In situ decoration of stainless steel nanoparticles for synergistic enhancement of α-Ni(OH)2 oxygen evolution reaction catalysis†
Anirudh
Balram
 ,
Hanfei
Zhang
,
Hanfei
Zhang
 and
Sunand
Santhanagopalan
and
Sunand
Santhanagopalan
 *
*
Multi-Scale Energy Systems (MuSES) Laboratory, Mechanical & Aerospace Engineering Department, The University of Texas at Arlington, 500 W. First Street, Arlington, TX 76019, USA. E-mail: ssanthan@uta.edu
First published on 30th August 2017
Abstract
Alkaline water-splitting is a promising clean technology for hydrogen production. However, reducing the oxygen evolution reaction (OER) overpotential is critical to the overall process efficiency and economic feasibility. To this end, we demonstrate novel 3D hierarchical α-Ni(OH)2 nanoparticle decorated stainless steel nanoparticle (SSNP) catalyst deposits on Ni foam substrates. SSNP deposition along with simultaneous in situ Ni(OH)2 decoration of the SSNP is facilitated via a facile single-step electrophoretic deposition (EPD) based co-deposition method. The enhanced OER catalytic activity of α-Ni(OH)2 owing to the synergistic SSNP support, which possibly serves as a dopant source to the metal hydroxide, could sustain current densities of 10 and 125 mA cm−2 at overpotentials of 220 and 250 mV respectively, in 1 M KOH. These robust deposits could survive accelerated cycling and prolonged oxygen generation at higher current densities despite the lack of any polymeric binders. In 10 M KOH, a current density of 500 mA cm−2 could be maintained at an overpotential of 450 mV (iR-uncorrected).
Water splitting is an extremely promising route to sustainable and clean hydrogen production.1 The present challenge is to economically minimize the overpotentials required to facilitate the two electrode reactions: the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). The overall system overpotential, however, is typically dominated by the OER side due to its comparatively sluggish kinetics.2 Thus, reducing OER overpotentials is a crucial component in the economic viability of electrolytic water splitting.3 From a practical viewpoint, the improved OER kinetics and the remarkable performance of non-precious catalysts in reducing OER potentials in alkaline media make alkaline water splitting particularly attractive.4 Nickel based catalysts such as Ni(OH)2,2 Ni2P/NiOx,5 Ni–Co hydroxide,6 especially Ni–Fe hydroxide7etc. in various nanostructured morphologies have been found to perform exceptionally well. More recently, the excellent catalytic activity observed in the cases of pure Ni(OH)2,8 NiO,5 Ni,9 Co(OH)2 structures10 has also been attributed primarily to the inadvertent Fe doping via contaminants present in the KOH electrolyte.11 Catalytic activity has also been found to be significantly influenced by the deposition substrate and support materials.9,12–15 Generally, noble metal substrates and supports have been observed to greatly enhance the catalytic activity of OER catalysts.16–21 However, more interesting from a commercial perspective have been reports of high activity of such Ni-based deposits on relatively inexpensive stainless steel (SS) substrates.3,9,22
Presumably, in these instances, the unintended doping of the deposit with trace iron via the substrate during the fabrication process9 greatly amplifies the OER activity of the catalyst. For scalability, consistent production of such high activity deposits as a serendipitous consequence of synergistic interactions naturally engendered during electrode fabrication is very appealing. For example, co-deposition of desired Ni–Fe materials requires precise monitoring and control of the deposition conditions such as potential, pH, Ni and Fe ion concentrations within the electrodeposition bath etc. to correct for anomalous deposition rates of one species over another during deposition.23–25 However, when the steel substrate itself acts as the dopant source by its mere presence, only the relatively facile deposition of Ni(OH)2 needs to be controlled. In our work, we observed a similar enhancement in Ni(OH)2 catalyst performance for the OER when deposited on SS substrates. We hypothesized that utilizing steel nanoparticles (SSNP) as the Ni(OH)2 catalyst support could provide greatly increased SS/Ni(OH)2 interfacial area and improve performance due to nanostructuring effects.26 Additionally, the stability of stainless steel under OER conditions makes an excellent choice for support material.27 Furthermore, steel itself has been observed by several researchers to be a highly active OER catalyst in alkaline water electrolysis.28–32
Thus, to realize a truly hierarchical 3D structure with maximized SS/Ni(OH)2 interface, we prepared a catalyst composed of Ni(OH)2 nanoparticle decorated SS nanoparticles deposited on a 3D Ni foam substrate. With scalability in mind, the widespread use and relatively inexpensive nature of Ni foam would make it an obvious candidate for the substrate. We electrophoretically co-deposited SS nanoparticles (SSNP) while simultaneously decorating them with Ni(OH)2in situ on the Ni foam in a single-step process requiring no pre or post-treatment. The decoration of individual nanoparticles, along with the intimate contact between all the deposit components can facilitate excellent electrochemical performance. We expect the SSNP to behave as a conductive pathway from the Ni(OH)2 to the Ni foam backbone helping improve charge transfer. In particular for higher current density applications, it is necessary to produce deposits with low internal resistance and robustness to survive the rigors of violent gas evolution. EPD allows us to achieve these results while avoiding polymeric binder materials that typically hamper performance33,34 and can lead to increased internal resistances due to bubble coverage.35 This highly accessible 3D Ni foam structure coated with the unique binderless SS/Ni(OH)2 nanocomposite rendered a highly active OER catalyst deposit.
