Ni/NiOx-decorated carbon nanofibers with enhanced oxygen evolution activity for rechargeable zinc–air batteries†
Bing
Li
a,
Sheau-Wei
Chien
a,
Xiaoming
Ge
a,
Jianwei
Chai
a,
Xin-Yi
Goh
b,
Kei-Teng
Nai
b,
T. S.
Andy Hor
ac,
Zhaolin
Liu
*a and
Yun
Zong
 *a
*a
aInstitute of Materials Research and Engineering (IMRE), A*STAR (Agency for Science, Technology and Research), 2 Fusionopolis Way, Innovis #08-03, Singapore 138634, Republic of Singapore. E-mail: zl-liu@imre.a-star.edu.sg; y-zong@imre.a-star.edu.sg
bRaffles Girls’ School (Secondary), 20 Anderson Road, Singapore 259978, Republic of Singapore
cDepartment of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong SAR, China
First published on 7th October 2016
Abstract
We report Ni/NiOx nanoparticle-decorated carbon nanofibers, produced via electrospinning with subsequent heat treatments, as efficient oxygen evolution reaction catalysts for rechargeable Zn–air batteries with superior performances.
Oxygen evolution reaction (OER) is of great importance to water-splitting1 and secondary metal–air battery2 technologies. Being a kinetically sluggish process, it demands good catalysts to lower the overpotential on the air-electrode to improve the overall charge–discharge energy efficiency. Ir/C and RuO2 are known as the two most efficient OER catalysts, showing low overpotentials and small Tafel slopes.1b,3 However, scarcity and prohibitive cost of the precious metals are the bottlenecks which have limited their applications only to high value niche areas. The efforts devoted to developing their alternatives with acceptable performance and low cost are tremendous, leading to the discovery of a number of transition metal based OER catalysts.4 Nickel (Ni), one of such metals with earth-abundance, is particularly attractive due to the additional merit of high corrosion-resistance in alkaline aqueous solution, a commonly used electrolyte in zinc–air batteries (ZnABs).5 The efficacy of Ni-based nanostructured materials in OER catalysis is supported by several recently reported successes, represented by NiP,6 Ni(OH)2,5,7 NiO5,8 and their composites.9 As the good electrical conductivity required for efficient electron transfer in an electrocatalysis reaction is often absent in such catalysts, conductive agents, e.g. nanostructured carbons and their derivative materials, are incorporated into the air-electrode to improve the electron transfer behaviours for enhanced OER performance.10 The introduction of such carbon-based “additives” concurrently brings along the additional merits of boosting oxygen reduction reaction (ORR) activity as inherited properties of the nanostructured carbon materials and their hetero-atom doped counterparts, producing a bifunctional air-electrode to facilitate both charge and discharge processes of the resultant metal–air batteries. The close-contact of the nanoparticle metal oxide with conductive nanostructured carbon is reported to further enhance the OER and ORR activities of the composite catalyst via synergistic coupling in the electrocatalytic reactions.9a,10a
For instance, a composite of ultrathin Ni(OH)2 nanoplates grown on multi-walled carbon nanotubes (MWCNTs) by Zhou et al.9a showed a smaller overpotential of 474 mV at a current density of 10 mA cm−2 in 0.1 M KOH, which is notably lower than that of Ni(OH)2 nanoplates (595 mV) or its mechanical blends with MWCNTs (540 mV). In addition, the smaller Tafel slope and higher stability observed on the very same composite electrode proved its usefulness in OER catalysis. Similar observations were reported by Lee and co-workers on a hybrid electrode composed of mesoporous NiCo2O4 nanoplatelets and graphene nanosheets (NiCo2O4–G),9b and by the Qiao group on a 3-dimensional hybrid paper constructed using N-doped graphene (NG) with in- and out-of-plane pores (NG-NiCo2O4).10c The use of graphene or CNTs in a hybrid catalyst often involves tedious preparation procedures with a high cost, which would naturally diminish the opportunity of its adoption in applications with the need for a large quantity of OER catalysts. Recently, we developed Co3O4 nanoparticle (NP) decorated carbon nanofibers (CNFs) using an electrospinning technique with subsequent heat treatments. The resultant Co3O4/CNFs hybrid catalyst showed great promise as an integrated electrode for rechargeable ZnABs.11 The merits of such catalysts, apart from their simple preparation at a low cost, are their high electrocatalytic activity, low contact resistance, short electron transfer pathway, mitigated detachment/agglomeration of the active NPs, rich voids in the large electrolyte/nanofiber interfaces for rapid ion transport and fast oxygen gas diffusion, etc. These desirable features likely originated from the Co3O4 NP decorated one-dimensional (1D) highly conductive CNFs, which could be further intertwined to form an interconnected 3D network as an interesting integrated binder-free electrode.
