New AIEgens with delayed fluorescence for fluorescence imaging and fluorescence lifetime imaging of living cells†
Shifeng
Gan
a,
Jian
Zhou
 *b,
Trevor A.
Smith
*b,
Trevor A.
Smith
 c,
Huifang
Su
d,
Wenwen
Luo
a,
Yuning
Hong
c,
Huifang
Su
d,
Wenwen
Luo
a,
Yuning
Hong
 ce,
Zujin
Zhao
ce,
Zujin
Zhao
 *a and
Ben Zhong
Tang
*ad
*a and
Ben Zhong
Tang
*ad
aState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou, 510640, China. E-mail: mszjzhao@scut.edu.cn
bCollege of Material, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036, China. E-mail: zhoujian@hznu.edu.cn
cSchool of Chemistry, The University of Melbourne, Victoria, 3010, Australia
dDepartment of Chemistry, Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk
eDepartment of Chemistry and Physics, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, VIC 3083, Australia
First published on 12th September 2017
Abstract
A series of luminogens with both aggregation-induced emission and delayed fluorescence features are synthesized and characterized. Biocompatible fluorescent nanoparticles are fabricated by encapsulating them within a bovine serum albumin matrix, and perform well in fluorescence imaging and fluorescence lifetime imaging of living cells.
Fluorescence microscopy is a powerful technique applied in complex biological environments to visualize the organs, tissues, cells, etc., owing to its high sensitivity and good spatial resolution.1 However, the autofluorescence of the tested samples and the light scattering in the instruments always reduce the sensitivity or give rise to false signals of this detection method.2 To overcome the disadvantages, fluorescence lifetime imaging microscopy is now emerging as a promising supplementor alternative to conventional fluorescence microscopy. By introducing a suitable delay between the pulsed excitation light and the measurement of the fluorescence signal of the dyes, the short-lived background fluorescence can be completely eliminated, and thus the signal-to-noise ratios are greatly improved.2 So far, most fluorescent dyes utilized in time-resolved fluorescence microscopy are organometallic complexes containing heavy metals, such as europium, terbium, and iridium.3 Usually, these complexes with long excited state lifetimes (microseconds or milliseconds) are expensive, unstable3d and even toxic, which severely limits their practical applications in biosystems.1a Therefore, metal-free dyes with long fluorescence lifetimes are highly desired.
Recently, pure organic molecules with delayed fluorescence have drawn considerable attention because of their high exciton utilization efficiency in organic light-emitting diodes.4 These molecules generally have small energy splitting (ΔEST) between the lowest triplet excited state (T1) and the lowest singlet excited state (S1),5 which enables T1 to be used for radiation via a reverse intersystem crossing (RISC) process from T1 to S1 under thermal activation. Their highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) are separately distributed on different units of the molecules, which can decrease ΔEST and promote RISC. Designing an electron donor–acceptor system with a highly twisted conformation is a possible way to realize effective HOMO–LUMO separation and achieve delayed fluorescence. The delayed fluorescence molecules have long fluorescence lifetimes in the order of microseconds or even milliseconds,6 which are much longer than the lifetimes of autofluorescence or background fluorescence in biosystems. However, the application of delayed fluorescence molecules for fluorescence lifetime bioimaging is relatively rare.2b,7 This is because they commonly suffer from severe concentration or aggregation-caused quenching (ACQ) of their fluorescence, owing to the strong intermolecular interactions.8,9 In addition, their fluorescence lifetimes can be shortened to nanoseconds in aggregates, which is not favored for time-resolved fluorescence imaging.9a
To solve the ACQ problem, an effective method is to design luminogens with aggregation-induced emission (AIEgens).10 They could be almost nonfluorescent in solution but become highly emissive on aggregate formation with elongated fluorescence litetimes.9,10 In view of this, integrating AIE and delayed fluorescence characteristics within a molecule should be a feasible way to create new luminescent materials for bioimaging. Herein, we prepared a series of luminogens with AIE and delayed fluorescence features. They show great potential in both fluorescence imaging and fluorescence lifetime imaging based on confocal laser scanning microscopy (CLSM) and the time correlated single photon counting (TCSPC) technique, respectively.
