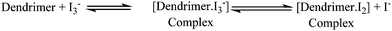Synthesis and application of stilbenoid phenothiazine dendrimers as additives for dye-sensitized solar cells†
Mahalingam
Ravivarma
a,
Chinnadurai
Satheeshkumar
a,
Shanmugam
Ganesan
b and
Perumal
Rajakumar
 *a
*a
aDepartment of Organic Chemistry, University of Madras, Chennai-600 025, Tamil Nadu, India. E-mail: perumalrajakumar@gmail.com
bDepartment of Chemistry, SRM University, Chennai-603 203, Tamil Nadu, India
First published on 25th July 2017
Abstract
Stilbenoid dendrimers with phenothiazine as a surface group were synthesized in good yields by the Heck and Horner–Wadsworth–Emmons coupling reaction and characterized by spectral and analytical data. Various core units such as trimethylbenzene, pyridine, and triphenylamine with phenothiazine as the surface unit were employed for the synthesis of dendrimers. The photophysical properties of the dendrimers reveal that as the generation increases the light absorption ability also increases. Furthermore, as the generation increases the absorption maxima show a hypsochromic shift for the n–π* transition and a bathochromic shift for the π–π* transition. However, the fluorescence emission in general increases as the dendrimer generation increases. When the synthesized stilbenoid dendrimers are utilized as an additive in the redox couple (I−/I3−) of a Dye Sensitized Solar Cell (DSSC), the solar energy conversion efficiency increases with an increase in generation and reaches a maximum of 7.3% with the higher generation dendrimer 10.
Introduction
Dendrimers have evolved as a powerful class of material with increasing applications in various fields.1–3 The π-conjugated dendritic systems with specified dendrons or core units can exhibit intrinsic physical properties,4 which bring new applications in electrical,5 and optical6 as well as biomedical7 fields. Such highly conjugated dendritic structures find their applications as thin film materials with desired properties.8 Hence, such conjugated dendritic structures are the most promising among the π-conjugated materials and have been used as the active material in electronic and photoelectronic devices.9 Therefore, developing such new conjugated dendrimers can further allow us to gain a deep insight into the photophysical properties of various novel materials. Stilbenoid chromophores exhibit many interesting photophysical phenomena and are hence suitable for various applications in materials science.10 Incorporation of stilbenoid units into dendrimers generates extended conjugation in the macrostructures11 which is an important structural feature for opto-electronic applications such as light-emitting diodes (LEDs),12 electron transporting materials,13 optical images,14 storage,15 light harvesting16 and switching techniques.17 Hence, the design of