In this contribution, we extend the strategy to nickel-based material systems for their remarkable OER performance with an extra merit of corrosion-resistance in alkaline aqueous solution, the commonly used electrolyte for ZnABs. The preparation of nickel/nickel oxide (Ni/NiOx) NP decorated CNFs involves 3 successive steps, i.e. electrospinning the “precursor” polymeric fibres with a controlled loading of nickel cations from a mixed solution of polyacrylonitrile (PAN) and Ni(OAc)2, pyrolyzing the PAN/Ni2+ fibres at 900 °C under a nitrogen atmosphere to convert them to Ni NP decorated CNFs (C-NiPAN900), and thermally annealing C-NiPAN900 in air at 300 °C to form Ni/NiOx NP decorated CNFs as the final product, denoted as C-NiPAN900-300. The morphologies of C-NiPAN900 and C-NiPAN900-300 were studied using a field-emission scanning electron microscope (FE-SEM) and a transmission electron microscope (TEM), and their phase structure and chemical compositions were elucidated via X-ray diffraction (XRD) analysis and X-ray photoelectron spectroscopy (XPS), respectively. The electrochemical study results suggest excellent OER activity for C-NiPAN900-300 which is comparable to that of the benchmark catalyst of Ir/C, but with superior cycling stability over the latter. The steady performance of ZnABs with C-NiPAN900-300 as a catalyst in the air-electrode is reflected by the lower charge voltage and the narrower charge–discharge voltage gap, as well as the greater cycling stability, all of which are critical for practical applications.
The as-prepared C-NiPAN900-300 consists of fibres with a diameter of 100–500 nm, as shown in Fig. 1A. Their surfaces are decorated with NPs of sizes in the range of 20–30 nm (Fig. 1B, bright spots). The representative TEM images (Fig. 1C, and Fig. S1, ESI†) confirm a high loading of the metal-based NPs (darker ones as contrast to carbon for higher electron density) with the majority being about 20 nm in diameter. Some “giant” NPs of 100–150 nm were likely a consequence of “Oswald ripening”12 growth in the carbonization process. The lattice spacings are found to be about 2.12 and 2.42 Å (Fig. 1D, and Fig. S1, ESI†), ascribed to the (200) and (111) planes of NiO,13 respectively.
