A one-pot, metal-free approach to bicyclic 2-pyridones†
Abstract
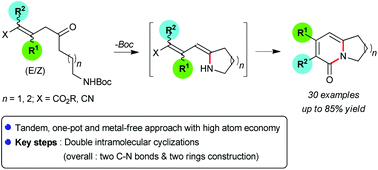
A one-pot, metal-free approach for the synthesis of 3,4-disubstituted, bicyclic 2-pyridones was developed. The reaction occurred through the intramolecular, two-fold sequential cyclization of Michael adducts under thermal basic conditions. The methodology proceeded with a range of substrates to afford bicyclic 2-pyridones in up to 85% yield, and provided direct and easy access to two Tylophora alkaloid core structures.


 Please wait while we load your content...
Please wait while we load your content...