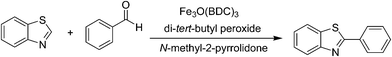Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct arylation of benzoazoles with aldehydes utilizing metal–organic framework Fe3O(BDC)3 as a recyclable heterogeneous catalyst†
Son H. Doan,
Khoa D. Nguyen,
Tung T. Nguyen and
Nam T. S. Phan*
Faculty of Chemical Engineering, HCMC University of Technology, VNU-HCM, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam. E-mail: ptsnam@hcmut.edu.vn; Fax: +84 8 38637504; Tel: +84 8 38647256 ext. 5681
First published on 5th January 2017
Abstract
A metal–organic framework Fe3O(BDC)3 was synthesized and used as a recyclable heterogeneous catalyst for the direct arylation of azoles with benzaldehydes. Following this protocol, inexpensive and commercially available benzaldehydes could act as an aryl source for the transformation, replacing the aryl halides normally employed in the conventional cross-coupling reactions. The catalyst disclosed higher catalytic productivity for the formation of aryl-substituted azoles than various MOFs and diverse homogeneous iron catalysts. The conversion was considerably regulated by the solvent and the oxidant, and the combination of N-methyl-2-pyrrolidone with di-tert-butyl peroxide offered the best yield. Heterogeneous catalysis was verified for the reaction, and the production of aryl-substituted azoles was only feasible in the presence of the iron framework. It was possible to reuse the catalyst for the direct arylation of azoles with aldehydes while its catalytic activity was preserved for multiple cycles. To our best judgment, this direct arylation of azoles with aldehydes utilizing solid catalysts has not been previously reported.
1. Introduction
Aryl-substituted azoles and their derivatives are popular structural scaffolds found in miscellaneous series of biologically active compounds, agricultural chemicals, pharmaceutical chemicals, and organic materials.1–5 These skeletons were previously synthesized via the reaction between metalloheteroarenes and haloarenes, or metalloarenes and haloheteroarenes, hence experiencing disadvantages with regard to green chemistry points of view.3 Recently, different strategies for these structures have been developed and mentioned in the literature.6 Wang et al. achieved aryl-substituted azoles by [Pd(π-allyl)Cl]2-catalyzed coupling of thioethers with azoles.7 This research group also implemented the transformation between aryltrimethylammonium triflates and benzoazoles utilizing the same palladium complex catalyst.8 Pooniya et al. performed the direct arylation of azaheterocycles with diaryliodonium salts using copper salts as the catalyst.9 Walsh et al. demonstrated a productive room-temperature Pd(OAc)2/NiXantphos-catalyzed arylation reaction of benzoxazoles with aryl bromides.10 Hoover et al. synthesized a series of arylated benzoxazoles via the CuI-catalyzed oxidative coupling reaction of 2-nitrobenzoic acids and benzoxazoles.11 Maiti et al. published a copper salts-mediated decarboxylative arylation reaction of benzoic acids and heteroarenes.12 Li et al. previously disclosed the first illustration of the direct arylation of benzoazoles utilizing inexpensive and commercially available aldehydes as the arylating reagent and FeSO4 as the catalyst.13 However, the iron salt catalyst could not be reused.Metal–organic frameworks (MOFs) have sprung up as a new type of crystalline material, assembled by appending metal ions or metal clusters with organic connecting linkers.14–17 It is possible to regulate the characteristics of MOFs by integrating diverse metal cations with varied organic components.18–20 The temperament of MOFs results in different advantages as correlated to customary inorganic materials.21–24 Despite the fact that critical attempts are necessitated for the expansion of the field, encouraging applications of these frameworks in abundant domains have been substantially analyzed, diversifying from gas storage to catalysis.25–30 The utilization of MOFs as heterogeneous catalysts has latterly allured momentous curiosity from many research groups around the world.31–34 Theoretically, it would be possible to constitute the catalytic active site either on organic connecting linkers or at metal cations in MOFs, consequently optimizing the dispersion of effective sites on the catalyst.35–37 Certainly, diverse organic reactions employing MOFs as heterogeneous catalysts have been claimed in the literature.38–44 MOFs possessing iron sites were formerly utilized as heterogeneous catalysts for miscellaneous organic conversions.31,45–50 In this manuscript, we would like to present the direct arylation of benzoazoles utilizing inexpensive and commercially available benzaldehydes as arylating reagents and Fe3O(BDC)3 as the productive heterogeneous catalyst. To our best knowledge, this reaction was not previously implemented utilizing heterogeneous catalysts.
