High rate Li-ion storage properties of MOF-carbonized derivatives coated on MnO nanowires†
Zhen-Dong
Huang‡
a,
Zhen
Gong‡
a,
Qi
Kang
a,
Yanwu
Fang
a,
Xu-Sheng
Yang
 b,
Ruiqing
Liu
a,
Xiujing
Lin
a,
Xiaomiao
Feng
b,
Ruiqing
Liu
a,
Xiujing
Lin
a,
Xiaomiao
Feng
 a,
Yanwen
Ma
*a and
Dan
Wang
*ac
a,
Yanwen
Ma
*a and
Dan
Wang
*ac
aKey Laboratory for Organic Electronics and Information Displays & Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, Nanjing 210023, China. E-mail: iamywma@njupt.edu.cn
bAdvanced Manufacturing Technology Research Centre, Department of Industrial and Systems Engineering, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong, China
cState Key Laboratory of Multiphase Complex Systems Institute of Process Engineering Chinese Academy of Sciences, No. 1, Beiertiao, Zhongguancun, Beijing, 100190, P. R. China. E-mail: danwang@ipe.ac.cn
First published on 2nd June 2017
Abstract
Recently, metal–organic framework (MOF) derived porous carbon-based composites have become one of the most advanced electrode materials for high performance energy storage systems. In this work, zeolitic imidazolate framework (ZIF) types of MOF strung by MnO2 NWs, forming an interesting structure like Chinese candied hawthorn fruit on a stick, are used as precursors to prepare C/Co-coated MnO nanowires (C/Co-MnO NWs). It is interesting and exciting to observe that the simultaneously formed carbon coating derived from the ZIFs significantly promotes the cyclic and rate performances of manganese oxide because of the synergistic effect of the highly conductive uniform carbon coating and the high capacity contribution from the MnO NWs. The obtained C/Co-MnO NWs could deliver 848.4 and 718 mA h g−1 at 500 and 5000 mA g−1 after 40 charge/discharge cycles, respectively, which is superior to other reported MOF-derived nanostructured materials, and makes it a very promising candidate anode material for future high-power lithium ion batteries.
Introduction
Worldwide recognition of the importance of green energy storage and distribution for people's daily lives and the atmospheric environment has now emerged alongside the decay of the urban environment. The incomplete combustion of fossil fuels to power traditional vehicles and to supply heat for space heating is considered as one of the main reasons for the more and more serious fog and haze conditions in large cities, especially in developing countries. Therefore, the ever-increasing demand for electrical vehicles (EVs) and low cost green energy harvest stations is accelerating the development of advanced energy storage and energy conversion systems. Currently, lithium-ion batteries (LIBs) are the leading power sources for EVs and still predominate in the battery market for portable electronic devices and green energy distribution because of their high energy density, long life span, lack of memory effect, and environmental benignity.1–3 However, traditional LIBs that use graphite as the anode material are not able to satisfy the rapidly expanding demand due to the limited theoretical capacity of graphite (372 mA h g−1).2–6 Thus, one of the urgent research interests for materials scientists delving into next generation energy storage technology is to develop a novel low cost anode material with higher capacity and better cycle stability at higher power states.Nowadays, other than various advanced carbon materials, nanostructured silicon-based hybrids and nanostructured transition metals and their oxides or sulfides have attracted intensive interest because of their much higher theoretical lithium ion storage capacities.5–16 However, their semi-conductive or even insulating nature and huge volume change during the (de)lithiation process are two of their intrinsic drawbacks as anode materials for LIBs. Therefore, Mn-based oxides, such as MnO, MnO2 and Mn3O4,16–25 have been extensively studied as promising candidate anode materials due to their smaller volume change relative to Si materials and their environmentally benign nature. However, in the same way as for other transition metal oxide anode materials for LIBs, the rate capability and cyclic stability of Mn-based oxide anodes also have to be solved before their practical application. As for the less conductive Mn-based oxides, the significant volume change during the charge–discharge process will again lead to the pulverization of active particles and their detachment from the conductive network or current collector, in turn increasing the system charge transfer resistance and reducing the availability of the active substance.