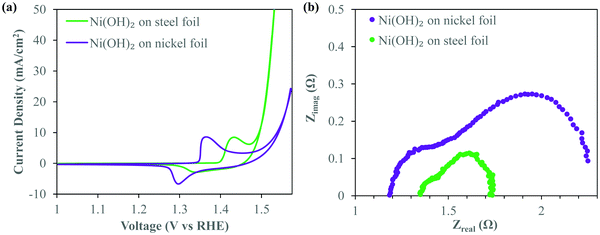
First, to illustrate the effect of the deposition substrate on electrochemical performance, particularly on the OER, we first deposited Ni(OH)2 on planar nickel and stainless steel foil. For consistency, both substrates were cleaned for 10 minutes in 6 M HCl prior to deposition. Ni(OH)2 was then deposited via electrodeposition potentiostatically at 200 V for 2 min from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of ethanol and isopropanol (IPA) with 0.2 mg ml−1 of dissolved NiCl2·6H2O. Highly localized pH at the electrode–electrolyte interface36 during deposition causes Ni2+ to deposit in the form of nickel hydroxide. Thermogravimetric analysis (TGA) of the deposit (Fig. S1, ESI†) confirms the characteristic two step weight loss associated with α-Ni(OH)2,37 the hydrated phase of Ni(OH)2 containing water molecules intercalated within the Ni(OH)2 crystal. We note here that despite the relatively high voltage used, the ethanol/IPA electrolyte itself does not appear to dissociate due to the applied field. On the basis of the formation of Ni(OH)2, it is obvious that the small amount of water content from the dissolved water of crystallization of the salt dissociates and forms H2 gas. These bubbles are presumably too small to be seen visually during deposition. However, this small amount of water electrolysis is controlled enough to not affect the integrity of the deposit and provide localized alkaline conditions at the electrode to precipitate Ni(OH)2. Fig. 1a shows the iR-corrected cyclic voltammograms (CV) of these α-Ni(OH)2 deposits made on stainless steel (SS) and nickel foil substrates under similar deposition conditions. Details of the post-run iR correction technique are provided in the Experimental methods section. These CV curves were recorded at 5 mV s−1 in oxygen purged 1 M KOH. Both the Ni(OH)2 deposits show anodic peaks signifying a conversion to NiOOH. However, there is a clear anodic shift of the redox peaks of Ni(OH)2 on the SS substrate. The anodic peak and cathodic peak of Ni(OH)2 deposited on SS shift anodically by ∼70 and 45 mV respectively. This anodic shift of the redox peaks suggests Fe-doping of the Ni(OH)2 crystal8,38,39 which is known to play a critical role in OER overpotential reduction. It is readily apparent that the deposit on the SS foil shows a greatly enhanced OER performance as witnessed by the steep increase in current density around 1.45 V vs. RHE, associated with oxygen gas evolution, after the oxidation of Ni(OH)2. At the figure of merit typically considered for OER applications i.e. the overpotential required to evolve oxygen at a current density of 10 mA cm−2, the SS requires merely 275 mV, over 50 mV lower than that for the Ni(OH)2 deposit prepared on the nickel substrate.
1 solution of ethanol and isopropanol (IPA) with 0.2 mg ml−1 of dissolved NiCl2·6H2O. Highly localized pH at the electrode–electrolyte interface36 during deposition causes Ni2+ to deposit in the form of nickel hydroxide. Thermogravimetric analysis (TGA) of the deposit (Fig. S1, ESI†) confirms the characteristic two step weight loss associated with α-Ni(OH)2,37 the hydrated phase of Ni(OH)2 containing water molecules intercalated within the Ni(OH)2 crystal. We note here that despite the relatively high voltage used, the ethanol/IPA electrolyte itself does not appear to dissociate due to the applied field. On the basis of the formation of Ni(OH)2, it is obvious that the small amount of water content from the dissolved water of crystallization of the salt dissociates and forms H2 gas. These bubbles are presumably too small to be seen visually during deposition. However, this small amount of water electrolysis is controlled enough to not affect the integrity of the deposit and provide localized alkaline conditions at the electrode to precipitate Ni(OH)2. Fig. 1a shows the iR-corrected cyclic voltammograms (CV) of these α-Ni(OH)2 deposits made on stainless steel (SS) and nickel foil substrates under similar deposition conditions. Details of the post-run iR correction technique are provided in the Experimental methods section. These CV curves were recorded at 5 mV s−1 in oxygen purged 1 M KOH. Both the Ni(OH)2 deposits show anodic peaks signifying a conversion to NiOOH. However, there is a clear anodic shift of the redox peaks of Ni(OH)2 on the SS substrate. The anodic peak and cathodic peak of Ni(OH)2 deposited on SS shift anodically by ∼70 and 45 mV respectively. This anodic shift of the redox peaks suggests Fe-doping of the Ni(OH)2 crystal8,38,39 which is known to play a critical role in OER overpotential reduction. It is readily apparent that the deposit on the SS foil shows a greatly enhanced OER performance as witnessed by the steep increase in current density around 1.45 V vs. RHE, associated with oxygen gas evolution, after the oxidation of Ni(OH)2. At the figure of merit typically considered for OER applications i.e. the overpotential required to evolve oxygen at a current density of 10 mA cm−2, the SS requires merely 275 mV, over 50 mV lower than that for the Ni(OH)2 deposit prepared on the nickel substrate.
