 170 s−1 for 30 min. It was indicated that the system still possesses high viscosity and a good shear-resistance in spite of the addition of salt. This is analogous to the case of the salt effect on the viscosity (see Fig. 6).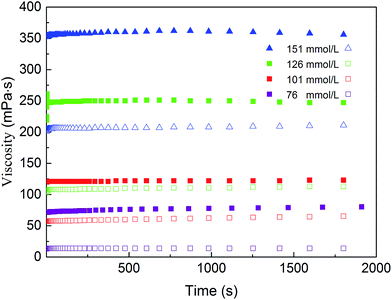 | ||
| Fig. 8 Viscosity as a function of shearing time for CTAB/NPEC-5 with various concentrations of NaCl (solid symbol: with NaCl; open symbol: without NaCl; shear rate, 170 s−1). | ||
The static screening effect of the counter-ions was beneficial to the growth of the micelles. Entangled WLMs enable the formation of the network and make the system more structured, which results in a better viscoelasticity and shear-resistance in the solution. Fig. 2a and 7b are the images before and after the addition of 855 mmol L−1 NaCl. In Fig. 2a, there is a large quantity of spherical aggregates, whereas in Fig. 7b, entangled WLMs can be observed. By combining the rheological behaviour, the viscosity, and viscoelasticity, the shear-resistance can be explained. It is obvious that the formation of spherical aggregates enables low viscosity and viscoelasticity in the sample (see Fig. 2a and 10a). After adding 855 mmol L−1 NaCl, the spherical aggregates quickly grow into the entangled WLMs (see Fig. 7b), forming a tight network structure. Consequently, the saturated network endows the solution with a good shear-resistance and the solution behaves as a non-Newtonian fluid.
Thermal-sensitivity versus the EO moieties
The viscosity and transmittance as a function of temperature for the mixed systems are illustrated in Fig. 9a and b, respectively. When the temperature was below 35 °C, the viscosities of CTAB/NPEC-7, CTAB/NPEC-5 without NaCl, and CTAB/NPEC-5 with NaCl gradually decreased (Fig. 9a). However, the transmittance of three systems rapidly increased before 30 °C and then remained almost constant between 30 and 35 °C (Fig. 9b). This means before the critical temperature (Tc), the aggregates in the solutions gradually change. Once the temperature reaches Tc (35 °C), significant changes occur. Thus, all the viscosities of the three systems sharply decrease. Furthermore, the transmittance of the CTAB/NPEC-7 solution shows a sharp decrease and the solution becomes turbid, which are in accordance with the reduction in viscosity. The transmittance of CTAB/NPEC-5 with NaCl continues to slowly decrease, whereas an increase in the transmittance of CTAB/NPEC-5 without NaCl occurs and no cloudy phenomenon was observed, albeit the temperature reached 45 °C. On the one hand, water increases with temperature due to the enhancement of hydration on the surface of the aggregates.64 Therefore, the systems with more EO moieties possess more hydrated water and thus a higher viscosity and better temperature resistance. On the other hand, because the thermal motion speeds up with an increase in temperature, the electrostatic interactions between the molecules are weakened. Correspondingly, the area of the head-group enlarges, the P value diminishes, and the aggregates convert into spherical micelles for all the systems at the experimental concentration. In addition, the temperature-resistant property of the CTAB/NPEC-5 with NaCl system improves to some degree (see Fig. 9a).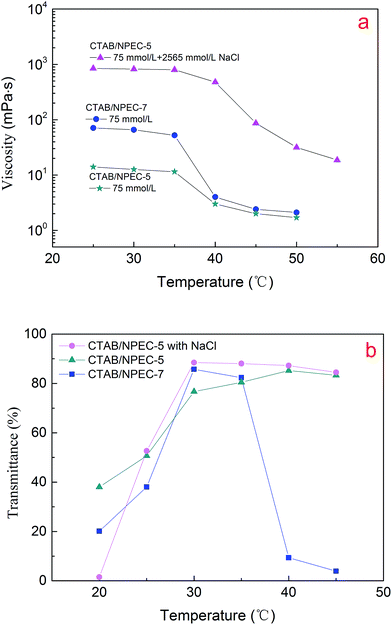 | ||