conjugated dendrimers using stilbenoid chromophores has received much attention during recent times. Moreover, conjugated dendrimers exhibit several advantages such as monodispersity, stereospecificity etc. The development of π-conjugated dendritic molecules with desired surfaces and core units is used for target applications, and detailed analysis of the structure–activity relationship of dendrimers is still challenging.18The non-planar and bowl-shaped structure19 of phenothiazine (PTZ) prevents molecular aggregation and reduces intermolecular interactions, and furthermore its good electron-donating properties with thermal stability20 and favorable electrochemical properties21 make it a good surface unit in dendrimers. PTZ is widely used for manufacturing organic light-emitting diodes (OLEDs)22 and semiconducting23 materials. Phenothiazine-labeled dendrimers are well known as an efficient electroluminescence material in display devices.24 In addition, the phenothiazine ring system has been chemically modified to exhibit various bio-activities such as anti-malarial,25 anti-cancer,26 anti-helminthic,27 anti-histamine28 and anti-psychotic effects.29
Increasing energy consumption is causing a more serious crisis of environmental pollution and driving us to search for new and renewable energy sources. Solar energy is widely recognized as the most promising source to solve environmental pollution.30 Dye-sensitized solar cells (DSSCs) are potential tools for solar energy conversion due to their low cost processing and impressive photovoltaic performance. Several factors influence the power conversion efficiency in DSSCs. In dye-sensitized solar cells, the redox couple plays a major role in determining the photo-voltaic properties and stability. Generally, a liquid medim such as acetonitrile is employed as an electrolyte solvent to dissolve the I−/I3− redox couple.31 However, sublimation of iodine in the redox couple is a major drawback and hence many research groups have developed a new electrolyte base with an organic nitrogenous compound as an additive to achieve the long-term stability of DSSCs.32 During the last two decades, most of the research groups focused on the modification of the electrolyte in dye-sensitized solar cells by the addition of organic and inorganic compounds as a plasticizer and the addition of organic compounds enhances the efficiency of dye-sensitized solar cells.33 Nitrogen-containing organic molecules are commonly used as additives in electrolytic solutions to improve the performance of DSSCs.34,35 Recently, various 1,2,3-triazole based dendrimers have been used as additives in the redox couple, and an improved power conversion efficiency has been reported by our research group.36,37
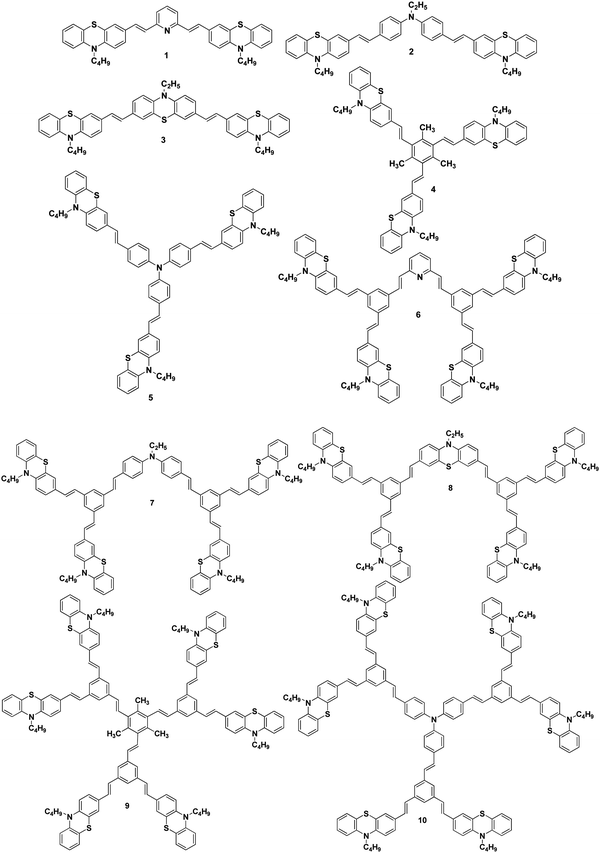