In recent years, great efforts have been made by materials scientists to solve the aforementioned problems encountered by Mn-based oxide anodes. For example, binary manganese oxides (MxMnyOz) have been developed as promising anode candidates with enhanced electrochemical performance,20 since most research results indicate that binary metal oxides exhibit better conductivity and structure stability than mono-metal oxides. The development of nanostructured hierarchical Mn-based oxides is also one of the important strategies to promote the cyclic and rate capability of Mn-based anode materials.16 Furthermore, constructing a uniform 3 dimensional conductive network via the introduction of uniform surface coating and formation of a nanostructured composite with carbon is considered as one of the most important and effective ways to achieve superior rates and cyclic stabilities for Mn-based oxide anode materials.17–21 The introduction of carbon materials, such as carbon nanotubes, graphene and nitrogen-doped carbon coatings, can not only help to accelerate charge transport, but is also helpful for the formation of a stable solid electrolyte interphase and the remission of inner stress caused by the huge volume change.
In the last few years, metal–organic frameworks (MOFs), constructed with metal ions or clusters and polyfunctional organic ligands, have attracted increasing attention both from fundamental scientific research and for practical multifunctional applications.9–29 Zeolitic imidazolate framework (ZIF) types of MOFs, such as ZIF-67 and ZIF-8,9–14 have high porosity and are considered as promising MOFs for commercial applications. Carbonized derivatives of ZIFs show especially good potential for applications in LIBs. For instance, ZIF nanocrystal derived carbon anode materials show good cyclic stability and superior rate capability.27–29 However, the very low tap density of highly porous nanostructures, together with their relatively low capacity, limits their practicability as anode materials for commercial LIBs. Nevertheless, the unique structure and composition of ZIFs and their carbonized derivatives are very helpful to design different nanostructured transition metals and their oxides and to improve their rate and cyclic stability.
Based on the above concerns, an interesting structure of ZIF-67 MOFs on MnO2 nanowires (NWs) is designed and prepared by a controllable way in this work, as inspired by the interesting structure of candied hawthorn fruit on a stick. Furthermore, the simultaneously formed carbon coating derived from ZIF-67 is designed to promote the cyclic and rate performances of manganese oxide. Benefiting from the synergistic effect of the highly conductive uniform carbon coating and the high capacity contribution from MnO, the obtained C/Co-MnO NW composite shows a high reversible capacity of 848.4, 836.5, 834 and 718 mA h g−1 at 500, 1000, 2000 and 5000 mA g−1 after 40 charge/discharge cycles, respectively.