| Fig. 9 Viscosity (a) (shear rate, 6 s−1) and transmittance (b) as a function of temperature for the bicomponent surfactants (the total concentration of all the mixtures is 75 mmol L−1). | ||
The mechanism of the rheological properties of the mixtures with various EO moieties
The viscosities of CTAB/NPEC-5, CTAB/NPEC-7, and CTAB/NPEC-10 were studied to investigate the effect of the EO moieties on the rheological behaviour.For CTAB/NPEC-10, there are 10 EO moieties in the hydrophobic chain of NPEC-10, and the viscosity remains almost constant and behaves like water even when the concentration reaches 150 mmol L−1 (see Fig. 10a). It is clear that the 10 EO moieties were not well aligned anymore. The molecular conformation results in larger steric hindrance than those of NPEC-5 and NPEC-7, resulting in an increased area of the head-group. Thus, the CTAB and NPEC-10 molecules tend to form micelles with small size instead of assembling into long micelles.
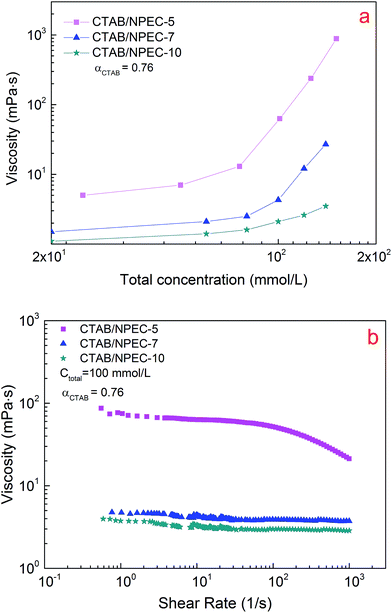 | ||
| Fig. 10 The viscosity of the solutions as a function of the total concentration (a) (shear rate, 6 s−1) and (b) the shear rate for the systems with various EO numbers. | ||
For CTAB/NPEC-7, the viscosity gradually increases with the total concentration. There are 7 EO moieties in the hydrophobic chain of NPEC-7. The conformation of the NPEC-7 molecule is similar to that of NPEC-10. However, the steric hindrance of NPEC-7 is smaller due to less EO moieties and so is the area of the head-group. Therefore, spherical micelles were no longer the only aggregates in the solution. That is the reason for the higher viscosity of CTAB/NPEC-7 when compared to that of the CTAB/NPEC-10 systems.
For CTAB/NPEC-5, the viscosity can increase three orders of magnitude as the concentration increases up to 150 mmol L−1. Once the total concentration exceeds the micellar entanglement value, the viscosity increases significantly and a large quantity of non-spherical aggregates appear. The morphologies shown in Fig. 2a and 5 display the transformation of the aggregates. When the total concentration was 75 mmol L−1, spherical aggregates with uniform size were sparsely dispersed in the solution (see Fig. 2a). In the solution at the concentration of 150 mmol L−1, entangled WLMs mostly intertwined with each other and formed a network (see Fig. 5), which corresponds to the high viscoelasticity. When compared with NPEC-7, there are 5 EO moieties in the hydrophobic chain of NPEC-5 and the lower number of EO moieties leads to a smaller area of the head-group. Thus, a tighter arrangement among the molecules occurred, facilitating the growth of the aggregates along one dimension before its maximum length and then the viscosity greatly increased.
Our study reveals that an appropriate number of EO moieties contribute to the formation of the high viscoelastic fluids and satisfy practical applications well.
Conclusion
The number of EO moieties contribute to the diverse conformations of EO chains and the morphologies of the aggregates, leading to various molar ratios and rheological behaviours among CTAB/NPEC-n. The conformations of the EO moieties concern the head-group area and steric hindrance, which affect the arrangement of the surfactant molecules. The anionic–nonionic surfactant with 5 EO moieties and 7 EO moieties can form entangled WLMs, which enables a higher viscoelasticity in the mixtures under an appropriate external environment. Upon increasing the total concentration, the aggregates transform from spherical micelles to vesicles, WLMs, and other micelles. Accordingly, the viscoelasticity of the solution varies. The viscosities and transmittance of the CTAB/NPEC-5 and CTAB/NPEC-7 solutions drop at the transition temperature of 35 °C. NaCl facilitates the formation of entangled WLMs and networks, leading to an increase in the viscosity. A high NaCl concentration prompts an increase in the elasticity, enabling a good shear-resistance for the network. However, the viscosity decreases when precipitation occurs at a high NaCl concentration (∼3520 mmol L−1). This study has extended the understanding of rheological behaviour of anionic–nonionic carboxylate/cationic surfactant systems as well as the effect of the EO moieties. The EO moieties play an important role in the formation of aggregates and the rheological behaviour can be effectively regulated by introducing EO moieties with appropriate numbers.Acknowledgements
This work was financially by the National Natural Science Foundation of China (No. 21273286, 51574267) and Science Foundation of China University of Petroleum, Beijing (No. 2462015YJRC033).Notes and references
- P. Parekh, D. Varade, J. Parikh and P. Bahadur, Colloids Surf., A, 2011, 385, 111–120 CrossRef CAS.
- V. B. Fainerman, R. Miller and E. V. Aksenenko, Adv. Colloid Interface Sci., 2002, 96, 295 CrossRef CAS PubMed.
- S. Ghosh, J. Colloid Interface Sci., 2001, 244, 128–138 CrossRef CAS.
- C. Das, T. Chakraborty, S. Ghosh and B. Das, Colloid J., 2010, 72, 788–798 CrossRef CAS.
- D. Tikariha, B. Kumar, S. Ghosh, A. K. Tiwari, S. K. Saha, N. Barbero, P. Quagliotto and K. K. Ghosh, J. Nanofluids, 2013, 2, 316–324 CrossRef CAS.
- P. K. Khatua, S. Ghosh, S. K. Ghosh and S. C. Bhattacharya, J. Dispersion Sci. Technol., 2005, 25, 741–748 CrossRef.
- P. Sharma, S. Sachar, G. Kaur, P. Thakur, S. B. Mandeep and T. S. Banipal, J. Surf. Sci. Technol., 2007, 23, 131–147 CAS.
- M. Prasad, A. M. Donald, R. Palepu and S. P. Moulik, J. Surf. Sci. Technol., 2003, 19, 125–138 CAS.
- H. Oda, S. Nagadome, S. Lee, F. Ohseto, Y. Sasaki and G. Sugihara, J. Surf. Sci. Technol., 1998, 14, 1–22 CAS.
- S. K. Suri and H. S. Randhawa, J. Surf. Sci. Technol., 1989, 5, 355–363 CAS.
- S. B. Sulthana, S. G. T. Bhat and A. K. Rakshit, J. Surf. Sci. Technol., 1997, 13, 20–31 CAS.
- P. Brown, C. P. Butts and J. Eastoe, Soft Matter, 2013, 9, 2365 RSC.
- S. W. Liu, X. W. Wang, L. M. Chen, L. X. Hou and T. H Zhou, Soft Matter, 2014, 10, 9177–9186 RSC.
- G. Palazzo, Soft Matter, 2013, 9, 10668 RSC.
- C. A. Dreiss, Soft Matter, 2007, 3, 956–970 RSC.
- T. Chakraborty and S. Ghosh, J. Surfactants Deterg., 2008, 11, 323–334 CrossRef CAS.
- R. Angelico, S. Amin, M. Monduzzi, S. Murgia, U. Olsson and G. Palazzo, Soft Matter, 2012, 8, 10941 RSC.