We report herein the cost-effective synthesis of a new type of phenothiazine-based conjugated stilbenoid dendrimer, 1 to 10 (Fig. 1), by the Heck and Horner–Wadsworth–Emmons coupling technique using a convergent synthetic methodology along with their photophysical properties. Furthermore, when the synthesized phenothiazine conjugated dendrimers were utilized as an additive in DSSC electrolyte systems, better power conversion efficiency and stability have been observed.
Result and discussion
The Wittig reaction of 10-n-butyl-10H-phenothiazine-3-carbaldehyde 11 with methyltriphenylphosphonium bromide in the presence of potassium tert-butoxide afforded 10-n-butyl-3-vinyl-10H-phenothiazine 12 in 65% yield (Scheme 1). The 1H NMR spectrum of 10-n-butyl-3-vinyl-10H-phenothiazine 12 displayed two doublets at δ 5.02 (J = 10.8 Hz) and δ 5.49 (J = 17.7 Hz) for cis and trans protons, respectively, and a doublet of a doublet at δ 6.47 (J = 17.4 Hz, J = 10.8 Hz) which confirmed the presence of the vinyl group in compound 12. Heck coupling between 1.0 equiv. of various symmetrical dibromo and tribromo compounds 13–17 and 2.1/3.1 equiv. of vinyl functionalized phenothiazine 12 using Pd(OAc)2 in the presence of K2CO3 and tetrabutylammonium bromide resulted in the formation of the stilbenoid phenothiazine dendrimers 1–5, respectively, as shown in Scheme 1.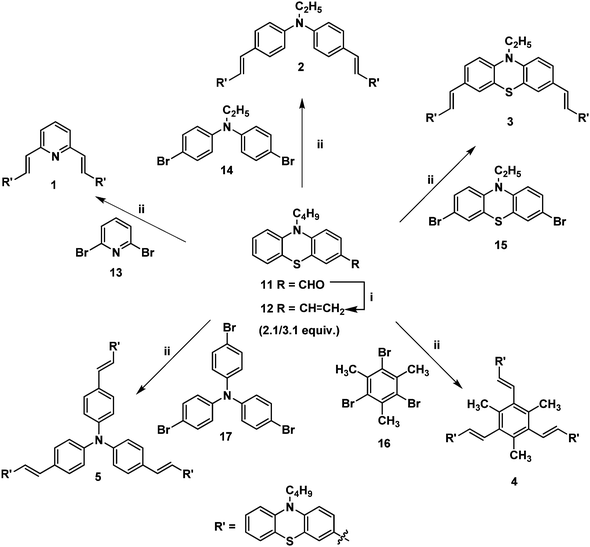 | ||
| Scheme 1 (i) Methyltriphenylphosphonium bromide (1 equiv.), KOtBu, THF, 12 h, 12 (65%); (ii) Pd(OAc)2, TBAB, K2CO3, DMF, 90 °C, 12 h, 1 (68%), 2 (64%), 3 (62%), 4 (68%) and 5 (71%). | ||
The 1H NMR spectrum of the stilbenoid phenothiazine dendrimer 1 displayed a triplet at δ 3.77 (J = 6.6 Hz) for N-methylene protons and one doublet at δ 6.95 (J = 16.2 Hz) for the olefinic protons in addition to the other aliphatic and aromatic proton signals. The 13C NMR spectrum of the stilbenoid phenothiazine dendrimer 1 showed a signal for an N-methylene carbon at δ 46.3 in addition to the other aliphatic and aromatic carbon signals. The mass spectrum (ESI-MS) of 1 showed the molecular ion peak at m/z 638.3 [M + H]+. The structure of 1 was further confirmed from elemental analysis. Similarly, the structure of dendrimers 2–5 was also confirmed from spectral and analytical data.
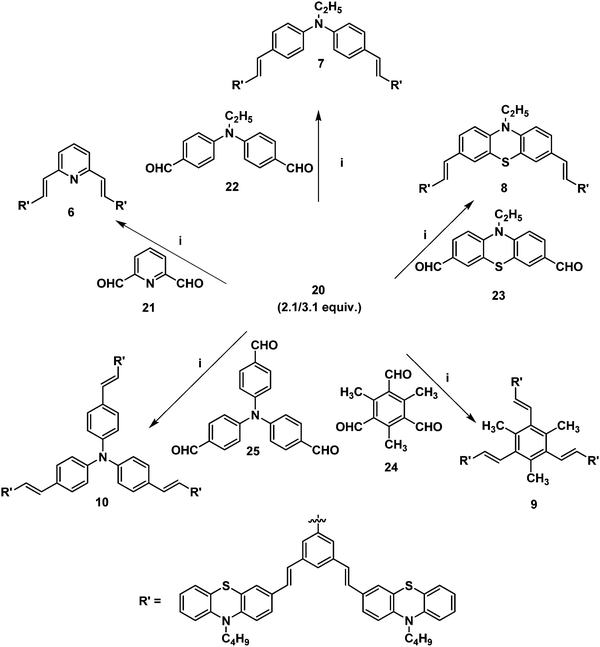
Furthermore, it is of interest to synthesize dendritic structures with extended conjugation. The first-generation stilbenoid conjugated dendron 20 was prepared as shown in Scheme 2. The Michaelis–Arbuzov reaction of 1,3-dibromo-5-(bromomethyl)benzene 18 with 3.0 equiv. of triethylphosphite at 160 °C for 3 h afforded the phosphonate ester 19 in good yields. Furthermore, Heck coupling between the phosphonate ester 19 and 2.1 equiv. of 10-n-butyl-3-vinyl-10H-phenothiazine 12 gave the G1 dendron 20 in 64% yield (Scheme 2).