2. Experimental
The catalyst was synthesized according to a literature protocol51,52 (see ESI†). In an exemplary experiment, a solution of benzothiazole (0.135 g, 1 mmol) and benzaldehyde (0.204 ml, 2 mmol) in N-methyl-2-pyrrolidone (4 ml) was added into a round bottom flask containing the catalyst (0.015 g, 5 mol%). Diphenyl ether (0.1 ml), as an internal standard, was then added to the flask. The catalyst amount was enumerated concerning the iron/benzothiazole molar proportion. The reaction mixture was magnetically stirred for 5 min to distribute the catalyst in the solvent. Afterwards, di-tert-butyl peroxide (tBuOOtBu, 0.42 ml, 2 mmol) was added to the flask. The solution was vigorously stirred at 100 °C for 3 h on a magnetic stirrer. Samples were withdrawn at various time periods, then quenched with KOH solution (5% w/w, 1 ml). The extraction was then executed using ethyl acetate (2 ml), and the organic phase was strongly stirred with Na2SO4, then analyzed by GC for diphenyl ether. 2-Phenylbenzo[d]thiazole was isolated by column chromatography on silica gel. GC-MS, 1H NMR, and 13C NMR analyses were implemented to validate the product identity. To test the recyclability of the catalyst, the framework was collected by centrifugation, washed intensively with methanol to remove excess reagents, activated under vacuum in a Schlenk line at 140 °C for 3 h, and then reused for a new catalytic run.3. Results and discussion
The framework was utilized as a catalyst for the direct arylation between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole as the major product (Scheme 1). Following this protocol, the inexpensive and commercially available benzaldehyde could act as an aryl source for the transformation, replacing the aryl halides normally employed in the conventional cross-coupling reactions. Elementary studies were aimed at the influence of temperature on the generation of 2-phenylbenzo[d]thiazole. The reaction was performed in N-methyl-2-pyrrolidone at 5 mol% catalyst for 3 h, with a benzothiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde mole proportion of 1
benzaldehyde mole proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
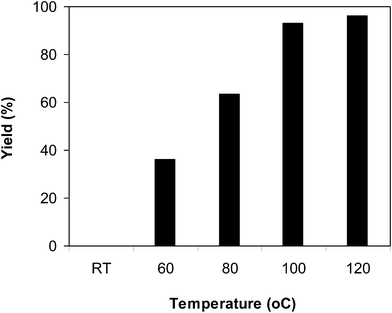
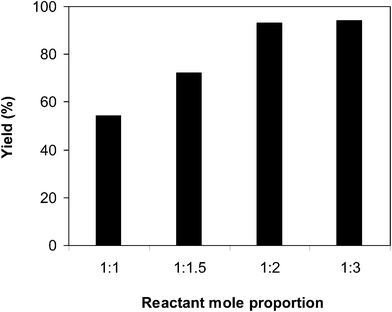
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, utilizing 2 equivalents of tBuOOtBu, at ambient temperature, 60 °C, 80 °C, 100 °C and 120 °C. It was impossible for the reaction to proceed at ambient temperature, with no evidence of product being noted after 3 h. The reaction executed at 60 °C offered 36% yield after 3 h. As expected, boosting the temperature caused a noteworthy enrichment in the yield of the expected product. Certainly, 63% yield was recorded after 3 h for the reaction implemented at 80 °C. The yield of 2-phenylbenzo[d]thiazole could be increased to 93% after 3 h when the temperature was raised to 100 °C. It was noticed that augmenting the temperature to 120 °C did not magnify the yield of the anticipated product dramatically (Fig. 1). Furthermore, the amount of benzaldehyde also had a noteworthy impact on the conversion, and the best yield of the anticipated product was recorded for the reaction utilizing 2 equivalents of benzaldehyde (Fig. 2).
2, utilizing 2 equivalents of tBuOOtBu, at ambient temperature, 60 °C, 80 °C, 100 °C and 120 °C. It was impossible for the reaction to proceed at ambient temperature, with no evidence of product being noted after 3 h. The reaction executed at 60 °C offered 36% yield after 3 h. As expected, boosting the temperature caused a noteworthy enrichment in the yield of the expected product. Certainly, 63% yield was recorded after 3 h for the reaction implemented at 80 °C. The yield of 2-phenylbenzo[d]thiazole could be increased to 93% after 3 h when the temperature was raised to 100 °C. It was noticed that augmenting the temperature to 120 °C did not magnify the yield of the anticipated product dramatically (Fig. 1). Furthermore, the amount of benzaldehyde also had a noteworthy impact on the conversion, and the best yield of the anticipated product was recorded for the reaction utilizing 2 equivalents of benzaldehyde (Fig. 2).
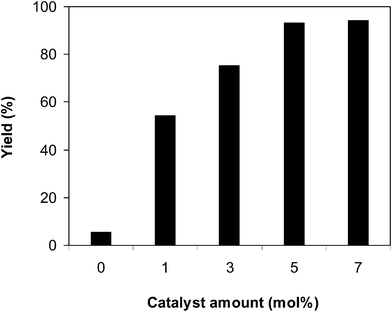
Another point that should be explored for the reaction between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole is the catalyst amount. In the first illustration of the direct arylation of benzoazoles utilizing inexpensive and commercially available aldehydes as the arylating agent, Li et al. employed up to 20 mol% FeSO4 catalyst.13 The reaction was executed in N-methyl-2-pyrrolidone at 100 °C for 3 h, with a benzothiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde mole proportion of 1
benzaldehyde mole proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, utilizing 2 equivalents of tBuOOtBu, at 1 mol%, 3 mol%, 5 mol%, and 7 mol% catalyst. Interestingly, it was noticed that a high yield of 2-phenylbenzo[d]thiazole could be obtained by employing a lower catalyst amount as compared to the corresponding homogeneous iron catalyst. The reaction deploying 1 mol% catalyst could progress to 54% yield after 3 h. Expanding the catalyst quantity to 3 mol% amended the yield of the anticipated product to 75% after 3 h. Up to 93% yield of 2-phenylbenzo[d]thiazole was attained for the transformation in the presence of 5 mol% catalyst. Extending the catalyst quantity to more than 5 mol% was noted to be wasteful as the yield of the predicted product was not augmented enormously. Certainly, the reaction utilizing 7 mol% catalyst reached 94% yield after 3 h. It must be emphasized that in the absence of the framework, the reaction between benzothiazole and benzaldehyde provided only 5% yield after 3 h (Fig. 3). Therefore, these data verified that employing the iron framework for the conversion was compulsory.