Experimental
Materials and synthetic procedures
| 2KMnO4 + 3MnSO4 + 2H2O → 5MnO2 + K2SO4 + 2H2SO4 |
Characterization
The morphologies of the MnO2 NWs, ZIF-67, ZIF-67-MnO2 NWs, C/Co and C/Co-MnO NWs were characterized by field emission scanning electron microscopy (FE-SEM, Hitachi S-4800) at an acceleration voltage of 3 kV and field emission transmission electron microscopy (FE-TEM, JEOL 2010F) at an accelerating voltage of 200 kV. Nitrogen adsorption/desorption isotherms were obtained at 77 K using an automated adsorption apparatus (Micromeritics ASAP2020). The surface area was calculated based on the Brunauer–Emmett–Teller (BET) equation. The X-ray diffraction patterns of the MnO2 NWs, ZIF-67, ZIF-67-MnO2 NWs, C/Co and C/Co-MnO NWs were measured on an X-ray diffractometer (RIGAKU, RINT-ULTIMA III) using Cu Kα radiation (λ = 1.54051 Å). The diffraction patterns were recorded in the 2θ range of 5–75° with a step size of 0.01°. The elemental mapping of the C/Co-MnO NWs was characterized by energy-dispersive X-ray spectroscopy (EDS, Oxford instruments X-Max). The Co and Mn content within the as-prepared C/Co-MnO NWs was measured by following the general rules of JY/T 015-1996 for inductively coupled plasma-atomic emission spectrometry (ICP-AES, Prodigy, Teledyne Leeman Labs).To investigate the electrochemical performances, the as-prepared active materials, including the MnO2 NWs, ZIF-67, ZIF-67-MnO2 NWs, C/Co and C/Co-MnO NWs, were homogeneously mixed with a conductive additive (acetylene black) and binder (PVDF) in a weight ratio of 75%/15%/10% in a suitable amount of N-methylpyrrolidinone solvent to form a uniform slurry. The working electrodes were prepared by coating the obtained slurry on copper foil, which acted as a current collector. The electrode was then punched out into disks 10 mm in diameter. The average mass loading of the active materials was about 1.27 mg cm−2. Two-electrode lithium ion batteries were assembled in an ultrapure Ar gas-filled glove box to investigate the lithium ion storage performance of the MnO2 NWs, ZIF-67, ZIF-67-MnO2 NWs, C/Co and C/Co-MnO NWs. The electrolyte used was 1 mol L−1 LiPF6 in ethylene carbonate (EC) + dimethyl carbonate (DMC) + ethyl methyl carbonate (EMC) in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Lithium discs were used as the counter electrodes. Cyclic voltammetry (CV) and galvanostatic charge and discharge measurements were carried out in a voltage range of 0.01 to 3 V vs. Li/Li+ at a current density ranging from 0.5 to 5 A g−1, respectively. Electrochemical impedance spectroscopy was carried out in a frequency range of 0.01 Hz to 100 kHz, and the perturbation amplitude was controlled at 5 mV. Galvanostatic charge/discharge tests were performed on a battery testing system (CT2001A, Wuhan Land).
1. Lithium discs were used as the counter electrodes. Cyclic voltammetry (CV) and galvanostatic charge and discharge measurements were carried out in a voltage range of 0.01 to 3 V vs. Li/Li+ at a current density ranging from 0.5 to 5 A g−1, respectively. Electrochemical impedance spectroscopy was carried out in a frequency range of 0.01 Hz to 100 kHz, and the perturbation amplitude was controlled at 5 mV. Galvanostatic charge/discharge tests were performed on a battery testing system (CT2001A, Wuhan Land).
Results and discussions
Morphology and structure
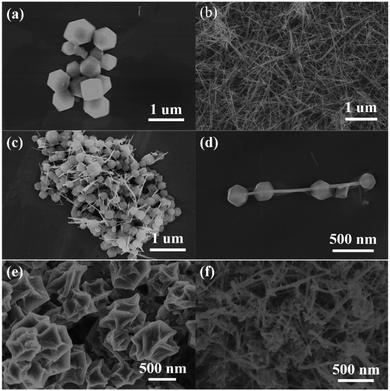
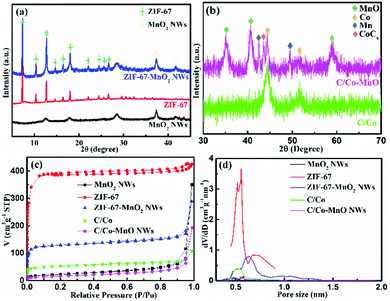
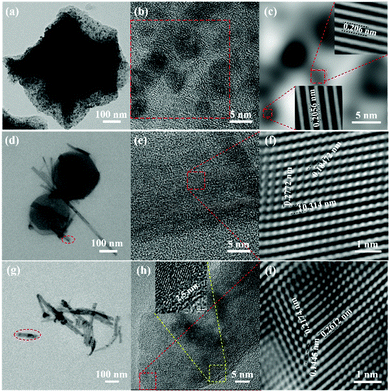
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (225). This observation indicates that the cobalt components within ZIF-67 are reduced to elemental metallic cobalt nanoparticles after a carbothermic reduction reaction. Furthermore, the cubic metallic cobalt particles dispersed into the carbon matrix are about 2–5 nm. During the carbonization process, the structure becomes dense. As shown in Fig. 2c and d, the specific surface area and the pore volume decrease from 1158.4 m2 g−1 and 0.66 cm3 g−1 for ZIF-67 to 193.755 m2 g−1 and 0.078 cm3 g−1 for the C/Co composites, respectively.