The molecular structures of these new luminogens are shown in Fig. 1. They have a D–A or D–A–D structure, in which the electron-deficient benzophenone (BP) is chosen as the electron acceptor (A), and the electron-rich nonplanar phenoxazine (PXZ) and phenothiazine (PTZ) are adopted as electron donors (D). The details of the synthetic routes (Scheme S1, ESI†), procedures and characterization data are given in the ESI.† Single crystals of BP-2PXZ and BP-PXZ are grown from dichloromethane/methanol and analyzed by X-ray single-crystal diffraction. As illustrated in Fig. 1, the crystal structures unveil that BP-2PXZ and BP-PXZ adopt a highly twisted conformation, which can efficiently inhibit close π–π stacking (Fig. S1, ESI†), and thus reduce nonradiative decay in the aggregated state. The large torsion angles between the D and A groups (68.2–74.3°) can lower the overlap of the HOMOs and LUMOs, and facilitate the occurrence of delayed fluorescence.4b,8a,9c
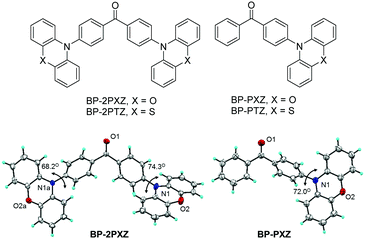 | ||
| Fig. 1 Molecular structures of the luminogens and crystal structures of BP-2PXZ (CCDC 1455109) and BP-PXZ (CCDC 1455110).† | ||
Calculations are carried out to predicate the ΔESTs of these luminogens based on time-dependent density functional theory (TD-DFT) at the M062X/6-31G(d,p) level. The geometrical parameters of the optimized ground state for BP-PXZ are in good agreement with those derived from the crystal structure: the dihedral angle between the donor BP and acceptor PXZ is calculated to be 70.3° (76.6° and 74.4° for BP-2PXZ), which matches well with the measured value (72.0° for BP-PXZ; 74.3° and 68.2° for BP-2PXZ) from its crystal structure. The HOMOs and LUMOs of the luminogens are clearly separated because of the large dihedral angles between the D and A groups (Fig. 2). The HOMOs are mainly located on PXZ or PTZ units, while the LUMOs are delocalized on the BP moiety. Principally, the obvious separations of the HOMOs and LUMOs can lead to a small ΔEST and thus cause delayed fluorescence.11 The ΔESTs of BP-2PXZ, BP-PXZ, BP-2PTZ, and BP-PTZ are computed to be 0.24, 0.33, 0.47 and 0.46 eV, respectively, which are small enough for the occurrence of upconversion from T1 to S1.8,9
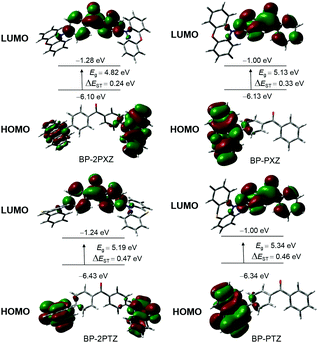 | ||
| Fig. 2 Frontier orbital distributions, energy levels, and energy splitting (ΔEST) between singlet and triplet states for the luminogens, calculated by DFT in the ground state. | ||
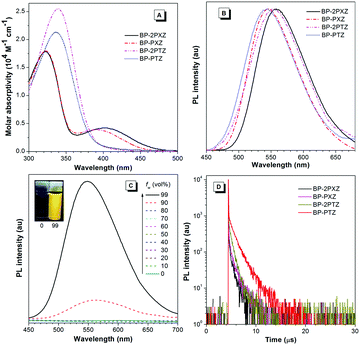
BP-2PTZ and BP-PTZ show main absorption maxima at 339 and 336 nm, respectively, associated with the π–π* transition of the luminogens (Fig. 3A). However, in addition to strong absorption bands at 322 nm, BP-2PXZ and BP-PXZ exhibit long-wavelength absorption peaks at 398 and 390 nm, respectively, which should result from the intramolecular charge transfer (ICT) from PXZ to BP. The absence of an ICT band in the absorption spectra of BP-2PTZ and BP-PTZ is probably owing to the weaker electron-donating ability of PTZ than PXZ.9c The emission maximum of BP-2PXZ is 558 nm, which is red-shifted by 7 nm relative to that of BP-2PTZ (551 nm). A small red-shift is also observed for BP-PXZ (546 nm), compared to that of BP-PTZ (544 nm). These results once again indicate that PXZ is a better electron-donating moiety than PTZ. The fluorescence quantum yields (ΦF) of BP-2PXZ, BP-PXZ, BP-2PTZ and BP-PTZ in dilute THF solutions are 0.9, 0.4, 0.8 and 0.9%, respectively (Table 1), revealing that they emit weakly in the solution state. The ΦFs of BP-2PXZ, BP-PXZ, BP-2PTZ and BP-PTZ in films are increased to 20.3, 13.6, 24.1 and 20.8%, respectively, unveiling that they are AIE-active. In order to further confirm the AIE characteristics, their PL changes in THF–water mixtures are measured. Fig. 3C illustrates the PL spectra of BP-PTZ in THF–water mixtures as an example. It can be seen that the emission intensity of BP-PTZ enhances greatly when a large amount of water is added to its pure THF solution. Other luminogens show similar emission enhancements in aqueous media (Fig. S2, ESI†). These luminogens must have formed aggregates in the mixtures with high water fractions, because they are insoluble in water. The intramolecular motions that are active at a low water fraction are restricted at a high water fraction. The nonradiative excited state decay is blocked in the aggregated state, which enables the molecules to emit strongly.10 Thus, these results verify the AIE nature of the luminogens.
| Compound | λ abs (nm) | λ em (nm) | Φ F (%) | τ (μs) | τ p (ns) | τ d (μs) | R (%) | ΔESTf (eV) | |
|---|---|---|---|---|---|---|---|---|---|
| THF | Film | ||||||||
| a In THF solution (10−5 M). b In solid film. c Fluorescence quantum yield determined by a calibrated integrating sphere. d Total PL lifetimes (τ) and PL lifetimes of prompt (τp) and delayed (τd) decay components for the compounds measured in a film under ambient conditions. e The ratio of delayed components. f Calculated by TD-DFT at M062X/6-31G(d,p). | |||||||||
| BP-2PXZ | 398, 322 | 558 | 0.9 | 20.3 | 0.16 | 24 | 0.73 | 18.6 | 0.24 |
| BP-PXZ | 390, 322 | 546 | 0.4 | 13.6 | 0.25 | 27 | 0.96 | 24.1 | 0.33 |
| BP-2PTZ | 339 | 551 | 0.8 | 24.1 | 0.31 | 25 | 0.66 | 44.9 | 0.47 |
| BP-PTZ | 336 | 544 | 0.9 | 20.8 | 1.06 | 36 | 1.36 | 77.1 | 0.46 |
The fluorescence decay curves of the luminogens in solid films are displayed in Fig. 3D. They can be well fitted in a biexponential manner, indicative of the presence of prompt and delayed fluorescence components. BP-2PXZ, BP-PXZ, BP-2PTZ, and BP-PTZ have delayed lifetimes of 0.73, 0.96, 0.66 and 1.36 μs, respectively, which fall in with the properties of typical delayed fluorescence molecules.4–8 The ratios of the delayed component of BP-2PXZ, BP-PXZ, BP-2PTZ and BP-PTZ are calculated to be 18.6, 24.1, 44.9 and 77.1%, respectively. The total lifetimes of BP-2PXZ, BP-PXZ, BP-2PTZ, and BP-PTZ (0.16–1.06 μs) are much longer than those of common organic fluorescent dyes as well as biosystem background,3e revealing their potential application in fluorescence lifetime imaging.