2, utilizing 2 equivalents of tBuOOtBu, at 1 mol%, 3 mol%, 5 mol%, and 7 mol% catalyst. Interestingly, it was noticed that a high yield of 2-phenylbenzo[d]thiazole could be obtained by employing a lower catalyst amount as compared to the corresponding homogeneous iron catalyst. The reaction deploying 1 mol% catalyst could progress to 54% yield after 3 h. Expanding the catalyst quantity to 3 mol% amended the yield of the anticipated product to 75% after 3 h. Up to 93% yield of 2-phenylbenzo[d]thiazole was attained for the transformation in the presence of 5 mol% catalyst. Extending the catalyst quantity to more than 5 mol% was noted to be wasteful as the yield of the predicted product was not augmented enormously. Certainly, the reaction utilizing 7 mol% catalyst reached 94% yield after 3 h. It must be emphasized that in the absence of the framework, the reaction between benzothiazole and benzaldehyde provided only 5% yield after 3 h (Fig. 3). Therefore, these data verified that employing the iron framework for the conversion was compulsory.
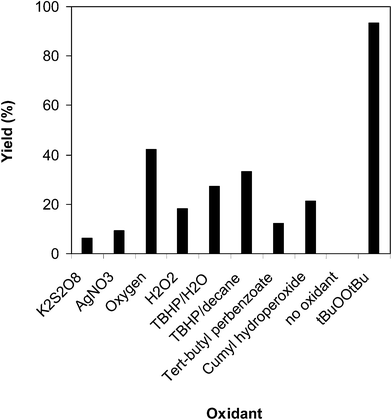
Similar to other oxidative transformations, the existence of an oxidant in the catalytic cycle is compulsory for the reaction between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole. We accordingly investigated the influence of various oxidants on the transformation. The reaction was conducted in N-methyl-2-pyrrolidone at 100 °C for 3 h, with benzothiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde mole proportion of 1
benzaldehyde mole proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
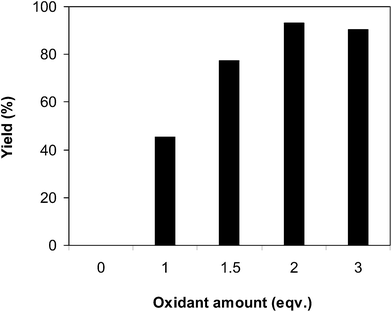
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, at 5 mol% catalyst, utilizing 2 equivalents of oxidant. The reaction utilizing potassium peroxodisulfate and silver nitrate proceeded with difficulty, with 6% and 9% yields, respectively, found after 3 h. tert-Butyl perbenzoate and cumyl hydroperoxide were also not appropriate for the reaction, and 12% and 21% yields, respectively, were recorded after 3 h. The reaction utilizing aqueous tert-butyl hydroperoxide provided 27% yield after 3 h, while the corresponding oxidant in decane expressed better efficiency. It was noticed that oxygen was not an effective oxidant for the reaction, producing 2-phenylbenzo[d]thiazole in 42% yield after 3 h under these conditions. Li et al. previously employed pure oxygen as the oxidant, though up to 20 mol% catalyst was required, the reaction time was extended to 20 h, and the temperature was boosted to 150 °C.13 In this work, increasing the temperature from 100 °C to 150 °C under oxygen with 5 mol% catalyst did not enhance the reaction yield after 3 h. Compared with these oxidants, tBuOOtBu displayed the best performance for the arylation reaction, furnishing a 93% yield of the anticipated product after 3 h (Fig. 4). In addition, it was noted that the quantity of the oxidant disclosed a considerable impression on the generation of 2-phenylbenzo[d]thiazole. The reaction employing 1 equivalent of tBuOOtBu proceeded with difficulty, with 45% yield being observed after 3 h. The best result was recorded for the reaction deploying 2 equivalents of the oxidant. It must be emphasized that no evidence of product was found in the absence of the oxidant (Fig. 5).
2, at 5 mol% catalyst, utilizing 2 equivalents of oxidant. The reaction utilizing potassium peroxodisulfate and silver nitrate proceeded with difficulty, with 6% and 9% yields, respectively, found after 3 h. tert-Butyl perbenzoate and cumyl hydroperoxide were also not appropriate for the reaction, and 12% and 21% yields, respectively, were recorded after 3 h. The reaction utilizing aqueous tert-butyl hydroperoxide provided 27% yield after 3 h, while the corresponding oxidant in decane expressed better efficiency. It was noticed that oxygen was not an effective oxidant for the reaction, producing 2-phenylbenzo[d]thiazole in 42% yield after 3 h under these conditions. Li et al. previously employed pure oxygen as the oxidant, though up to 20 mol% catalyst was required, the reaction time was extended to 20 h, and the temperature was boosted to 150 °C.13 In this work, increasing the temperature from 100 °C to 150 °C under oxygen with 5 mol% catalyst did not enhance the reaction yield after 3 h. Compared with these oxidants, tBuOOtBu displayed the best performance for the arylation reaction, furnishing a 93% yield of the anticipated product after 3 h (Fig. 4). In addition, it was noted that the quantity of the oxidant disclosed a considerable impression on the generation of 2-phenylbenzo[d]thiazole. The reaction employing 1 equivalent of tBuOOtBu proceeded with difficulty, with 45% yield being observed after 3 h. The best result was recorded for the reaction deploying 2 equivalents of the oxidant. It must be emphasized that no evidence of product was found in the absence of the oxidant (Fig. 5).