m (225). This observation indicates that the cobalt components within ZIF-67 are reduced to elemental metallic cobalt nanoparticles after a carbothermic reduction reaction. Furthermore, the cubic metallic cobalt particles dispersed into the carbon matrix are about 2–5 nm. During the carbonization process, the structure becomes dense. As shown in Fig. 2c and d, the specific surface area and the pore volume decrease from 1158.4 m2 g−1 and 0.66 cm3 g−1 for ZIF-67 to 193.755 m2 g−1 and 0.078 cm3 g−1 for the C/Co composites, respectively.
Similar to the obtained C/Co composites, the carbonized derivative of the ZIF-67-MnO2 NWs becomes a complex composite of carbon, cobalt nanoparticles and MnO NWs (C/Co-MnO NWs) with a small amount of Mn and CoCx formed during the carbonization process, as indicated by the pink color X-ray diffraction pattern shown in Fig. 2b. As shown in Fig. S1b (ESI†), the morphology of ZIF-67 on MnO2 NWs is still maintained after 3 h annealing at 300 °C. However, after being calcined at 550 °C for 3 h, the ZIF-67 polyhedrons became molten and formed a uniform carbon coating on the surface of the MnO NWs reduced from the MnO2 NWs, which can be observed from the SEM image shown in Fig. 1f and the high resolution TEM images in Fig. 3g–i, and further confirmed by the element mapping results shown in Fig. S2 (ESI†). The measured lattice fringes (Fig. 3h and i) with interplane distances of 0.2612, 0.2174 and 0.1445 nm can be indexed to the (111), (200) and (311) crystallographic planes of the cubic MnO structure in the space group of Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (225), respectively. The uniform carbon coating, about 2–3 nm in thickness, can be observed from the inset of Fig. 3h and is good for the construction of a 3D conductive network. As for the Mn and CoCx impurity phases, it is very difficult to define them from the high resolution TEM images together with MnO, due to their small amounts and similar interlayer spaces. Based on the distribution of Co shown in Fig. S2c (ESI†), cobalt mainly gathers at the locations of polyhedron sites. The ICP-AES measurement result indicates that the elemental content of Co and Mn within the finally obtained C/Co-MnO NWs is about 16.0% and 43.8%, respectively. The nitrogen adsorption/desorption isotherm and pore-size distribution curves presented in Fig. 2c and d confirm that the BET specific surface area of the C/Co-MnO NWs further decreased to 57.8 m2 g−1, which is good for obtaining a higher volumetric energy density.
m (225), respectively. The uniform carbon coating, about 2–3 nm in thickness, can be observed from the inset of Fig. 3h and is good for the construction of a 3D conductive network. As for the Mn and CoCx impurity phases, it is very difficult to define them from the high resolution TEM images together with MnO, due to their small amounts and similar interlayer spaces. Based on the distribution of Co shown in Fig. S2c (ESI†), cobalt mainly gathers at the locations of polyhedron sites. The ICP-AES measurement result indicates that the elemental content of Co and Mn within the finally obtained C/Co-MnO NWs is about 16.0% and 43.8%, respectively. The nitrogen adsorption/desorption isotherm and pore-size distribution curves presented in Fig. 2c and d confirm that the BET specific surface area of the C/Co-MnO NWs further decreased to 57.8 m2 g−1, which is good for obtaining a higher volumetric energy density.