- A. D. Wang, W. Y. Shi, J. B. Huang and Y. Yan, Soft Matter, 2016, 12, 337–357 RSC.
- H. F. Shi, W. Ge, H. Oh, S. M. Pattison, J. T. Huggins, Y. Talmon, D. J. Hart, S. R. Raghavan and J. L. Zakin, Langmuir, 2013, 29, 102–109 CrossRef CAS PubMed.
- N. Hassan, J. M. Ruso and A. González-Pérez, Soft Matter, 2011, 7, 5194 RSC.
- S. Ghosh, A. Das Burman, G. C. De and A. R. Das, J. Phys. Chem. B, 2011, 115, 11098–11112 CrossRef CAS PubMed.
- S. Ghosh, G. B. Ray and S. Mondal, Fluid Phase Equilib., 2015, 405, 46–54 CrossRef CAS.
- S. Das, S. Maiti and S. Ghosh, RSC Adv., 2014, 4, 12275–12286 RSC.
- H. Afifi, G. Karlsson, R. K. Heenan and C. A. Dreiss, Langmuir, 2011, 27, 7480–7492 CrossRef CAS PubMed.
- L. S. Hao, P. Hu and Y. Q. Nan, Colloids Surf., A, 2010, 361, 187–195 CrossRef CAS.
- Y. Y. Lin, X. H. Cheng, Y. Qiao, C. L. Yu, Z. B. Li, Y. Yan and J. B. Huang, Soft Matter, 2010, 6, 902–908 RSC.
- E. F. Marques, R. O. Brito, S. G. Silva, J. E. Rodríguez-Borges, M. L. D. Vale, P. Gomes and O. Söderman, Langmuir, 2008, 24, 11009–11017 CrossRef CAS PubMed.
- H. Q. Yin, Y. Y. Lin, J. B. Huang and J. P. Ye, Langmuir, 2007, 23, 4225–4230 CrossRef CAS PubMed.
- Y. Q. Nan, H. L. Liu and Y. Hu, Colloids Surf., A, 2005, 269, 101–111 CrossRef CAS.
- J. Mata, D. Varade, G. Ghosh and P. Bahadur, Colloids Surf., A, 2004, 245, 69–73 CrossRef CAS.
- D. Varade, T. Joshi, V. K. Aswal, P. S. Goyal, P. A. Hassan and P. Bahadur, Colloids Surf., A, 2005, 259, 103–109 CrossRef CAS.
- X. Chen, T. J. Young, M. Sarkari, R. O. Williams III and K. P. Johnston, Int. J. Pharm., 2002, 242, 3–14 CrossRef CAS PubMed.
- M. Á. Valenzuela, M. P. Gárate and A. F. Olea, Colloids Surf., A., 2007, 307, 28–34 CrossRef CAS.
- A. Mehreteab and F. J. Loprest, J. Colloid Interface Sci., 1988, 125, 602–609 CrossRef CAS.
- Y. J. Chen and G. Y. Xu, Colloids Surf., A, 2013, 424, 26–32 CrossRef CAS.
- M. Hato and K. Shinoda, J. Phys. Chem., 1973, 77, 378–381 CrossRef CAS.
- R. Varadaraj, J. Bock, P. Geissler, S. Zushma, N. Brons and T. Colletti, J. Colloid Interface Sci., 1991, 147, 396–402 CrossRef CAS.
- M. Dahanayake, A. W. Cohen and M. J. Rosen, J. Phys. Chem., 1986, 90, 2413–2418 CrossRef CAS.
- Y. F. Wang and J. B. Huang, Acta Phys.-Chim. Sin., 2001, 488–490 Search PubMed.
- C. Minero, E. Pramauro, E. Pelizzetti, V. Degiorgio and M. Corti, J. Phys. Chem., 1986, 90, 1620–1625 CrossRef CAS.
- A. Masuyama, T. Kawano, Y. Zhu and Y. N. Toshiyuki Kida, Chem. Lett., 1993, 22, 2053–2056 CrossRef.