Electrochemical impedance spectra (EIS) obtained at an oxygen evolving overpotential of 370 mV (Fig. 1b) clearly show the lower Faradaic resistances40 associated with the deposits on SS as opposed to nickel. The smaller size of the first semicircle is an indicator of easier charge transfer between the deposit and the substrate. The smaller diameter of the second semicircle41 signifies improved OER kinetics related to easier formation of intermediates,40 critical to efficient OER catalysis. Clearly, the deposit on SS has a significantly lower overall Faradaic resistance and greatly superior OER kinetics enabling excellent OER catalysis. The electrochemical behavior seen in Fig. 1 suggests that Ni(OH)2 deposits on SS are clearly OER catalysts compared to similar deposits on Ni. We attribute this to the well-studied interaction of Fe and Ni(OH)2 leading to excellent OER catalysis.8,11 As mentioned previously, while typically Fe incorporation induced enhancement of OER catalysis performance of Ni(OH)2 occurs due to incidental iron inclusion from within the alkaline electrolyte used in electrolysis, use of a steel substrate during our electrodeposition allows for immediate exploitation of this synergy. To maximally harness this activity enhancement observed while utilizing a planar steel substrate and significantly lower the overpotential further, it is imperative to somehow increase the interfacial surface area between the deposit and the substrate. An approach that allows for nanostructuring while enabling thicker deposits and higher loading, without compromising on performance, would be highly beneficial. An EPD based co-deposition strategy would greatly simplify the controls required during deposition as compared to traditional Ni/Fe electrodeposition systems.
EPD allows for facile voltage induced deposition of a wide range of nanoparticles when optimally suspended within a suitable dispersion medium.42 Additionally, EPD was selected as the method to deposit the hybrid SS/Ni(OH)2 given its ability to produce high performance and robust deposits that can withstand the demands of electrochemical applications.43,44 A particular advantage of using electrophoresis based co-deposition, as we do here, is that it facilitates deposition of SSNP onto the Ni foam electrode while simultaneously decorating those deposited SSNP with Ni(OH)2in situ during a single-step deposition process. Briefly, 0.25 mg ml−1 SS nanoparticles (SSNP) were suspended in an ethanol–isopropanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution containing 0.2 mg ml−1 nickel chloride. A schematic of the deposition working mechanism is provided in Fig. S2 (ESI†). Upon application of an electric field, the SSNP charged positively on account of adsorbed Ni2+ ions migrate towards and deposit upon the negatively polarized Ni foam electrode. Upon making contact with the Ni foam electrode, given the highly localized pH at the electrode–electrolyte interface, Ni2+ ions get deposited in the form of Ni(OH)2 nanoparticles on the SSNP surface, following a mechanism similar to the case with the planar substrate previously discussed.
1) solution containing 0.2 mg ml−1 nickel chloride. A schematic of the deposition working mechanism is provided in Fig. S2 (ESI†). Upon application of an electric field, the SSNP charged positively on account of adsorbed Ni2+ ions migrate towards and deposit upon the negatively polarized Ni foam electrode. Upon making contact with the Ni foam electrode, given the highly localized pH at the electrode–electrolyte interface, Ni2+ ions get deposited in the form of Ni(OH)2 nanoparticles on the SSNP surface, following a mechanism similar to the case with the planar substrate previously discussed.