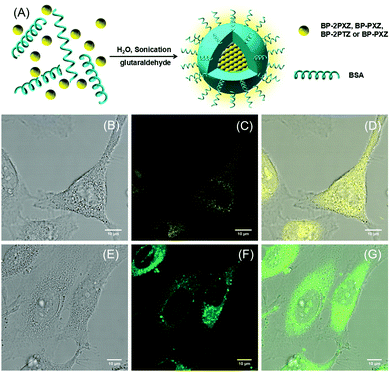
To improve the water solubility, we encapsulate these luminogens within bovine serum albumin (BSA) to generate fluorescent nanoparticles (NPs) (Fig. 4A). The sizes of the NPs are in the range of 109–150 nm, measured in aqueous solutions by dynamic light scattering (Fig. S3, ESI†). In order to evaluate the cytotoxicity of these NPs, MTT assays in the HeLa cell line are carried out.12 These NPs show low cytotoxicity towards the cells at different concentrations (Fig. S4, ESI†). For example, the cell viability stained with BP-2PTZ-BSA NPs at a high concentration of 20 μM can reach 95% after 24 h incubation. The confocal fluorescence images of BP-2PTZ-BSA NPs and BP-PXZ-BSA NPs are obtained by CLSM and shown in Fig. 4, and those of BP-2PXZ-BSA NPs and BP-PTZ-BSA NPs are given in Fig. S5 (ESI†). The results show that these NPs stain living HeLa cancer cells easily, and exhibit strong green or yellow fluorescence.12,13 The BSA functionalized NPs can readily enter the cells and light up the intracellular compartments. Interestingly, these NPs can even pass through the nucleur membrane and accumulate at certain areas in the nucleus.
To get more information on the local environment in cells, fluorescence lifetime imaging is further carried out based on the TCSPC technique using a homemade time-resolved spectroscopic imaging system. The fluorescence lifetime images of HeLa cancer cells stained with BP-2PTZ-BSA NPs and BP-PXZ-BSA NPs are shown in Fig. 5 and those for BP-2PXZ-BSA NPs and BP-PTZ-BSA NPs are shown in Fig. S6 (ESI†). The features of the HeLa cells can be observed clearly, which is consistent with the observation from fluorescence imaging, while the signals from background are depressed greatly. From Fig. 5D, we can see that the lifetime of BP-PXZ NPs ranges from about 700–2000 ps in the cellular environment. In particular, two distinctive lifetime signals around 1000 and 2000 ps can be observed well, indicating the different intracellular viscosity in different areas in the cellular environment. This is because the lifetimes of the luminogens are sensitive to the intracellular viscosity around them.13b–d The high viscosity will retard the intramolecular motion and result in longer lifetime of the molecule. In particular, the delayed fluorescence will become more prominent, with longer lifetimes and higher ratios.14 When more luminogens are aggregated in the local environment with higher intracellular viscosity, longer lifetime signals will be detected, enabling them to map the viscosity difference of the cellular environment. However, due to the limitation of the repetition rate of the laser, we are not able to record lifetime signals in the μs range with our current set-up.
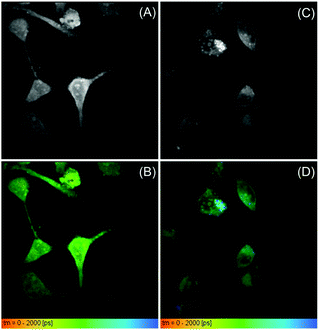 | ||
| Fig. 5 The fluorescence lifetime images of (A and B) HeLa cells stained with BP-2PTZ-BSA NPs (10 μM of the BP-2PTZ) and (C and D) BP-PXZ-BSA NPs (10 μM of the BP-PXZ) at 37 °C for 0.5 h. | ||
In summary, a series of luminogens with a D–A or D–A–D structure are synthesized and characterized. These luminogens show intriguing AIE and delayed fluorescence characteristics, enabling them to emit strongly in the aggregated state with long fluorescence lifetimes. They are encapsulated within a BSA matrix to generate water-soluble NPs for bioimaging applications. The obtained NPs show low cytotoxicity and can stain living cells efficiently, exhibiting strong green or yellow fluorescence in cells. They also show good performance in fluorescence lifetime imaging, providing a clear map of intracellular viscosity according to varied lifetimes. Although the luminogens with AIE and delayed fluorescence have been rarely explored for fluorescence lifetime bioimaging, they should have great potential as a time-resolved fluorescent sensing platform to overcome the drawbacks of luminescent complexes containing heavy metals.