- Z. X. Chen, S. P. Deng and X. K. Li, J. Colloid Interface Sci., 2008, 318, 389–396 CrossRef CAS PubMed.
- Z. Y. Liu, L. Zhang, X. L. Cao, X. W. Song, Z. Q. Jin, L. Zhang and S. Zhao, Energy Fuels, 2013, 27, 3122–3129 CrossRef CAS.
- Z. X. Cheng, X. B. Dong, Q. Y. Pan, J. C. Zhang and X. W. Dong, Mater. Lett., 2006, 60, 3137–3140 CrossRef CAS.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., 1976, 72, 1525–1568 Search PubMed.
- J. M. Corkill, J. F. Goodman, C. P. Ogden and J. R. Tate, Proc. R. Soc. A, 1963, 273, 84–102 CrossRef CAS.
- B. A. Schubert, N. J. Wagner, E. W. Kaler and S. R. Raghavan, Langmuir, 2004, 20, 3564–3573 CrossRef CAS PubMed.
- X. M. Pei, J. X. Zhao, Y. Z. Ye, Y. You and X. L. Wei, Soft Matter, 2011, 7, 2953–2960 RSC.
- X. M. Pei, Z. H. Xu, B. L. Song, Z. G. Cui and J. X. Zhao, Colloids Surf., A, 2014, 443, 508–514 CrossRef CAS.
- J. H. Huang, S. X. Zhang, Y. H. Feng, J. C. Li, H. Q. Yan, F. R. He, G. Z. Wang, Y. F. Liu and L. N. Wang, Colloids Surf., A, 2016, 500, 222–229 CrossRef CAS.
- Y. X. Han, Y. J. Feng, H. Q. Sun, Z. Q. Li, Y. G. Han and H. Y. Wang, J. Phys. Chem. B, 2011, 115, 6893–6902 CrossRef CAS PubMed.
- A. A. Dar, A. Garai, A. R. Das and S. Ghosh, J. Phys. Chem. A, 2010, 114, 5083–5091 CrossRef CAS PubMed.
- D. Wang, R. H. Dong, P. F. Long and J. C. Hao, Soft Matter, 2011, 7, 10713–10719 RSC.
- D. P. Acharya, D. Varade and K. Aramaki, J. Colloid Interface Sci., 2007, 315, 330–336 CrossRef CAS PubMed.
- M. E. Cates, Macromolecules, 1987, 20, 2289–2296 CrossRef CAS.
- S. R. Raghavan and E. W. Kaler, Langmuir, 2001, 17, 300–306 CrossRef CAS.
- C. Oelschlaeger, P. Suwita and N. Willenbacher, Langmuir, 2010, 26, 7045–7053 CrossRef CAS PubMed.
- F. Chen, Y. Wu, M. Wang and R. L. Zha, Colloid Polym. Sci., 2015, 293, 687–697 CAS.
- A. Parker and W. Fieber, Soft Matter, 2013, 9, 1203–1213 RSC.
- D. F. Yu, X. Huang, M. L. Deng, Y. Y. Lin, L. X. Jiang, J. B. Huang and Y. L. Wang, J. Phys. Chem. B, 2010, 114, 14955–14964 CrossRef CAS PubMed.
- T. Lu, L. G. Xia, X. D. Wang, A. Q. Wang and T. Zhang, Langmuir, 2011, 27, 9815–9822 CrossRef CAS PubMed.
- S. Förster, M. Konrad and P. Lindner, Phys. Rev. Lett., 2005, 94, 017803 CrossRef PubMed.
- R. A. Araujo, D. J. B. Villegas, R. G. Larson and U. M. Cordova-Figueroa, Soft Matter, 2016, 12, 4071–4081 RSC.
- F. N. Padia, M. Yaseen, B. Gore, S. Rogers, G. Bell and J. R. Lu, J. Phys. Chem. B, 2013, 118, 179–188 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28071d |
| This journal is © The Royal Society of Chemistry 2017 |