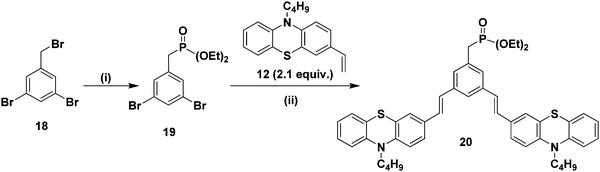 | ||
| Scheme 2 (i) Triethylphosphite (3.0 equiv.), 160 °C, reflux, 3 h, 19 (85%). (ii) Pd(OAc)2, TBAB, K2CO3, DMF, 90 °C, 24 h, 20 (64%). | ||
2.1/3.1 equiv. of the first-generation conjugated dendron 20 underwent Horner–Wadsworth–Emmons coupling with various di- and tri-aldehydes 21–25 to afford the first-generation counjugated dendrimers 6–10 as shown in Scheme 3. The 1H NMR spectrum of the stilbenoid dendrimer 10 displayed the N-methylene protons as a triplet at δ 3.79 (J = 6.6 Hz) in addition to the signals for the other aliphatic and aromatic protons. In the 13C NMR spectrum, dendrimer 10 showed the N-methylene carbon at δ 47.2 in addition to the aliphatic and aromatic carbon signals. The mass spectrum (MALDI-TOF) of 10 showed the molecular ion peak at m/z 2227.5. The structure of dendrimer 10 was also confirmed from analytical data. Similarly, the structure of the conjugated dendrimers 6–9 was also confirmed from spectral and analytical data.
Photophysical properties
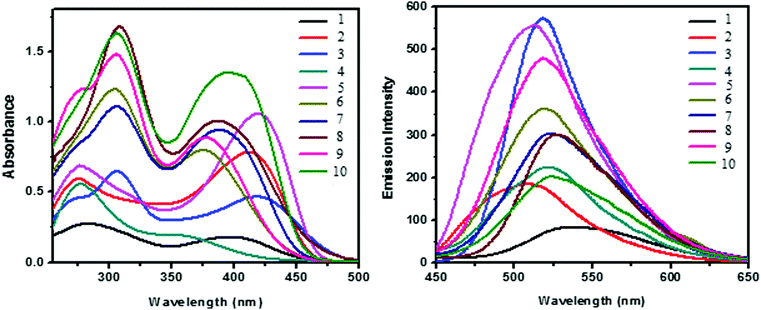
The UV-vis absorption spectra of dendrimers 1–10 were recorded in DMSO at a concentration of 1 × 10−5 M. The photophysical data are given in Table 1 and the UV absorption spectrum is shown in Fig. 2a. Two major absorption bands are observed around 274–308 and 375–421 nm due to the π–π* and n–π* transition of the stilbene and phenothiazine units, respectively. The molar extinction coefficient of both the absorption bands of the dendrimers has been found to increase significantly as the generation of the dendrimer increases (Fig. 2a), which may be due to the valence effect. The absorption maxima (λmax) of the n–π* transition for all the dendrimers decrease as the generation of the dendrimer increases whereas the absorption maxima (λmax) of the π–π* transition increase as the generation of the dendrimer increases. Hence, for the n–π* transition a hypsochromic shift is observed whereas for the π–π* transition a bathochromic shift is observed. Fig. 2b shows the fluorescence spectra of dendrimers 1–10 in DMSO and the fluorescence parameters of all the dendrimers are shown in Table 1. All the dendrimers exhibit an intense fluorescence emission in the region of 508–537 nm upon excitation at 400 nm. A red shift of around 10 nm was observed for the zeroth generation dendrimers 1–5 when compared with the corresponding first-generation dendrimers 6–10 due to an increase in the number of phenothiazine units which is otherwise known as the multivalency effect in dendrimer chemistry.| Stilbenoid dendrimers | λ abs max (nm) | ε (104 M−1 cm−1) | λ em max (nm) |
|---|---|---|---|
| 1 | 401, 282 | 1.8, 2.7 | 537 |
| 2 | 414, 274 | 7.8, 5.9 | 508 |
| 3 | 420, 305 | 4.6, 6.5 | 518 |
| 4 | 365, 276 | 1.7, 5.5 | 512 |
| 5 | 421, 274 | 10.5, 6.9 | 512 |
| 6 | 375, 304 | 7.9, 12.2 | 518 |
| 7 | 390, 305 | 9.3, 11.0 | 522 |
| 8 | 389, 308 | 10.0, 16.7 | 527 |
| 9 | 377, 305 | 8.8, 14.6 | 519 |
| 10 | 398, 305 | 13.4, 16.2 | 524 |
DSSC studies
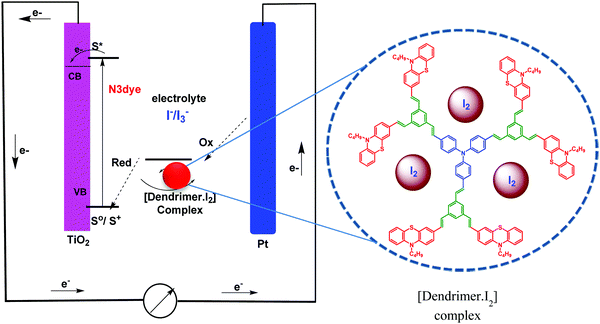