We acknowledge the financial support from the National Natural Science Foundation of China (21673082 and 21404029), the Guangdong Natural Science Funds for Distinguished Young Scholar (2014A030306035), the Innovation and Technology Commission of Hong Kong (ITC-CNERC14SC01), and the Fundamental Research Funds for the Central Universities (2017ZD001 and 2015ZY013).
Conflicts of interest
There are no conflicts to declare.References
- (a) K. Hanaoka, K. Kikuchi, S. Kobayashi and T. Nagano, J. Am. Chem. Soc., 2007, 129, 13502 CrossRef CAS PubMed; (b) P. Nalbant, L. Hodgson, V. Kraynov, A. Toutchkine and K. M. Hahn, Science, 2004, 305, 1615 CrossRef CAS PubMed.
- (a) B. Song, G. Wang and J. Yuan, Chem. Commun., 2005, 3553 RSC; (b) X. Xiong, F. Song, J. Wang, Y. Zhang, Y. Xue, L. Sun, N. Jiang, P. Gao, L. Tian and X. Peng, J. Am. Chem. Soc., 2014, 136, 9590 Search PubMed.
- (a) Q. Zhao, F. Li and C. Huang, Chem. Soc. Rev., 2010, 39, 3007 RSC; (b) C. Li, Y. Liu, Y. Wu, Y. Sun and F. Li, Biomaterials, 2013, 34, 1223 CrossRef CAS PubMed; (c) G. Zhang, J. Chem, S. J. Payne, S. E. Kooi, J. N. Demas and C. L. Fraser, J. Am. Chem. Soc., 2007, 129, 8942 CrossRef CAS PubMed; (d) S. Y. Lee, T. Yasuda, Y. S. Yang, Q. Zhang and C. Adachi, Angew. Chem., Int. Ed., 2014, 53, 6402 CrossRef CAS PubMed; (e) Y. Ma, S. Liu, H. Yang, Y. Wu, H. Sun, J. Wang, Q. Zhao, F. Li and W. Huang, J. Mater. Chem. B, 2013, 1, 319 RSC.
- (a) H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234 CrossRef CAS PubMed; (b) H. Tanaka, K. Shizu, H. Miyazaki and C. Adachi, Chem. Commun., 2012, 48, 11392 RSC.
- (a) H. Nakanotani, T. Higuchi, T. Furukawa, K. Masui, K. Morimoto, M. Numata, H. Tanaka, Y. Sagara, T. Yasuda and C. Adachi, Nat. Commun., 2014, 5, 4016 CAS; (b) Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Adv. Mater., 2014, 29, 22 Search PubMed.
- (a) P. Rajamalli, N. Senthilkumar, P. Gandeepan, P. Y. Huang, M. J. Huang, C. Z. Ren Wu, C. Y. Yang, M. J. Chiu, L. K. Chu, H. W. Lin and C. H. Cheng, J. Am. Chem. Soc., 2016, 138, 628 CrossRef CAS PubMed; (b) T.-A. Lin, T. Chatterjee, W.-L. Tsai, W.-K. Lee, M.-J. Wu, M. Jiao, K.-C. Pan, C.-L. Yi, C.-L. Chung and K.-T. Wong, Adv. Mater., 2016, 28, 6976 CrossRef CAS PubMed.
- (a) T. Li, D. Yang, L. Zhai, S. Wang, B. Zhao, N. Fu, L. Wang, Y. Tao and W. Huang, Adv. Sci., 2017, 4, 1600166 CrossRef PubMed; (b) Z. Yang, Z. Mao, Z. Xie, Y. Zhang, S. Liu, J. Zhao, J. Xu, Z. Chi and M. P. Aldred, Chem. Soc. Rev., 2017, 46, 915 RSC.