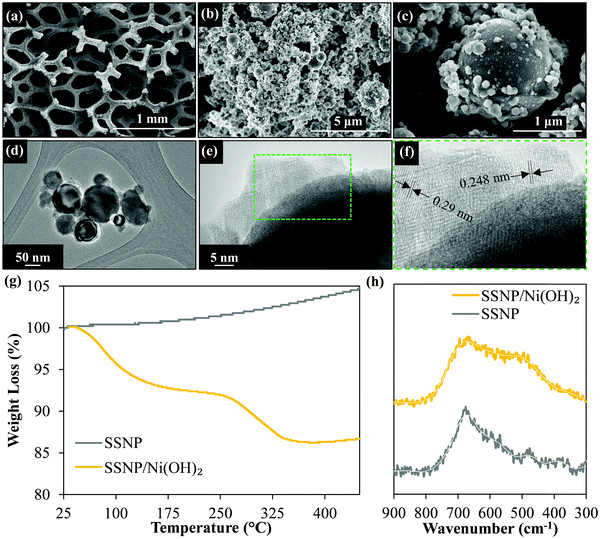
The electron micrographs (Fig. 2) of the resultant SSNP/Ni(OH)2 deposit on the Ni foam substrate show the dense deposit of SSNP on the Ni foam decorated by smaller Ni(OH)2 nanoparticles. Fig. 2d and e show the high-resolution transmission electron microscopy (HRTEM) images which indicate some nanoparticle decoration on the surface of SSNP. The X-ray diffraction (XRD) data obtained (Fig. S3, ESI†) show no obvious Ni(OH)2 peaks with only sharp well defined SS related peaks visible. This may be attributable to the generally disordered and small particle size of the Ni(OH)2 particles on the SSNP. However, Fig. 2f shows lattice spacing of 0.29 and 0.248 nm attributable to α-Ni(OH)2.45,46 Further confirmation of formation of a SSNP/Ni(OH)2 hybrid was attained via TGA as seen in the curves in Fig. 2g obtained under air flow (10 ml min−1) at a ramp rate of 10 °C min−1. As-purchased SSNP show only a gradual increase in weight as the temperature increases due to the oxidation of the SS surface. The SSNP/Ni(OH)2 hybrid on the other hand shows three distinct features, the two weight loss steps as seen previously in Fig. S1 (ESI†), associated with Ni(OH)2 and an additional feature of weight increase after the complete conversion to NiO that is attributable to the previously observed oxidation of the exposed SSNP surfaces. The Raman spectra of as-purchased SSNP and the hybrid deposit are shown in Fig. 2h. Due to the low Raman scattering intensities obtained from Ni(OH)2, no clear signal of Ni(OH)2 could be obtained at lower powers of the 532 nm laser source. Higher Raman intensities could be obtained at slightly higher laser powers (≥3 mW); however, laser induced heating appears to convert the hydroxide into the corresponding oxide,47 NiO, showing a broad feature around 508 cm−1.48 Both bare SSNP and SSNP/Ni(OH)2 deposits show a similar feature around 690 cm−1 attributable to the FeCr2O4 spinel phase in the SS.49 The material characterization data in Fig. 2 support the formation of a SSNP/Ni(OH)2 hybrid during the electrophoretic co-deposition process.
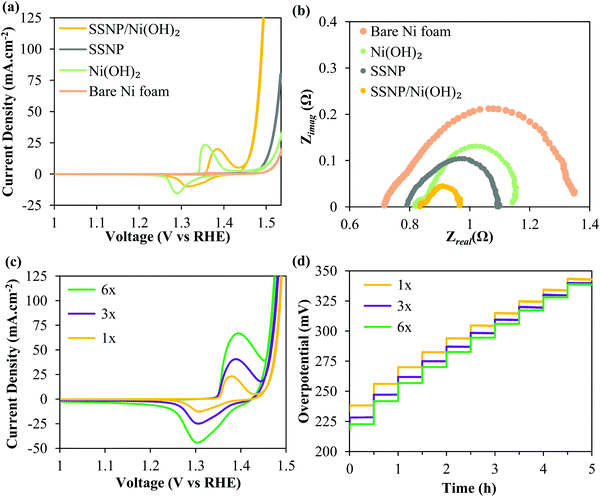
Again, all the OER performance characterization was performed in O2 purged 1 M KOH. Fig. 3 shows that the SSNP/Ni(OH)2 comprehensively outperforms two control samples – electrophoretically deposited bare SSNP as well as electrodeposited bare Ni(OH)2 – deposited under similar conditions at 200 V for 2 min. The hybrid deposit required an ultralow overpotential of ∼255 mV for a current density of 10 mA cm−2. Now, the nanostructuring of the Ni(OH)2 surface could have potentially increased the electrochemically active area. Although we refrained from directly calculating the electrochemical surface area (ECSA), we estimated double-layer capacitances (Cdl) of the deposits as previously reported elsewhere.22,50,51 More details of the Cdl estimation are provided in the Experimental methods section. The electrochemical double-layer capacitance results (Fig. S4, ESI†) suggest that pure Ni(OH)2 and the SSNP/Ni(OH)2 hybrid have very similar Cdl. ECSA is typically calculated as the ratio of Cdl and specific capacitance (Cs) i.e. capacitance of an atomically smooth layer of the material.50 Given the complexity of our hybrid SSNP/Ni(OH)2 deposit on Ni foam, a reliable estimate for Cs for our deposit is difficult to find in the literature. However, comparing the average Cs values provided in the literature for Ni and stainless steel in alkaline medium (∼30 μF cm−2), it may be reasonable to estimate that the hybrid and pure Ni(OH)2 deposits have similar ECSA, given the similar Cdl values derived from Fig. S4 (ESI†).50 We stress here that these are mere estimates and note that ECSA determination is prone to error.50 Assuming our ECSA estimates are valid, the overpotential required by the hybrid at all higher current densities is significantly lower than that required by the individual Ni(OH)2 deposit despite having similar ECSA. Yet again, the Ni(OH)2 redox peaks in the hybrid SSNP/Ni(OH)2 deposit show an anodic shift seen previously suggesting some Fe infiltration within the Ni(OH)2. This indicates significant influence of the intimate interface formed between SSNP/Ni(OH)2 during deposition on the greatly enhanced OER catalysis properties of the deposit. The EIS data acquired at an oxygen evolving overpotential of 370 mV, seen in Fig. 3b, corroborate the trends seen in the cyclic voltammograms. The composite deposit shows the lowest total Faradaic resistances compared to both the exclusively SSNP and Ni(OH)2 deposits, leading to excellent OER performance characteristics. The inordinate reduction in resistance to the OER as represented by the greatly diminished second semicircle is indicative of the synergy between SSNP and Ni(OH)2 within the hybrid deposit to facilitate facile oxygen evolution.