Lithium ion storage properties
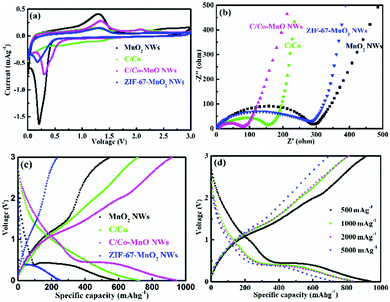
In this work, galvanostatic charge/discharge measurements at different current densities, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were carried out in lithium half-cells to investigate and understand the electrochemical behaviour of the as-prepared active materials. Fig. 4 and 5 indicate that the MnO2 NWs, ZIF-67-MnO2 NWs, C/Co, and C/Co-MnO NWs are electrochemically active, and capable of lithium ion storage. As shown in Fig. 4a, the typical CV curves of the second cycle scanning between 0.01 and 3.0 V at 0.1 mV s−1, which are similar to those of reported MnO/C materials,17,18 demonstrate that the electrodes made of C/Co and C/Co-MnO NWs show a smaller voltage gap between the reduction and oxidation peaks. Meanwhile, the CV curve of the C/Co-MnO NWs shows a larger peak current density than that of C/Co, resulting from a higher fraction of high capacity transition metal components. A pair of weak typical redox peaks could be found from the CV curve of C/Co shown in Fig. 4a, which indicates that Co has a relatively minor contribution to the capacity of the C/Co electrode. Therefore, the CV analysis results clarify that the C/Co-MnO NWs show better electrochemical performance due to the synergistic effect of the MnO NWs and their uniform MOF derived carbon coating. It can be found from Fig. 4b that the C/Co and C/Co-MnO NWs show much lower charge transfer resistance than the MnO2 NWs and ZIF-67-MnO2 NWs. After fitting with the equivalent circuit shown in the inset of Fig. S3c (ESI†), the calculated resistance, including the electrolyte resistance and the contact resistance of the active materials to the current collector, is around 4.2 Ω. The charge transfer resistance (Rct), which is equal to the diameter of the semicircle in the high frequency range, is about 65.9 Ω. Furthermore, in the Bode plots given in Fig. S3d (ESI†), the characteristic frequencies f0 at a phase angle of −45° for the C/Co-MnO NWs, C/Co, ZIF-67-MnO2 NWs, and MnO2 NWs are 0.071, 0.067, 0.026 and 0.010 Hz, corresponding to time constants τ0 (τ0 = 1/f0) of 14.1, 14.9, 38.5 and 100 s, respectively. The values of the C/Co-MnO NWs are much smaller than those of the materials mentioned earlier, indicating that the C/Co-MnO NWs have a faster charge–discharge rate than the other materials. This observation further supports the fact that the improved conductivity of the C/Co-MnO NWs, arising from the uniform carbon coating on the MnO NWs, is responsible for its better electrochemical performance.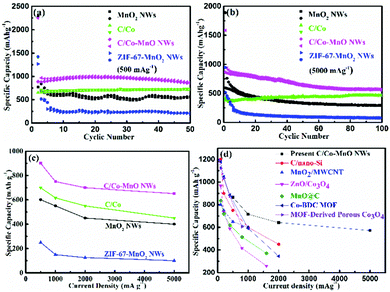 | ||
| Fig. 5 (a and b) The cyclic performances of the C/Co-MnO NWs at a current density of 500 and 5000 mA g−1, respectively, (c) comparison of the rate and capacity retention after 50 cycles of the as-prepared MnO2 NWs (in black), ZIF-67-MnO2 NWs (in blue), C/Co (in green) and C/Co-MnO NWs (in pink), and (d) comparison of the rate and capacity retention after 50 cycles of the as-prepared C/Co-MnO NWs with other reported similar materials, such as C/nano-Si,9 MOF-derived porous Co3O4,14 ZnO/Co3O4,15 MnO@C,17 MnO2/MWCNT,21 and Co-BDC MOF.34 | ||
The typical charge/discharge profiles presented in Fig. S3 (ESI†) and Fig. 4c and d for the first cycle and the 40th cycle, respectively, also evidently prove that the electrode made of C/Co-MnO NWs delivers an excellent rate capability and a much higher capacity than other electrodes prepared with other controlled materials. The Li//C/Co-MnO NW half-cell exhibits a much higher capacity, higher coulombic efficiency for the first cycle and better cyclic performance than the Li//MnO2 NW half-cell, which is promoted by the ZIF-67-derived surface layer of C/Co. With respect to ZIF-67-derived C/Co, a typical discharge plateau and