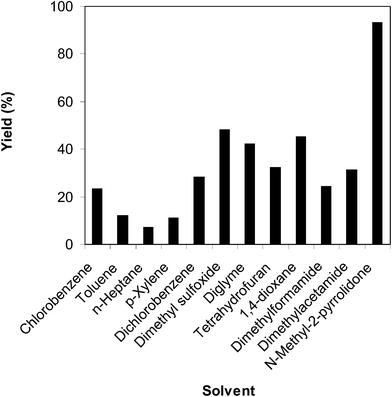
The solvent showed a noticeable influence, being conditional on the disposition of the solid catalyst. Consequently, the impact of miscellaneous solvents on the direct arylation between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole was examined. The reaction was executed at 100 °C for 3 h, with a benzothiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde mole proportion of 1
benzaldehyde mole proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu in different organic solvents. Nonpolar solvents such as toluene, p-xylene, n-heptane were inappropriate for the conversion, granting the expected product in 12%, 11%, and 5% yields, respectively, after 3 h. Chlorobenzene and dichlorobenzene also exhibited low performance, producing 2-phenylbenzo[d]thiazole in 23% and 28% yields after 3 h, respectively. The reaction implemented in N,N-dimethylformamide proceeded to 24% yield, while that performed in N,N-dimethylacetamide offered 31% yield after 3 h. Tetrahydrofuran displayed a similar performance, generating the anticipated product in 32% yield. This number could be enhanced to 42% for the conversion executed in diglyme, and 45% for that carried out in 1,4-dioxane. Performing the reaction in dimethyl sulfoxide, the reaction yield was increased to 48% after 3 h. Compared with these solvents, N-methyl-2-pyrrolidone revealed the best performance, with 93% yield of 2-phenylbenzo[d]thiazole being recorded after 3 h (Fig. 6).
2, at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu in different organic solvents. Nonpolar solvents such as toluene, p-xylene, n-heptane were inappropriate for the conversion, granting the expected product in 12%, 11%, and 5% yields, respectively, after 3 h. Chlorobenzene and dichlorobenzene also exhibited low performance, producing 2-phenylbenzo[d]thiazole in 23% and 28% yields after 3 h, respectively. The reaction implemented in N,N-dimethylformamide proceeded to 24% yield, while that performed in N,N-dimethylacetamide offered 31% yield after 3 h. Tetrahydrofuran displayed a similar performance, generating the anticipated product in 32% yield. This number could be enhanced to 42% for the conversion executed in diglyme, and 45% for that carried out in 1,4-dioxane. Performing the reaction in dimethyl sulfoxide, the reaction yield was increased to 48% after 3 h. Compared with these solvents, N-methyl-2-pyrrolidone revealed the best performance, with 93% yield of 2-phenylbenzo[d]thiazole being recorded after 3 h (Fig. 6).
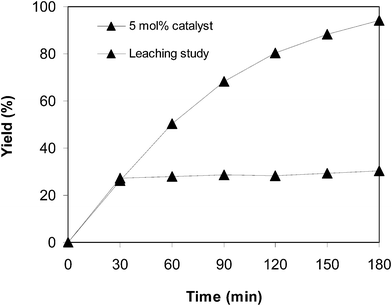
Since the direct arylation between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole was implemented in solution, it is critical to conduct a leaching test. In a number of circumstances, the conversion might carry on via fractionally homogeneous catalysis as a portion of catalyst was dissolved throughout the course of the reaction. The leaching analysis was accordingly executed to verify that the arylation occurred through genuinely heterogeneous catalysis. The reaction was performed in N-methyl-2-pyrrolidone at 100 °C for 3 h, with a benzothiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde mole proportion of 1
benzaldehyde mole proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu. Following a 30 min reaction period with a 30% yield of 2-phenylbenzo[d]thiazole being recorded, the iron framework catalyst was segregated. The liquid phase was thereafter relocated to a new flask, and the reaction was allowed to proceed for a further 150 min. The generation of the predicted product throughout this period, if any, was confirmed by GC analysis. It was spotted that after the removal of the iron framework, no more 2-phenylbenzo[d]thiazole was produced (Fig. 7). These experimental data validated that the arylation between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole was able to carry on only if the framework catalyst was present in the reaction blend. Certainly, the offering of homogeneous catalysis to the arylation reaction was inconsequential.
2 at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu. Following a 30 min reaction period with a 30% yield of 2-phenylbenzo[d]thiazole being recorded, the iron framework catalyst was segregated. The liquid phase was thereafter relocated to a new flask, and the reaction was allowed to proceed for a further 150 min. The generation of the predicted product throughout this period, if any, was confirmed by GC analysis. It was spotted that after the removal of the iron framework, no more 2-phenylbenzo[d]thiazole was produced (Fig. 7). These experimental data validated that the arylation between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole was able to carry on only if the framework catalyst was present in the reaction blend. Certainly, the offering of homogeneous catalysis to the arylation reaction was inconsequential.
To comprehend the mechanism of the arylation between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole, additional experiments were implemented. The reaction was executed in N-methyl-2-pyrrolidone at 100 °C for 3 h, with a benzothiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde mole proportion of 1
benzaldehyde mole proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
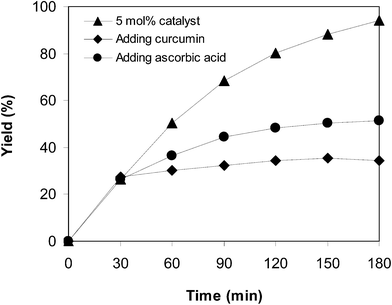
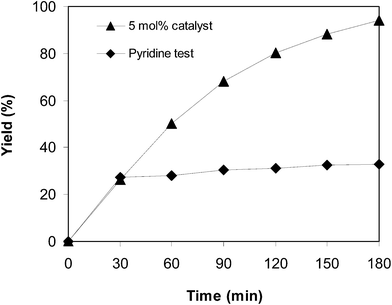
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu. To validate the necessity of the oxidant for the transformation, curcumin ((1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione) or ascorbic acid in the role of the antioxidant were injected into the catalytic reaction after the first 30 min reaction period. The consequent mixture was magnetically stirred at 100 °C for an additional 150 min. It was noticed that the existence of curcumin in the reaction mixture restrained the arylation, and 34% yield of the predicted product was observed after 3 h. Ascorbic acid also had a negative effect on the conversion (Fig. 8). These data intimated that curcumin or ascorbic acid snared the radicals created in the cycle of the catalytic transformation, thus ceasing the arylation. In another test, a catalyst poison, pyridine, was utilized. After 30 min reaction period, pyridine was injected into the reaction mixture, and the consequent mixture was magnetically stirred at 100 °C for an additional 150 min. It was also recognized that the conversion was discontinued after the addition of pyridine (Fig. 9).