In dye-sensitized solar cells, the electrolyte (I−/I3−) plays an important role in improving the efficiency of solar cell performance. The electrolyte solution must have long term stability, including electrochemical and interfacial stability, which does not cause desorption of the dye from the titanium oxide surface and helps to maintain a high enough I3− diffusion coefficient, which is one of the main requirements for high performance in DSSCs. The electron donor–acceptor system in the redox couple of triiodide can be explained by considering the following reaction equilibrium equation (Scheme 4) in the electrolyte solution.38 The addition of dendrimers to the iodine electrolyte results in the formation of a dendrimer iodine complex which is highly stable in nature. Strong bonding between iodine and the electronegative nitrogen and sulfur atoms (Fig. 3) in the dendrimer suppresses the sublimation of iodine from the dendrimer complex, which is useful for the efficient recycling process of the electrolyte.The solar cell efficiency was calculated by using the formula
| η = Voc × Jsc × ff/P |
The current–voltage curve when the conjugated dendrimer 1–10 is used as an additive in the redox couple of the dye-sensitized solar cell is shown in Fig. 4, and the photoelectrochemical values are summarised in Table 2. The dendrimer bearing nitrogen and sulphur atoms can easily form charge transfer complexes very effectively with iodine in the redox reaction, which results in a decrease in the sublimation of iodine and also enhances the performance of DSSCs. From Table 2, it is clear that when dendrimer 10 is used as an additive, the efficiency increases to the maximum of 7.3% which is due to the presence of more electron-donating phenothiazine groups and triphenyl amine core units. The sublimation of iodine in the redox couple is minimized by the incorporation of phenothiazine units into the dendrimers and hence such additives can enhance the efficiency of DSSCs.39 The variation of the core unit in the dendrimers causes no significant change in the performance of DSSCs. Dendrimer 1 shows a low power conversion efficiency as its has only two phenothiazine groups with pyridine which has an sp2 hybridized nitrogen atom at the core unit.
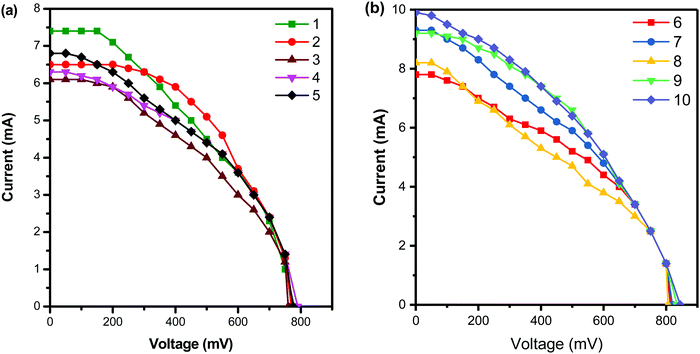 | ||
| Fig. 4 Current–voltage curves of DSSCs with the dendrimer additives (a) 1–5 and (b) 6–10 under simulated solar light at 60 mW cm−2. | ||
| S. no. | Electrolyte system/conjugated dendrimer | V oc (mV) | J sc (mA cm−2) | Fill factor (ff), % | (η), % |
|---|---|---|---|---|---|
| V oc = open circuit voltage, Jsc = short circuit current density, fill factor (ff), and η = efficiency. | |||||
| 1 | TiO2/N3dye/KI/I2/Pt | 648 | 4.2 | 49 | 2.2 |
| 2 | TiO2/N3dye/KI/I2/1/Pt | 760 | 6.1 | 49 | 3.8 |
| 3 | TiO2/N3dye/KI/I2/2/Pt | 770 | 6.5 | 49 | 4.1 |
| 4 | TiO2/N3dye/KI/I2/3/Pt | 780 | 7.4 | 49 | 4.7 |
| 5 | TiO2/N3dye/KI/I2/4/Pt | 775 | 6.8 | 49 | 4.3 |
| 6 | TiO2/N3dye/KI/I2/5/Pt | 790 | 6.3 | 50 | 4.2 |
| 7 | TiO2/N3dye/KI/I2/6/Pt | 814 | 7.8 | 50 | 5.3 |