reduction peak clearly observed at a voltage of 0.5 V should result from the reduction of Mn2+ to Mn0. The corresponding redox reaction of Mn2+/Mn0 should account for the higher capacity of the C/Co-MnO NWs over C/Co. This lithium ion storage mechanism is similar to that in other reported MnO@C composites.17,33 Moreover, the synergistic effect of the MnO NW and its uniform MOF derived carbon coating is responsible for its superior lithium ion storage performance, compared to the ZIF-67-MnO2 NWs. This result is consistent with the CV analysis results, seen in Fig. 4a and c. As shown in Fig. 4d, the specific charge capacities of the C/Co-MnO NWs are 848.4, 836.5, 834 and 718 mA h g−1 at 500, 1000, 2000 and 5000 mA g−1 after 40 charge/discharge cycles, respectively, which confirm the superior rate capability of the as-prepared C/Co-MnO NWs. Fig. 5 and Fig. S4 (ESI†) further exhibit that the as-prepared C/Co-MnO NWs not only deliver a much better rate and cyclic performance than the controlled C/Co, MnO2 NWs, ZIF-67-MnO2 NWs and C/Co-MnO2 physical mixture at a constant current density of 500, 1000, 2000 and 5000 mA g−1, but also show a superior rate and cyclic performance than other reported similar materials (see Fig. 5d), such as C/nano-Si,9 MOF-derived porous Co3O4,14 ZnO/Co3O4,15 MnO@C,17 MnO2/MWCNT,21 Co3O4/MnO232 and Co-BDC MOF.34
Furthermore, the as-prepared C/Co-MnO NWs also present a much better rate capability than the controlled mixture of C/Co-MnO2 NWs, as seen in Fig. 5 and Fig. S4a and b (ESI†). As shown in Fig. 1f and 3h, the in situ formed uniform carbon coating fully covered the surface of the MnO NWs. It has a strong interaction and created an integral electrical contact between MnO and the carbon coating. A similar mechanism could also be observed from the reported core–shell ZnO/ZnFe2O4@C mesoporous nanospheres.35 However, there is only point electrical contact between the physically mixed MnO2 NWs and the large size C/Co particles derived from ZIF-67, as shown in Fig. S4a and b (ESI†). Therefore, the synergistic effects of much improved conductivity and relatively suppressed volume change resulting from the integrally strong carbon coating are responsible for the much superior rate and cyclic performance of the C/Co-MnO NWs compared to the controlled mixture of C/Co-MnO2 NWs.
Conclusions
In this work, a novel and interesting structure of ZIF-67 MOFs on MnO2 nanowires (NWs), like candied hawthorn fruit on a stick, is designed and successfully prepared by a controllable multi-step chemical method. The observed experimental results demonstrate that the polyvinylpyrrolidone (PVP) modified surface is helpful for the growth of ZIF-67 on the MnO2 NWs and for minimizing the MOF size from 500 to 100 nm. It is also interesting and exciting to find that the simultaneously formed carbon coating derived from ZIF-67 significantly promotes the cyclic and rate performances of manganese oxide. Benefiting from the synergistic effects of the highly conductive uniform carbon coating and the high capacity contribution from MnO, the obtained C/Co-MnO NWs deliver 848.4, 836.5, 834 and 718 mA h g−1 at 500, 1000, 2000 and 5000 mA g−1 after 40 charge/discharge cycles, respectively. The achieved high rates and much enhanced cyclic performances make the present C/Co-MnO NWs superior to other reported MOF-derived nanostructured materials.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51402155), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (YX03002), National Synergistic Innovation Center for Advanced Materials (SICAM) and Foundation of NJUPT (NY214021).References
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928 CrossRef CAS PubMed.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, Nat. Mater., 2010, 9, 353 CrossRef CAS PubMed.