To further lower OER overpotentials, thicker deposits were produced by performing multiple depositions of SSNP/Ni(OH)2 on the Ni foam substrate using the same deposition parameters (200 V; 2 min) and a fresh deposition dispersion was used after every deposition step. The deposits were designated 1×, 3× and 6×, corresponding to a single deposit, and three and six consecutive deposits i.e. 2, 6 and 12 total minutes of deposition respectively. Fig. 3c shows the CV of the 1×, 3× and 6× deposits. The overpotential to achieve 10 mA cm−2 decreases by ∼35 mV to merely 220 mV for the 6× sample. Since typically hydrophobic binders are avoided in these EPD produced deposits, improved performance is witnessed even at higher overall mass loadings. Generally the binders and carbonaceous support can cause severe drops in performance due to increased bubble trapping, especially at higher loadings.35 The chronopotentiograms for the three samples performed in 10 mA cm−2 increments from 10 to 100 mA cm−2 every 30 min are shown in Fig. 3d. Similar to the trends in the CV curves, the thicker deposits outperform the 1× deposit and significantly lower the onset potential of the OER. At higher current densities there appears to be a slightly lower disparity in performance between the lower and higher loading samples, likely owing to mass transport limitations.3 Most significantly, the samples prove to be robust and able to survive the conditions of vigorous bubble generation without damage, as seen from the stability of the overpotentials in the chronopotentiometry curves.
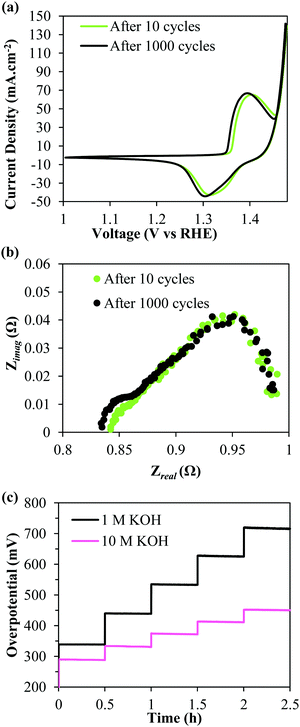
Prior to recording the chronopotentiograms seen in Fig. 3d, the deposits were also subjected to an accelerated stability testing protocol by cycling 1000 times between a voltage window of 1.3–1.8 V vs. RHE at 100 mV s−1. Fig. 4a shows iR-corrected CV curves of the 6× deposit. It is observable that there is no loss in performance even after being subjected to the harsh testing protocol. The EIS curves recorded at 370 mV similarly show near identical curves before and after accelerated cycling confirming the durability of the deposits. The same deposit was then tested for higher current density applications in 10 M KOH. Fig. 4c compares the iR uncorrected chronopotentiograms recorded in 1 M and 10 M KOH solutions. The current densities were stepped up from 100 to 500 mA cm−2 in 100 mA cm−2 increments. It is evident that the performance is significantly improved in the more concentrated KOH electrolyte. In 10 M KOH, the deposit only requires (iR uncompensated) 290 mV @ 100 mA cm−2 and 450 mV @ 500 mA cm−2. Most significantly, even at these high current densities, the SSNP/Ni(OH)2 deposit proves to be highly durable with no apparent increase in overpotential over time.
In conclusion, by electrophoretically co-depositing Ni(OH)2 nanoparticle decorated SS nanoparticles onto a 3D Ni foam substrate, we were able to maximize the synergistic interactions between the two components in the deposit. The performance of the composite deposit far exceeds the individual components, likely owing to some Fe doping of the Ni(OH)2 in the deposit induced during the deposition process itself. The engineered 3D nanostructuring of the SS/Ni(OH)2 interface (i.e. Ni(OH)2 nanoparticle decoration of the nanoscale SS particles) onto the 3D Ni foam support allows for a greater exposed catalyst surface. These robust deposits produced by EPD are able to sustain high current electrolysis despite the lack of typically used binder materials. This allows the entire deposit to be available for the catalytic action with improved wettability and catalyst accessibility. In typically studied 1 M KOH, merely 220 mV and 250 mV are required to sustain 10 and 125 mA cm−2 respectively. This allows for overpotentials as low as 450 mV (iR uncorrected) to generate 500 mA cm−2 in 10 M KOH.