2 at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu. To validate the necessity of the oxidant for the transformation, curcumin ((1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione) or ascorbic acid in the role of the antioxidant were injected into the catalytic reaction after the first 30 min reaction period. The consequent mixture was magnetically stirred at 100 °C for an additional 150 min. It was noticed that the existence of curcumin in the reaction mixture restrained the arylation, and 34% yield of the predicted product was observed after 3 h. Ascorbic acid also had a negative effect on the conversion (Fig. 8). These data intimated that curcumin or ascorbic acid snared the radicals created in the cycle of the catalytic transformation, thus ceasing the arylation. In another test, a catalyst poison, pyridine, was utilized. After 30 min reaction period, pyridine was injected into the reaction mixture, and the consequent mixture was magnetically stirred at 100 °C for an additional 150 min. It was also recognized that the conversion was discontinued after the addition of pyridine (Fig. 9).
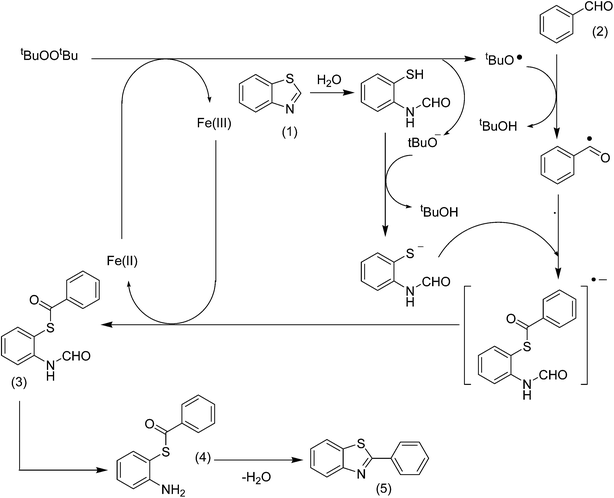
Li et al. previously recommended that this reaction would take a ring-opening but not a decarbonylative pathway.13 Indeed, the ring-opening transformation of benzoazoles was recently suggested by Doucet et al.53 A plausible mechanism via ring-opening pathway was proposed for the arylation between benzothiazole and benzaldehyde to generate 2-phenylbenzo[d]thiazole (Scheme 2). The hydrolysis of benzothiazole to N-(2-mercaptophenyl)formamide could be initiated by the water residue in the solvent. Indeed, MS analysis indicated the presence of S-2-formamidophenyl benzothioate (3) as an intermediate in the reaction mixture (Fig. S26†). However, a clear mechanism for this transformation still needs further studies. To gain insight about the location of the catalytic reaction, experiments using the catalyst with smaller particle size were conducted under identical conditions. Undoubtedly, smaller size crystals normally exhibit higher activity due to the extended external surface of the catalyst.54 It was noted that the yield of 2-phenylbenzo[d]thiazole was not improved significantly. Moreover, the cavity size of the framework is approximately 7 Å, while the sizes of the reactants and products are less than 7 Å.55,56 This observation would indicate that the transformation might occur inside the pores as well as on the external surface of the catalyst. Nevertheless, additional studies are needed to elucidate the location of the catalytic reaction.
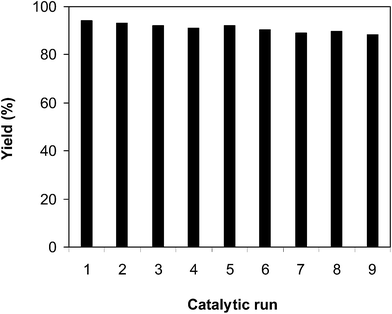
The catalyst showed better productivity in the generation of 2-phenylbenzo[d]thiazole than innumerable MOFs and miscellaneous customary iron salts. To accentuate the extraordinary attributes of the iron framework, its reusability must be investigated. In splendid circumstances, it must be possible to reutilize the iron framework as many times as possible while its efficiency could be maintained. We then explored the reusability of the catalyst in the arylation of benzothiazole with benzaldehyde over 8 consecutive catalytic cycles. The reaction was executed in N-methyl-2-pyrrolidone at 100 °C for 3 h, with a benzothiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde mole proportion of 1
benzaldehyde mole proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
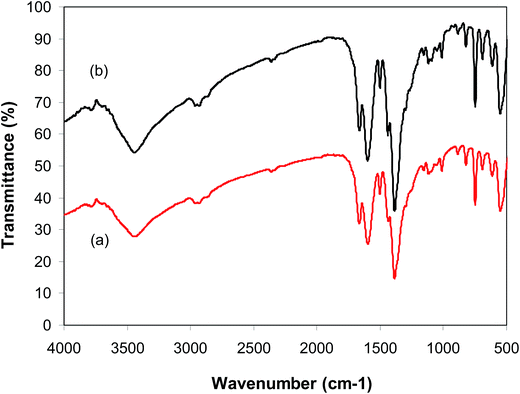
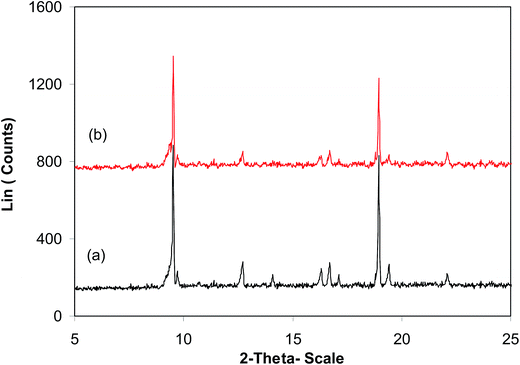
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu. At the end of each run, the catalyst was collected by centrifugation, washed intensively with methanol to remove excess reagents, activated under vacuum in a Schlenk line at 140 °C for 3 h, and reused for new catalytic reaction. Experimental data revealed that it was realistic to reutilize the catalyst without a critical decline in catalytic productivity. Undeniably, the arylation reaction still contributed an 88% yield of 2-phenylbenzo[d]thiazole in the 8th run (Fig. 10). In addition, FT-IR (Fig. 11) and XRD (Fig. 12) data of the reused framework showed that the framework integrity could be sustained throughout the experiments.