- (a) J. Guo, X.-L. Xiang, H. Nie, W. Luo, S. Gan, S. Hu, R. Hu, A. Qin, Z. Zhao, S.-J. Su and B. Z. Tang, Adv. Funct. Mater., 2017, 1606458 CrossRef; (b) R. Furue, T. Nishimoto, I. S. Park, J. Lee and T. Yasuda, Angew. Chem., Int. Ed., 2016, 55, 7171 CrossRef CAS PubMed; (c) I. H. Lee, W. Song and J. Y. Lee, Org. Electron., 2016, 29, 22 CrossRef CAS; (d) S. Wang, X. Yan, Z. Cheng, H. Zhang, Y. Liu and Y. Wang, Angew. Chem., Int. Ed., 2015, 54, 13068 CrossRef CAS PubMed; (e) N. Aizawa, C.-J. Tsou, I. S. Park and T. Yasuda, Polym. J., 2017, 49, 197 CrossRef CAS.
- (a) S. Xu, T. Liu, Y. Mu, Y.-F. Wang, Z. Chi, C. C. Lo, S. Liu, Y. Zhang, A. Lien and J. Xu, Angew. Chem., Int. Ed., 2015, 54, 874 CrossRef CAS PubMed; (b) Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki and C. Adachi, J. Am. Chem. Soc., 2012, 134, 14706 CrossRef CAS PubMed; (c) S. Gan, W. Luo, B. He, L. Chen, H. Nie, R. Hu, A. Qin, Z. Zhao and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 3705 RSC; (d) J. Guo, X.-L. Li, H. Nie, W. Luo, R. Hu, A. Qin, Z. Zhao, S.-J. Su and B. Z. Tang, Chem. Mater., 2017, 29, 3623 CrossRef CAS; (e) J. Hu, X. Zhang, D. Zhang, X. Cao, T. Jang and Y. Tao, Dyes Pigm., 2017, 137, 480 CrossRef CAS.
- (a) Z. Zhao, B. He and B. Z. Tang, Chem. Sci., 2015, 6, 5347 RSC; (b) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed; (c) Z. Zhao, S. Chen, C. Y. K. Chan, J. W. Y. Lam, C. K. W. Jim, P. Lu, Z. Chang, H. S. Kwok, H. Qiu and B. Z. Tang, Chem. – Asian J., 2012, 7, 484 CrossRef CAS PubMed; (d) L. Ma, X. Feng, S. Wang and B. Wang, Mater. Chem. Front., 2017 10.1039/C7QM00254H; (e) C. Wang and Z. Li, Mater. Chem. Front., 2017 10.1039/C7QM00201G.
- N. J. Turro, V. Ramamurthy and J. C. Scaiano, Modern Molecular Photochemistry of Organic Molecules, University Science Books, Saussalito, 2010 Search PubMed.
- (a) X. Zhang, K. Wang, M. Liu, X. Zhang, L. Tao, Y. Chen and Y. Mei, Nanoscale, 2015, 7, 11486 RSC; (b) M. Gao, J. Chen, G. Lin, S. Li, L. Wang, A. Qin, Z. Zhao, L. Ren, Y. Wang and B. Z. Tang, Nanoscale, 2016, 8, 17878 CAS.
- (a) D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441 CrossRef CAS PubMed; (b) S. Chen, Y. Hong, Y. Zeng, Q. Sun, Y. Liu, E. Zhao, G. Bai, J. Qu, J. Hao and B. Z. Tang, Chem. – Eur. J., 2015, 21, 4315 CrossRef CAS PubMed; (c) H. Soleimaninejad, M. Z. Chen, X. Lou, T. A. Smith and Y. Hong, Chem. Commun., 2017, 53, 2874 RSC; (d) W. Becker, J. Microsc., 2012, 247, 119 CrossRef CAS PubMed.
- J. Huang, H. Nie, J. Zeng, Z. Zhuang, S. Gan, Y. Cai, J. Guo, S.-J. Su, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2017 DOI:10.1002/anie.201706752.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, crystal data, PL spectra, particle size distribution, confocal fluorescence images, time-resolved fluorescence images and cytotoxicity. CCDC 1455109 and 1455110. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7qm00286f |
| This journal is © the Partner Organisations 2017 |