Experimental methods
Nanomaterial deposition
Electrodeposition of Ni(OH)2 was performed from an ethanol/isopropanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrolyte containing 0.2 mg ml−1 dissolved NiCl2·6H2O (Sigma-Aldrich, St. Louis, MO, USA). Deposition was carried out under an applied potential of 200 V using a high voltage power source (Matsusada Precision, Model EJ-2R100) for 2 min each on stainless steel and nickel foil substrates (McMaster-Carr, Elmhurst, IL, USA). 20 ml of dispersion was used in each deposition with 1 cm−2 exposed area on the substrate. Substrates were cleaned in 6 M HCl for 10 minutes, rinsed thoroughly in deionized water and dried prior to deposition.
1) electrolyte containing 0.2 mg ml−1 dissolved NiCl2·6H2O (Sigma-Aldrich, St. Louis, MO, USA). Deposition was carried out under an applied potential of 200 V using a high voltage power source (Matsusada Precision, Model EJ-2R100) for 2 min each on stainless steel and nickel foil substrates (McMaster-Carr, Elmhurst, IL, USA). 20 ml of dispersion was used in each deposition with 1 cm−2 exposed area on the substrate. Substrates were cleaned in 6 M HCl for 10 minutes, rinsed thoroughly in deionized water and dried prior to deposition.
Electrophoretic deposition (EPD) was performed using the same ethanol:isopropanol solution as the dispersion medium. Ni foam substrates (MTI Corporation, Richmond, CA, USA) were also cleaned using a similar protocol to the planar substrates. Stainless steel 316L nanopowder (40–100 nm) was purchased from US Research Nanomaterials, Inc., Houston, TX, USA. SS nanoparticles (SSNP) were dispersed in the dispersion medium using an ultrasonic probe sonicator for 5 minutes. The Ni foam substrate (1 cm2 projected area) was suspended between two graphite foil counter electrodes, spaced 1.5 cm from each other in a beaker containing 20 ml of dispersion. EPD was then performed by applying 200 V for two minutes. Pure Ni(OH)2 was deposited as described before. Pure SSNP deposition was performed using a dispersion containing only 0.25 mg ml−1 suspended SSNP with no additional surfactants added to the dispersion. The SSNP/Ni(OH)2 nanocomposite was deposited from a dispersion containing 0.25 mg ml−1 suspended SSNP and 0.2 mg ml−1 of dissolved NiCl2·6H2O. Typically, SSNP were dispersed first for 10 minutes, after which an appropriate amount of NiCl2 dissolved in ethanol was introduced into the dispersion and further sonicated for 10 minutes. For multiple depositions, after every 2 min deposition, fresh 20 ml of dispersion was used and the deposition process repeated. The deposit was not allowed to dry in between steps.
Material characterization
A Hitachi S-4800 field emission scanning electron microscope was used to obtain secondary electron images of the nanocomposite deposits on Ni foam. A Hitachi H-9500 HRTEM operated at 300 kV was used to record transmission electron microscopy images of the SSNP/Ni(OH)2 deposits. Deposits were removed from the foam substrate via sonication in isopropanol and then drop cast onto the TEM grid for imaging.Thermogravimetric analysis (TGA) curves were recorded using a TGA-51 (Shimadzu Scientific Instruments). Samples were heated in air flow (10 ml min−1) at a ramp rate of 10 °C min−1. Raman spectra were recorded using a Thermo Scientific™ DXR™ Raman imaging microscope. A 532 nm laser excitation source operated at 3 mW power was used to record the spectra (average of 5 exposures; 45 s each). XRD was performed using a Bruker D-8 Advance diffractometer (40 kV, 40 mA; Cu Kα radiation). A zero-background Si holder (MTI Corporation, Richmond, CA, USA) was used to avoid substrate interference.
Electrochemical testing
All electrochemical characterization was performed using a basic three-electrode setup (graphite rod counter electrode; Ag/AgCl (4 M KCl) reference electrode) and a Gamry Reference 3000 potentiostat (Gamry Instruments, Warminster, PA, USA). The 1 M KOH electrolyte (pH 13.6) was first saturated with oxygen by bubbling oxygen gas for 20 minutes. Voltages versus RHE are reported as follows: ERHE(V) = Evs.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag/AgCl + 0.20 + (0.059 × pH).
Ag/AgCl + 0.20 + (0.059 × pH).
95% iR correction was applied manually to all as-recorded cyclic voltammograms based on the uncompensated resistance (Ru) measured prior to testing using an inbuilt function of the potentiostat. (0.95 × i × Ru) V was subtracted from the recorded voltage values in the CV to apply the correction. The recorded uncompensated resistance value corresponded to the real intercept on the X axis of the Nyquist plots recorded subsequently. Electrochemical impedance spectra (EIS) data were recorded from 105–0.1 Hz under an AC perturbation of 10 mV at an applied voltage of ∼1.6 V vs. RHE. All deposits on Ni foam were stabilized by cycling 10 times at a scan rate of 5 mV s−1 in 1 M KOH. Scan rates used (5 mV s−1 or 2 mV s−1) are specified appropriately within the manuscript. For the accelerated stability test, the deposit was cycled 1000 times between 1.3–1.8 V vs. RHE at 100 mV s−1.