2, at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu. At the end of each run, the catalyst was collected by centrifugation, washed intensively with methanol to remove excess reagents, activated under vacuum in a Schlenk line at 140 °C for 3 h, and reused for new catalytic reaction. Experimental data revealed that it was realistic to reutilize the catalyst without a critical decline in catalytic productivity. Undeniably, the arylation reaction still contributed an 88% yield of 2-phenylbenzo[d]thiazole in the 8th run (Fig. 10). In addition, FT-IR (Fig. 11) and XRD (Fig. 12) data of the reused framework showed that the framework integrity could be sustained throughout the experiments.
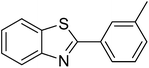
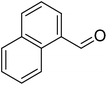
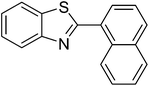
The scope of this work was subsequently extended to direct arylation between different aldehydes and benzothiazoles utilizing the iron framework as the catalyst. First, various aldehydes were allowed to join the reaction with benzothiazole. Aryl-substituted azoles were then isolated by column chromatography on silica gel. It was noticed that 2-phenylbenzo[d]thiazole was obtained in 90% isolated yield after 3 h from the reaction between benzothiazole and benzaldehyde. The benzaldehydes possessing a substituent were less reactive towards the transformation. Certainly, the reaction between benzothiazole and 4-methylbenzaldehyde produced 2-p-tolylbenzo[d]thiazole in 72% yield, while a 71% yield of 2-m-tolylbenzo[d]thiazole was attained for the case of 3-methylbenzaldehyde. Using identical conditions, 2-(4-methoxyphenyl)benzo[d]thiazole was generated in an 84% yield from the reaction between benzothiazole and 4-methoxybenzaldehyde, while an 81% yield of 2-(4-fluorophenyl)benzo[d]thiazole was recorded for the case of 4-fluorobenzaldehyde. It was found that 2-substituted benzaldehydes were less reactive towards the transformation, though 55% and 47% yields of 2-o-tolylbenzo[d]thiazole and 2-(benzo[d]thiazol-2-yl)phenol were achieved for the case of 2-methylbenzaldehyde and 2-hydroxybenzaldehyde, respectively. A bulky aldehyde, 1-naphthaldehyde, was also reactive, affording 2-(naphthalen-1-yl)benzo[d]thiazole in 78% yield. Furan-2-carbaldehyde was less reactive than other aldehydes, providing 2-(furan-2-yl)benzo[d]thiazole in 62% yield. Picolinaldehyde joined the reaction more readily than furan-2-carbaldehyde, with an 80% yield of 2-(pyridin-2-yl)benzo[d]thiazole being recorded. Reactions between benzaldehyde and different benzothiazoles were also performed. Under these conditions, 6-methyl-2-phenylbenzo[d]thiazole was produced in 71% yield, while 88% and 69% yields were achieved for 6-methoxy-2-phenylbenzo[d]thiazole and 6-nitro-2-phenylbenzo[d]thiazole, respectively. Furthermore, the reaction between 4,5-dimethylthiazole and benzaldehyde proceeded readily, providing 4,5-dimethyl-2-phenylthiazole in 87% yield (Table 1).
| Entry | Azoles | Aldehydes | Aryl-substituted azoles | Isolated yields (%) |
|---|---|---|---|---|
a The reaction was conducted in N-methyl-2-pyrrolidone at 100 °C for 3 h with a benzothiazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aldehyde mole proportion of 1 aldehyde mole proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu. 2 at 5 mol% catalyst, utilizing 2 equivalents of tBuOOtBu. |
||||
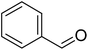
| 1 |  |
 |
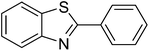 |
90 |
| 2 | 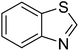 |
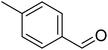 |
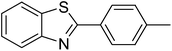 |
72 |
| 3 | 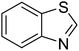 |
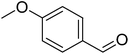 |
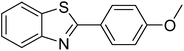 |
84 |
| 4 | 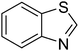 |
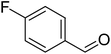 |
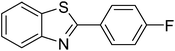 |
81 |
| 5 | 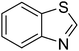 |
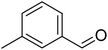 |
 |
71 |
| 6 | 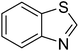 |
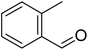 |
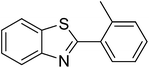 |
55 |
| 7 | 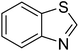 |
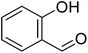 |
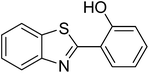 |
47 |
| 8 | 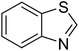 |
 |
 |
78 |
| 9 | 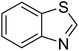 |
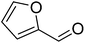 |
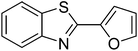 |
62 |
| 10 | 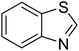 |
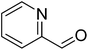 |
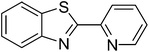 |
80 |
| 11 | 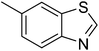 |
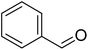 |
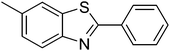 |
71 |
| 12 | 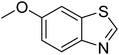 |
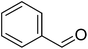 |
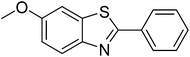 |
88 |
| 13 | 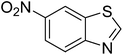 |
 |
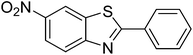 |
69 |
| 14 | 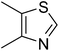 |
 |
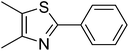 |
87 |
4. Conclusions
The metal–organic framework Fe3O(BDC)3 was synthesized and utilized as a recyclable heterogeneous catalyst for the direct arylation of azoles with aldehydes to generate aryl-substituted azoles. The catalyst revealed better productivity in the generation of 2-phenylbenzo[d]thiazole than innumerable MOFs as well as miscellaneous iron salts. The direct arylation transformation was substantially controlled by the solvent and the oxidant, and the combination of N-methyl-2-pyrrolidone as solvent with di-tert-butyl peroxide as oxidant furnished the best result. The reaction was able to carry on only if the iron framework catalyst was present in the reaction mixture, and the offering of homogeneous catalysis to the arylation reaction was inconsequential. It was possible to reutilize the catalyst for the direct arylation of azoles with aldehydes while its catalytic activity was preserved for multiple cycles. The highlight that aryl-substituted azoles could be generated utilizing the inexpensive and commercially available aldehydes as an aryl source instead of aryl halides in the presence of a recyclable catalyst will be important to the chemical industry.References
- P. Garg, S. Chaudhary and M. D. Milton, J. Org. Chem., 2014, 79, 8668–8677 CrossRef CAS PubMed.