- L. Qie, W. M. Chen, Z. H. Wang, Q. G. Shao, X. Li, L. X. Yuan, X. L. Hu, W. X. Zhang and Y. H. Huang, Adv. Mater., 2012, 24, 2047 CrossRef PubMed.
- Z. D. Huang, K. Zhang, T. T. Zhang, R. Q. Liu, X. J. Lin, Y. Li, T. Masese, X. M. Liu, X. M. Feng and Y. W. Ma, Energy Storage Mater., 2016, 5, 205 CrossRef.
- Z. J. Li, D. B. Kong, G. M. Zhou, S. D. Wu, W. Lv, C. Luo, J. J. Shao, B. H. Li, F. Y. Kang and Q. H. Yang, Energy Storage Mater., 2017, 6, 98 CrossRef.
- Y. L. Zhou, J. Tian, H. Y. Xu, J. Yang and Y. T. Qian, Energy Storage Mater., 2017, 6, 149 CrossRef.
- Z. D. Huang, K. Zhang, T. T. Zhang, X. S. Yang, R. Q. Liu, Y. Li, X. J. Lin, X. M. Feng, Y. W. Ma and W. Huang, Energy Storage Mater., 2016, 3, 36 CrossRef.
- Z. D. Huang, K. Zhang, T. T. Zhang, R. Q. Liu, X. J. Lin, Y. Li, X. M. Feng, Q. B. Mei, T. Masese, Y. W. Ma and W. Huang, Composites, Part A, 2016, 84, 386 CrossRef CAS.
- Y. H. Song, L. Zuo, S. H. Chen, J. F. Wu, H. Q. Hou and L. Wang, Electrochim. Acta, 2015, 173, 588 CrossRef CAS.
- Y. Z. Han, P. F. Qi, X. Feng, S. W. Li, X. T. Fu, H. W. Li, Y. F. Chen, J. W. Zhou, X. G. Li and B. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 2178 CAS.
- B. Liu, X. B. Zhang, H. Shioyama, T. Mukai, T. Sakai and Q. Xu, J. Power Sources, 2010, 195, 857 CrossRef CAS.
- C. Li, T. Q. Chen, W. J. Xu, X. B. Lou, L. K. Pan, Q. Chen and B. W. Hu, J. Mater. Chem. A, 2015, 3, 5585 CAS.
- G. Huang, F. F. Zhang, X. C. Du, Y. L. Qin, D. M. Yin and L. M. Wang, ACS Nano, 2015, 9, 1592 CrossRef CAS PubMed.
- D. Tian, X. L. Zhou, Y. H. Zhang, Z. Zhou and X. H. Bu, Inorg. Chem., 2015, 54, 8159 CrossRef CAS PubMed.
- D. Q. Zhu, F. C. Zheng, S. H. Xu, Y. G. Zhang and Q. W. Chen, Dalton Trans., 2015, 44, 16946 RSC.
- F. Cheng, W. C. Li and A. H. Lu, ACS Appl. Mater. Interfaces, 2016, 8, 27843 CAS.
- F. C. Zheng, G. L. Xia, Y. Yang and Q. W. Chen, Nanoscale, 2015, 7, 9637 RSC.
- Y. H. Wang, X. Ding, F. Wang, J. Q. Li, S. Y. Song and H. J. Zhang, Chem. Sci., 2016, 7, 4284 RSC.