ECSA measurements were performed by first cycling fresh samples 5 times at 50 mV s−1 between 0–0.6 V vs. Ag/AgCl electrode in 1 M KOH. Thereafter, CV curves were recorded at scan rates of 5, 10, 25, 50, 75, 100, 150, 200, 300, and 400 mV s−1 between 0–0.1 V vs. Ag/AgCl reference electrode. The differences between anodic and cathodic currents at 0.05 V vs. Ag/AgCl electrode were then plotted versus scan rate. The slope of this line represents twice the double layer capacitance (Cdl).
Chronopotentiograms were recorded with applied current densities of 10–100 mA cm−2 incremented by 10 mA cm−2 every half an hour. For higher current density testing (both in 1 M and 10 M KOH), current was stepped from 100–500 mA cm−2 in 100 mA cm−2 increments every 30 min. No iR correction was performed on any chronopotentiometry curves.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the financial support of the National Science Foundation (NSF Award 1444473). The authors sincerely thank Dr Hyejin Moon for reviewing a draft of this work.References
- M. Wang, Z. Wang, X. Gong and Z. Guo, Renewable Sustainable Energy Rev., 2014, 29, 573–588 CrossRef CAS.
- M. Gao, W. Sheng, Z. Zhuang, Q. Fang, S. Gu, J. Jiang and Y. Yan, J. Am. Chem. Soc., 2014, 136, 7077–7084 CrossRef CAS PubMed.
- F. J. Pérez-Alonso, C. Adán, S. Rojas, M. A. Peña and J. L. G. Fierro, Int. J. Hydrogen Energy, 2014, 39, 5204–5212 CrossRef.
- Y. Yan, B. Y. Xia, B. Zhao and X. Wang, J. Mater. Chem. A, 2016, 4, 17587–17603 CAS.
- L.-A. Stern, L. Feng, F. Song and X. Hu, Energy Environ. Sci., 2015, 8, 2347–2351 CAS.
- J. Nai, H. Yin, T. You, L. Zheng, J. Zhang, P. Wang, Z. Jin, Y. Tian, J. Liu, Z. Tang and L. Guo, Adv. Energy Mater., 2015, 5, 1401880 CrossRef.
- J. Luo, J. H. Im, M. T. Mayer, M. Schreier, M. K. Nazeeruddin, N. G. Park, S. D. Tilley, H. J. Fan and M. Gratzel, Science, 2014, 345, 1593–1596 CrossRef CAS PubMed.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS PubMed.
- T. T. H. Hoang and A. A. Gewirth, ACS Catal., 2016, 6, 1159–1164 CrossRef CAS.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed.
- S. Klaus, Y. Cai, M. W. Louie, L. Trotochaud and A. T. Bell, J. Phys. Chem. C, 2015, 119, 7243–7254 CAS.
- A. T. Swesi, J. Masud and M. Nath, Energy Environ. Sci., 2016, 9, 1771–1782 CAS.
- F. Dionigi and P. Strasser, Adv. Energy Mater., 2016, 6, 1600621 CrossRef.
- I. Najdovski, P. R. Selvakannan and A. P. O’Mullane, ChemElectroChem, 2015, 2, 106–111 CrossRef CAS.
- A. Balram, H. Zhang and S. Santhanagopalan, ACS Appl. Mater. Interfaces, 2017, 9, 28355–28365 CAS.
- Y. Gorlin, C. J. Chung, J. D. Benck, D. Nordlund, L. Seitz, T. C. Weng, D. Sokaras, B. M. Clemens and T. F. Jaramillo, J. Am. Chem. Soc., 2014, 136, 4920–4926 CrossRef CAS PubMed.
- L. C. Seitz, T. J. Hersbach, D. Nordlund and T. F. Jaramillo, J. Phys. Chem. Lett., 2015, 6, 4178–4183 CrossRef CAS PubMed.
- B. S. Yeo and A. T. Bell, J. Phys. Chem. C, 2012, 116, 8394–8400 CAS.
- M. A. Sayeed, T. Herd and A. P. O’Mullane, J. Mater. Chem. A, 2016, 4, 991–999 CAS.
- B. S. Yeo and A. T. Bell, J. Am. Chem. Soc., 2011, 133, 5587–5593 CrossRef CAS PubMed.
- Y. Zhou and H. C. Zeng, J. Phys. Chem. C, 2016, 120, 29348–29357 CAS.
- N. Naseri, A. Esfandiar, M. Qorbani and A. Z. Moshfegh, ACS Sustainable Chem. Eng., 2016, 4, 3151–3159 CrossRef CAS.
- D. Gangasingh and J. B. Talbot, J. Electrochem. Soc., 1991, 138, 3605–3611 CrossRef CAS.
- P. Tsay and C.-C. Hu, J. Electrochem. Soc., 2002, 149, C492 CrossRef CAS.