- T. Yamauchi, F. Shibahara and T. Murai, J. Org. Chem., 2014, 79, 7185–7192 CrossRef CAS PubMed.
- S.-S. Li, L. Qin and L. Dong, Org. Biomol. Chem., 2016, 14, 4554–4570 CAS.
- S. Ponra and K. C. Majumdar, RSC Adv., 2016, 6, 37784–37922 RSC.
- J. Jiang, H. Zou, Q. Dong, R. Wang, L. Lu, Y. Zhu and W. He, J. Org. Chem., 2016, 81, 51–56 CrossRef CAS PubMed.
- R. Rossi, M. Lessi, C. Manzini, G. Marianetti and F. Bellina, Adv. Synth. Catal., 2015, 357, 3777–3814 CrossRef CAS.
- F. Zhu and Z.-X. Wang, Org. Lett., 2015, 17, 1601–1604 CrossRef CAS PubMed.
- F. Zhu, J.-L. Tao and Z.-X. Wang, Org. Lett., 2015, 17, 4926–4929 CrossRef CAS PubMed.
- D. Kumar, M. Pilania, V. Arun, S. Pooniya and J. Article, Org. Biomol. Chem., 2014, 12, 6340–6344 CAS.
- F. Gao, B.-S. Kim and P. J. Walsh, Chem. Commun., 2014, 50, 10661–10664 RSC.
- L. Chen, L. Ju, K. A. Bustin and J. M. Hoover, Chem. Commun., 2015, 51, 15059–15062 RSC.
- T. Patra, S. Nandi, S. K. Sahoo and D. Maiti, Chem. Commun., 2016, 52, 1432–1435 RSC.
- S. Liu, R. Chen, X. Guo, H. Yang, G. Deng and C.-J. Li, Green Chem., 2012, 14, 1577–1580 RSC.
- Y.-C. Lai, C.-W. Kung, C.-H. Su, K.-C. Ho, Y.-C. Liao and D.-H. Tsai, Langmuir, 2016, 32, 6123–6129 CrossRef CAS PubMed.
- K. Banlusan and A. Strachan, J. Phys. Chem. C, 2016, 120, 12463–12471 CAS.
- H. Huang, M. Beuchel, Y. Park, P. J. Baesjou, S. C. J. Meskers, D. M. d. Leeuw and K. Asadi, J. Phys. Chem. C, 2016, 120, 11045–11048 CAS.
- M. Liu, H. S. Quah, S. Wen, Z. Yu, J. J. Vittal and W. Ji, Chem. Mater., 2016, 28, 3385–3390 CrossRef CAS.
- K. Wang, X.-L. Lv, D. Feng, J. Li, S. Chen, J. Sun, L. Song, Y. Xie, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 914–919 CrossRef CAS PubMed.
- J. D. Evans and F.-X. Coudert, J. Am. Chem. Soc., 2016, 138, 6131–6134 CrossRef CAS PubMed.
- P. Kucheryavy, N. Lahanas, E. Velasco, C.-J. Sun and J. V. Lockard, J. Phys. Chem. Lett., 2016, 7, 1109–1115 CrossRef CAS PubMed.
- V. Rubio-Giménez, S. Tatay, F. Volatron, F. J. Martínez-Casado, C. Martí-Gastaldo and E. Coronado, J. Am. Chem. Soc., 2016, 138, 2576–2584 CrossRef PubMed.
- J. Yuan, A. M. Fracaroli and W. G. Klemperer, Organometallics, 2016, 35, 2149–2155 CrossRef CAS.
- O. Fleker, A. Borenstein, R. Lavi, L. Benisvy, S. Ruthstein and D. Aurbach, Langmuir, 2016, 32, 4935–4944 CrossRef CAS PubMed.
- G. Zhang, R. Chenitz, M. Lefèvre, S. Sun and J.-P. Dodelet, Nano Energy, 2016, 29, 111–125 CrossRef CAS.
- J. K. Bristow, K. L. Svane, D. Tiana, J. M. Skelton, J. D. Gale and A. Walsh, J. Phys. Chem. C, 2016, 120, 9276–9281 CAS.
- G. Chang, H. Wen, B. Li, W. Zhou, H. Wang, K. Alfooty, Z. Bao and B. Chen, Cryst. Growth Des., 2016, 16, 3395–3399 CAS.