| 8 | TiO2/N3dye/KI/I2/7/Pt | 806 | 8.2 | 50 | 5.5 |
| 9 | TiO2/N3dye/KI/I2/8/Pt | 820 | 9.3 | 51 | 6.5 |
| 10 | TiO2/N3dye/KI/I2/9/Pt | 835 | 9.2 | 52 | 6.7 |
| 11 | TiO2/N3dye/KI/I2/10/Pt | 845 | 9.9 | 52 | 7.3 |
To prove that the dendrimer additive interacts with the redox couple of DSSCs by forming a charge transfer complex, we have studied the UV-vis spectra of the solutions of dendrimers 5 and 10 with different species of the electrolyte in dimethyl sulfoxide (DMSO) at room temperature (Fig. 5 and Table 3). Dendrimers 5 and 10 exhibited two absorption bands at 280 and 419, and 306 and 392 nm, respectively. The solution of iodide showed two absorption bands at 296 and 366 nm. Furthermore, for the solutions of iodide and triiodide with the dendrimer additive 5, new absorption bands at 282 and 417 nm are observed. Similarly, for the solutions of iodide and triiodide with the dendrimer additive 10, two new absorption bands were observed at 304 and 398 nm. The disappearance of the iodine absorption peaks at 296 and 366 nm in the presence of a dendrimer additive clearly shows the formation of a [dendrimer·I2] complex.
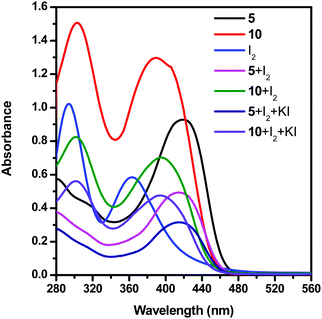 | ||
| Fig. 5 UV-vis spectra of solutions of dendrimers 5 and 10 and an electrolyte system in DMSO at room temperature. | ||
| Dendrimer/electrolite | λ abs max (nm) | Intensity |
|---|---|---|
| 5 | 280 | 0.57 |
| 419 | 0.93 | |
| 10 | 306 | 1.50 |
| 392 | 1.29 | |
| I2 | 296 | 1.02 |
| 366 | 0.58 | |
| 5 + I2 | 282 | 0.37 |
| 417 | 0.49 | |
| 10 + I2 | 304 | 0.82 |
| 398 | 0.70 | |
| 5 + I2 + KI | 282 | 0.27 |
| 417 | 0.31 | |
| 10 + I2 + KI | 303 | 0.56 |
| 396 | 0.47 |
The open circuit voltage and fill factor favour the DSSC system when dendrimer 10 is used as the additive and hence a higher efficiency of the DSSC is observed. Similarly, dendrimer 9 is rich in electron density comparable to that of dendrimer 8 and hence the efficiencies of 6.7% and 6.5% were observed, respectively.
Conclusion
In conclusion, we have synthesized the stilbenoid conjugated dendrimers using the Heck and Horner–Wadsworth–Emmons coupling reaction. The light absorption efficiency and the emission efficiency increase upon increasing dendrimer generation. Furthermore, as the number of phenothiazine units increases in the higher generation dendrimers, the DSSC performance also increases. DSSC studies showed a better power conversion efficiency of 7.3% with a short circuit current density (Jsc) of 9.9 mA cm−2, an open circuit voltage (Voc) of 845 mV, and a fill factor (ff) of 52% for dendrimer 10, which is higher than all the other synthesized phenothiazine dendrimers.Acknowledgements
The authors thanks DST-FIST, New Delhi, for providing the NMR spectral facility to the Department of Organic Chemistry, and MR thanks UGC for financial assistance and University of Madras for the infrastructure. The authors also acknowledge the financial assistance from DST Fast Track, New Delhi for the solar cell studies.References
- S. M. Grayson and J. M. J. Frechet, Chem. Rev., 2001, 101, 3819 CrossRef CAS PubMed.