- K. H. Kim, J. Y. Zheng, W. Shin and Y. S. Kang, RSC Adv., 2012, 2, 4759 RSC.
- F. M. Sapountzi, J. M. Gracia, C. J. Weststrate, H. O. A. Fredriksson and J. W. Niemantsverdriet, Prog. Energy Combust. Sci., 2017, 58, 1–35 CrossRef.
- F. Moureaux, P. Stevens, G. Toussaint and M. Chatenet, J. Power Sources, 2013, 229, 123–132 CrossRef CAS.
- F. Yu, F. Li and L. Sun, Int. J. Hydrogen Energy, 2016, 41, 5230–5233 CrossRef CAS.
- H. Schäfer, S. Sadaf, L. Walder, K. Kuepper, S. Dinklage, J. Wollschläger, L. Schneider, M. Steinhart, J. Hardege and D. Daum, Energy Environ. Sci., 2015, 8, 2685–2697 Search PubMed.
- H. Schäfer, D. M. Chevrier, K. Kuepper, P. Zhang, J. Wollschlaeger, D. Daum, M. Steinhart, C. Heß, U. Krupp, K. Müller-Buschbaum, J. Stangl and M. Schmidt, Energy Environ. Sci., 2016, 9, 2609–2622 Search PubMed.
- H. Schäfer, D. M. Chevrier, P. Zhang, J. Stangl, K. Müller-Buschbaum, J. D. Hardege, K. Kuepper, J. Wollschläger, U. Krupp, S. Dühnen, M. Steinhart, L. Walder, S. Sadaf and M. Schmidt, Adv. Funct. Mater., 2016, 26, 6402–6417 CrossRef.
- D. Tang, O. Mabayoje, Y. Lai, Y. Liu and C. B. Mullins, ChemistrySelect, 2017, 2, 2230–2234 CrossRef CAS.
- J. Ji, L. L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, X. Fan, F. Zhang and R. S. Ruoff, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
- X. Lu and C. Zhao, Nat. Commun., 2015, 6, 6616 CrossRef CAS PubMed.
- H. Wang, H. W. Lee, Y. Deng, Z. Lu, P. C. Hsu, Y. Liu, D. Lin and Y. Cui, Nat. Commun., 2015, 6, 7261 CrossRef CAS PubMed.
- M. Mishra, Y. Sakka, T. Uchikoshi and L. Besra, J. Ceram. Soc. Jpn., 2013, 121, 348–354 CrossRef CAS.
- M. Aghazadeh, M. Ghaemi, B. Sabour and S. Dalvand, J. Solid State Electrochem., 2014, 18, 1569–1584 CrossRef CAS.
- D. A. Corrigan, J. Electrochem. Soc., 1987, 134, 377 CrossRef CAS.
- M. W. Louie and A. T. Bell, J. Am. Chem. Soc., 2013, 135, 12329–12337 CrossRef CAS PubMed.
- R. L. Doyle and M. E. Lyons, Phys. Chem. Chem. Phys., 2013, 15, 5224–5237 RSC.
- Z. Zheng, W. Geng, Y. Wang, Y. Huang and T. Qi, Int. J. Hydrogen Energy, 2017, 42, 119–124 CrossRef CAS.
- A. R. Boccaccini, S. Keim, R. Ma, Y. Li and I. Zhitomirsky, J. R. Soc., Interface, 2010, 7(Suppl 5), S581–S613 CrossRef CAS PubMed.
- S. Santhanagopalan, A. Balram and D. D. Meng, ACS Nano, 2013, 7, 2114–2125 CrossRef CAS PubMed.
- D. H. Ha, M. A. Islam and R. D. Robinson, Nano Lett., 2012, 12, 5122–5130 CrossRef CAS PubMed.
- C. Wang, R. B. Moghaddam, M. J. Brett and S. H. Bergens, ACS Sustainable Chem. Eng., 2017, 5, 1106–1112 CrossRef CAS.
- D. Su, M. Ford and G. Wang, Sci. Rep., 2012, 2, 924 CrossRef PubMed.
- J. M. Gonçalves, R. R. Guimarães, C. V. Nunes, A. Duarte, B. B. N. S. Brandão, H. E. Toma and K. Araki, RSC Adv., 2016, 6, 102504–102512 RSC.
- M. Marciuš, M. Ristić, M. Ivanda and S. Musić, J. Alloys Compd., 2012, 541, 238–243 CrossRef.
- T. L. S. L. Wijesinghe and D. J. Blackwood, Appl. Surf. Sci., 2006, 253, 1006–1009 CrossRef CAS.
- C. C. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- K. Fan, H. Chen, Y. Ji, H. Huang, P. M. Claesson, Q. Daniel, B. Philippe, H. Rensmo, F. Li, Y. Luo and L. Sun, Nat. Commun., 2016, 7, 11981 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TGA curve of Ni(OH)2, schematic sketch of the electrophoretic co-deposition process, XRD curves of SSNP and the SSNP/Ni(OH)2 nanocomposite. See DOI: 10.1039/c7qm00299h |
| This journal is © the Partner Organisations 2017 |