- Y. Tian, S. Shen, J. Cong, L. Yan, S. Wang and Y. Sun, J. Am. Chem. Soc., 2016, 138, 782–785 CrossRef CAS PubMed.
- L. C. Gómez-Aguirre, B. Pato-Doldán, J. Mira, S. Castro-García, M. A. Señarís-Rodríguez, M. Sánchez-Andújar, J. Singleton and V. S. Zapf, J. Am. Chem. Soc., 2016, 138, 1122–1125 CrossRef PubMed.
- Y. Zhang, S. Yuan, X. Feng, H. Li, J. Zhou and B. Wang, J. Am. Chem. Soc., 2016, 138, 5785–5788 CrossRef CAS PubMed.
- X. Lu, X. Wang, L. Wu, L. Wu, Dhanjai, L. Fu, Y. Gao and J. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 16533–16539 CAS.
- X. Wang, H. Zhang, H. Lin, S. Gupta, C. Wang, Z. Tao, H. Fu, T. Wang, J. Zheng, G. Wu and X. Li, Nano Energy, 2016, 25, 110–119 CrossRef.
- G. H. Dang, T.T. Dang, D. T. Le, T. Truong and N. T. S. Phan, J. Catal., 2014, 319, 258–264 CrossRef CAS.
- F. Zhang, S. Zheng, Q. Xiao, Y. Zhong, W. Zhu, A. Lin and M. S. El-Shall, Green Chem., 2016, 18, 2900–2908 RSC.
- V. V. Torbina, I. D. Ivanchikova, O. A. Kholdeeva, I. Y. Skobelev and O. V. Vodyankina, Catal. Today, 2016, 278, 97–103 CrossRef CAS.
- L. Braglia, E. Borfecchia, L. Maddalena, S. Øien, K. A. Lomachenko, A. L. Bugaev, S. Bordiga, A. V. Soldatov, K. P. Lillerud and C. Lamberti, Catal. Today, 2016 DOI:10.1016/j.cattod.2016.02.039.
- Y. Zhang, Y. Zhou, Y. Zhao and C.-j. Liu, Catal. Today, 2016, 263, 61–68 CrossRef CAS.
- E. Plessers, D. E. D. Vos and M. B. J. Roeffaers, J. Catal., 2016, 340, 136–143 CrossRef CAS.
- X. Wang and Y. Li, J. Mol. Catal. A: Chem., 2016, 420, 56–65 CrossRef CAS.
- P. Hester, S. Xu, W. Liang, N. Al-Janabi, R. Vakili, P. Hill, C. A. Muryn, X. Chen, P. A. Martin and X. Fan, J. Catal., 2016, 340, 85–94 CrossRef CAS.
- W. T. Schumacher, M. J. Mathews, S. A. Larson, C. E. Lemmon, K. A. Campbell, B. T. Crabb, B. J.-A. Chicoine, L. G. Beauvais and M. C. Perry, Polyhedron, 2016, 114, 422–427 CrossRef CAS.
- K. A. Bussey, A. R. Cavalier, M. E. Mraz, K. D. Oshin, A. Sarjeant and T. Pintauer, Polyhedron, 2016, 114, 256–267 CrossRef CAS.
- O. A. Kholdeeva, Catal. Today, 2016, 278, 22–29 CrossRef CAS.
- X. Li, A. K. Tjiptoputro, J. Ding, J. M. Xue and Y. Zhu, Catal. Today, 2016, 279, 77–83 CrossRef.
- Z.-L. Wang, C. Li and Y. Yamauchi, Nano Today, 2016, 11, 373–391 CrossRef CAS.
- T. N. Lieu, K. D. Nguyen, D. T. Le, T. Truong and N. T. S. Phan, Catal. Sci. Technol., 2016, 6, 5916–5926 CAS.
- A. R. Oveisi, A. Khorramabadi-zad and S. Daliran, RSC Adv., 2016, 6, 1136–1142 RSC.
- L. Qin, Z. Li, Z. Xu, X. Guo and G. Zhang, Appl. Catal., B, 2015, 179, 500–508 CrossRef CAS.
- H. Sun, H. Su, X. Ma, P. Zhang, X. Zhang, X. Dai, J. Gao, C. Chen and S.-G. Sun, Electrochim. Acta, 2016, 205, 53–61 CrossRef CAS.
- V. T. Nguyen, H. Q. Ngo, D. T. Le, T. Truong and N. T. S. Phan, Chem. Eng. J., 2016, 284, 778–785 CrossRef CAS.
- T. D. Le, K. D. Nguyen, V. T. Nguyen, T. Truong and N. T. S. Phan, J. Catal., 2016, 333, 94–101 CrossRef CAS.
- E. Haque, J. W. Jun and S. H. Jhung, J. Hazard. Mater., 2011, 185, 507–511 CrossRef CAS PubMed.
- M. Anbia, V. Hoseini and S. Sheykhi, J. Ind. Eng. Chem., 2012, 18, 1149–1152 CrossRef CAS.
- F. Abdellaoui, C. Youssef, H. B. Ammar, T. Roisnel, J.-F. Soule and H. Doucet, ACS Catal., 2016, 6, 4248–4252 CrossRef CAS.
- R. Selvin, H.-L. Hsu and T.-M. Her, Catal. Commun., 2008, 10, 169–172 CrossRef CAS.
- H. Wang and M. Frenklach, Combust. Flame, 1994, 96, 163–170 CrossRef CAS.
- J. Jae, G. A. Tompsett, A. J. Foster, K. D. Hammond, S. M. Auerbach, R. F. Lobo and G. W. Huber, J. Catal., 2011, 279, 257–268 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24716d |
| This journal is © The Royal Society of Chemistry 2017 |