- J. M. J. Frechet, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4782 CrossRef CAS PubMed.
- C. C. Lee, J. A. MacKay, J. M. J. Frechet and F. C. Szoka, Nat. Biotechnol., 2005, 23, 1517 CrossRef CAS PubMed.
- K. T. Wong, Y. H. Lin, H. H. Wu and F. Fungo, Org. Lett., 2007, 9, 4531 CrossRef CAS PubMed.
- W. Wu, R. Tang, Q. Li and Z. Li, Chem. Soc. Rev., 2015, 44, 3997 RSC.
- O. V. Borshchev, S. A. Ponomarenko, N. M. Surin, M. M. Kaptyug, M. I. Buzin, A. P. Pleshkova, N. V. Demchenko, V. D. Myakushev and A. M. Muzafarov, Organometallics, 2007, 26, 5165 CrossRef CAS.
- M. Shen and X. Shi, Nanoscale, 2010, 2, 1596 RSC.
- M. Y. Cho, H. S. Kang, K. Kim, S. J. Kim, J. Joo, K. H. Kim, M. J. Cho and D. H. Choi, Colloids Surf., A, 2008, 313–314, 431 CrossRef.
- F. Fungo, S. A. Jenekhe and A. J. Bard, Chem. Mater., 2003, 15, 1264 CrossRef CAS.
- C. C. Lee, M. K. Leung, P. Y. Lee, T. L. Chiu, J. H. Lee, C. Liu and P. T. Chou, Macromolecules, 2012, 45, 751 CrossRef CAS.
- H. Meier and M. Lehmann, Angew. Chem., Int. Ed., 1998, 37, 643 CrossRef CAS.
- S. H. Hwang, C. N. Moorefield and G. R. Newkome, Chem. Soc. Rev., 2008, 37, 2543 RSC.
- S. Dayal, N. Kopidakis and G. Rumbles, Faraday Discuss., 2012, 155, 323 RSC.
- A. C. Vallejo, M. Vonlanthen, P. Porcu, A. Ruiu and E. Rivera, Tetrahedron Lett., 2017, 58, 1319 CrossRef.
- K. Mutkins, S. S. Y. Chen, A. Pivrikas, M. Aljada, P. L. Burn, P. Meredith and B. J. Powell, Polym. Chem., 2013, 4, 916 RSC.
- V. Balzani, G. Bergamini, P. Ceroni and E. Marchi, New J. Chem., 2011, 35, 1944 RSC.
- M. K. Leung, Y. S. Lin, C. C. Lee, C. C. Chang, Y. X. Wang, C. P. Kuo, N. Singh, K. R. Lin, C. W. Hu, C. Y. Tseng and K. C. Ho, RSC Adv., 2013, 3, 22219 RSC.
- F. C. Schryver, T. Vosch, M. Cotlet, M. V. Auweraer, K. Mllen and J. Hofkens, Acc. Chem. Res., 2005, 38, 514 CrossRef PubMed.
- Y. Zhan, Y. Xu, Z. Jin, W. Ye and P. Yang, Dyes Pigm., 2017, 140, 452 CrossRef CAS.
- D. H. Hwang, S. K. Kim, M. J. Park, J. H. Lee, B. W. Koo, I. N. Kang, S. H. Kim and T. Zyung, Chem. Mater., 2004, 16, 1298 CrossRef CAS.
- K. Mutkins, S. S. Y. Chen, A. Pivrikas, M. Aljada, P. L. Burn, P. Meredith and B. J. Powell, Polym. Chem., 2013, 4, 916 RSC.
- (a) A. P. Kulkarni, X. Kong and S. A. Jenekhe, Adv. Funct. Mater., 2006, 16, 1057 CrossRef CAS; (b) Y. Park, B. Kim, C. Lee, A. Hyun, S. Jang, J. H. Lee, Y. S. Gal, T. H. Kim, K. S. Kim and J. Park, J. Phys. Chem. C, 2011, 115, 4843 CrossRef CAS.