- J. G. Wang, D. D. Jin, R. Zhou, X. Li, X. R. Liu, C. Shen, K. Y. Xie, B. H. Li, F. Y. Kang and B. Q. Wei, ACS Nano, 2016, 10, 6227 CrossRef CAS PubMed.
- F. C. Zheng, D. Q. Zhu, X. H. Shi and Q. W. Chen, J. Mater. Chem. A, 2015, 3, 2815 CAS.
- S. J. Ee, H. C. Pang, U. Mani, Q. Y. Yan, S. L. Ting and P. Chen, ChemPhysChem, 2014, 15, 2445 CrossRef CAS PubMed.
- S. Z. Huang, Q. Zhang, W. B. Yu, X. Y. Yang, C. Wang, Y. Li and B. L. Su, Electrochim. Acta, 2016, 222, 561 CrossRef CAS.
- S. Z. Huang, Y. Cai, J. Jin, J. Liu, Y. Li, Y. Yu, H. E. Wang, L. H. Chen and B. L. Su, Nano Energy, 2015, 12, 833 CrossRef CAS.
- S. Z. Huang, J. Jin, Y. Cai, Y. Li, Z. Deng, J. Y. Zeng, J. Liu, C. Wang, T. Hasan and B. L. Su, Sci. Rep., 2015, 5, 14686 CrossRef CAS PubMed.
- S. Z. Huang, J. Jin, Y. Cai, Y. Li, Y. H. Tan, E. B. Wang, G. Tendeloo and B. L. Su, Nanoscale, 2014, 6, 6819 RSC.
- Y. Xia, B. B. Wang, G. Wang, X. X. J. Liu and H. Wang, ChemElectroChem, 2016, 3, 299 CrossRef CAS.
- P. Y. Wang, J. W. Lang, D. X. Liu and X. B. Yan, Chem. Commun., 2015, 51, 11370 RSC.
- Y. J. Yu, C. Yue, X. G. Lin, S. B. Sun, J. P. Gu, X. He, C. H. Zhang, W. Lin, D. H. Lin, X. L. Liao, B. B. Xu, S. T. Wu, M. S. Zheng, J. Li, J. Y. Kang and L. W. Lin, ACS Appl. Mater. Interfaces, 2016, 8, 3992 CAS.
- Z. L. Xiu, D. Y. Kim, M. H. Alfaruqi, J. H. Gim, J. J. Song, S. J. Kim, P. T. Duong, J. P. Baboo, V. Mathew and J. Kim, J. Mater. Chem. A, 2016, 4, 4706 CAS.
- M. Ramesh, H. S. Nagaraja, M. P. Rao, S. Anandan and N. M. Huang, Mater. Lett., 2016, 172, 85 CrossRef CAS.
- Q. F. Wang, R. Q. Zou, W. Xia, J. Ma, B. Qiu, A. Mahmood, R. Zhao, Y. Yang, D. G. Xia and Q. Xu, Small, 2015, 11, 2511 CrossRef CAS PubMed.
- Q. Zhu, Y. H. Li and Y. Gao, Chem. – Eur. J., 2016, 22, 6876 CrossRef CAS PubMed.
- S. Z. Huang, Q. Zhang, W. B. Yu, X. Y. Yang, C. Wang, Y. Li and B. L. Su, Electrochim. Acta, 2016, 222, 561 CrossRef CAS.
- X. S. Hu, H. P. Hu, C. Li, T. Li, X. B. Lou, Q. Chen and B. W. Hu, J. Solid State Chem., 2016, 242, 71 CrossRef CAS.
- C. Z. Yuan, H. Cao, S. Q. Zhu, H. Hua and L. R. Hou, J. Mater. Chem. A, 2015, 3, 20389 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qm00178a |
| ‡ Dr Huang and Mr Gong made equal contributions to this paper. |
| This journal is © the Partner Organisations 2017 |