- A. Tacca, R. Po, M. Caldararo, S. Chiaberge, L. Gila, L. Longo, P. R. Mussini, A. Pellegrino, N. Perin, M. Salvalaggio and A. S. Savoini, Electrochim. Acta, 2011, 56, 6638 CrossRef CAS.
- G. W. Kim, M. J. Cho, Y. J. Yu, Z. H. Kim, J. I. Jin, D. Y. Kim and D. H. Choi, Chem. Mater., 2007, 19, 42 CrossRef CAS.
- M. Kalkanidis, N. Klonis, L. Tilley and L. W. Deady, Biochem. Pharmacol., 2002, 63, 833 CrossRef CAS PubMed.
- K. Pluta, B. M. Mlodawska and M. Jelen, Eur. J. Med. Chem., 2011, 46, 3179 CrossRef CAS PubMed.
- C. Bodea and I. Silberg, Adv. Heterocycl. Chem., 1968, 9, 321 CrossRef CAS PubMed.
- K. Kubota, H. Kurebayashi, H. Miyachi, M. Tobe, M. Onishi and Y. Isobe, Bioorg. Med. Chem. Lett., 2009, 19, 2766 CrossRef CAS PubMed.
- A. Jaszczyszyn, K. Gasiorowski, P. Swiatek, W. Malinka, K. Cieslik-Boczula, J. Petrus and B. Czarnik-Matusewicz, Contemp. Oncol., 2012, 16, 332 CAS.
- S. Veerapandian, S. Amudha, S. A. Suthanthiraraj, M. A. Rahman and A. S. Nasar, RSC Adv., 2015, 5, 31404 RSC.
- H. G. Agrell, J. Lindgren and A. Hagfeldt, J. Photochem. Photobiol., A, 2004, 164, 23 CrossRef.
- H. Kusam, H. Orita and H. Sugihara, Langmuir, 2008, 24, 4411 CrossRef PubMed.
- (a) S. Ganesan, B. Muthuraaman, J. Madhavan, V. Mathew, P. Maruthamuthu and S. A. Suthanthiraraj, Electrochim. Acta, 2008, 53, 7903 CrossRef CAS; (b) S. Raja, C. Satheeshkumar, P. Rajakumar, S. Ganesan and P. Maruthamuthu, J. Mater. Chem., 2011, 21, 7700 RSC.
- S. Ganesan, B. Muthuraaman, J. Madhavan, V. Mathew, P. Maruthamuthu and S. A. Suthanthiraraj, Electrochim. Acta, 2008, 53, 7903 CrossRef CAS.
- (a) H. Kusama and H. Arakawa, Sol. Energy Mater. Sol. Cells, 2005, 85, 333 CrossRef CAS; (b) Z.-S. Huang, H. Meier and D. Cao, J. Mater. Chem. C, 2016, 4, 2404 RSC; (c) S. H. Aaron, K. C. C. Bikram, K. S. Navaneetha, A. K. Paul and D. S. Francis, ACS Appl. Mater. Interfaces, 2012, 4, 5813 CrossRef PubMed; (d) R. Y.-Y. Lin, T.-M. Chuang, F.-L. Wu, P.-Y. Chen, T.-C. Chu, J.-S. Ni, M.-S. Fan, Y.-H. Lo, K.-C. Ho and J. T. Lin, ChemSusChem, 2015, 8, 105 CrossRef CAS PubMed.
- P. Rajakumar, V. Kalpana, S. Ganesan and P. Maruthamuthu, Tetrahedron Lett., 2011, 52, 5812 CrossRef CAS.
- P. Rajakumar, A. Thirunarayanan, S. Raja, S. Ganesan and P. Maruthamuthu, Tetrahedron Lett., 2012, 53, 1139 CrossRef CAS.
- (a) F. Padinger, R. S. Rittberger and N. S. Sacriftci, Adv. Funct. Mater., 2003, 13, 85 CrossRef CAS; (b) D. L. Jinag and T. Aida, Nature, 1997, 388, 454 CrossRef.
- S. Ganesan, B. Muthuraaman, J. Madhavan, V. Mathew, P. Maruthamuthu and S. A. Suthanthiraraj, Electrochim. Acta, 2008, 53, 7903 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qm00238f |
| This journal is © the Partner Organisations 2017 |