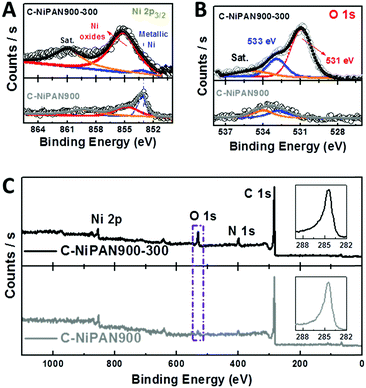
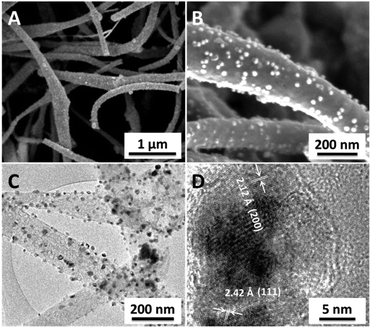 | ||
| Fig. 1 Morphological information about C-NiPAN900-300. (A and B): SEM images; (C and D): TEM images. | ||
The presence of NiO in C-NiPAN900-300 was supported by the XRD analysis (Fig. S2, ESI†). The characteristic diffraction peaks from the (200) planes of NiO (blue line, Fig. S2, ESI†) are readily identified (JCPDS #04-0835);14 while in C-NiPAN900, the sample prior to annealing at 300 °C, metallic Ni was clearly the dominant species (JCPDS #04-0850) (black line, Fig. S2, ESI†).15 The oxidation states of the Ni species in C-NiPAN900-300 were examined using X-ray photoelectron spectroscopy (XPS) analysis, and the two deconvoluted peaks in the Ni 2p3/2 spectrum (Fig. 2A, top curve) with binding energies centred at 853.1 and 855.2 eV are ascribed to the metallic Ni and nickel oxides (Ni2+ and Ni3+),16 respectively. This suggests that NiOx is the dominating species with the presence of a tiny amount of metallic Ni “residues”. In sharp contrast, C-NiPAN900 is found to be dominated by metallic Ni with a significantly lower content of NiOx (Fig. 2A, bottom curve). The high resolution O 1s spectra in Fig. 2B echo the Ni 2p3/2 spectra, and the peak associated with M–O bonding16 centred at a binding energy of 531 eV is very prominent for C-NiPAN900-300 (Fig. 2B, top curve) but much weaker for C-NiPAN900 (Fig. 2B, bottom curve). These data unambiguously prove the effective oxidization of the metallic Ni NPs (at least the outer shell10b) by annealing at 300 °C in air, which is consistent with the TEM and XRD results. It is worth noting that by using some advanced techniques, e.g. chemical mapping via electron energy-loss spectroscopy (EELS), one may resolve the O and Ni distribution in sub-nm resolution, which however, is not a focus of this work. If one takes the Ni content as a reference, it would not be difficult to spot the increase of the oxygen to carbon (O/C) atomic ratio (5.7 to 12.8%) upon annealing in air at 300 °C, as shown in the XPS survey spectra (Fig. 2C). The visible increase of the N peak intensity could be due to the removal of a small amount of amorphous carbon on the CNF surfaces upon annealing in air at 300 °C, which roughens the fibre surface and increases N exposure to XPS measurement, a surface-sensitive tool with a typical penetration depth below 10 nm. This, however, showed little impact on the C 1s peak intensity (Fig. 2C, insets), and hence excluded any noticeable adverse impacts on the CNFs during thermal conversion of Ni → NiOx for improved OER activity. C-NiPAN900-300 with a high loading of Ni/NiOx NPs on highly conductive CNFs is anticipated to be an excellent OER catalyst that equips ZnABs with an enhanced performance.
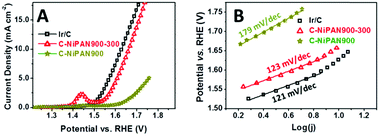
The electrochemical activities of C-NiPAN900-300 towards OER were investigated using linear sweep voltammetry (LSV). Ir/C was used as the benchmark OER catalyst, with C-NiPAN900 as the control. Unsurprisingly, notably improved OER activity was observed for C-NiPAN900-300 (vs. C-NiPAN900) as a clear negative shift of the LSV curve and higher current density at any given potential (Fig. 3A). For instance, at a potential of 1.7 V (vs. RHE) a current density of 14.1 mA cm−2 was obtained for C-NiPAN900-300, which is about 6 times as high as that of C-NiPAN900 (2.3 mA cm−2). As compared to the benchmark Ir/C, a slightly higher onset potential was found for C-NiPAN900-300. However, their LSV curves are almost parallel and of the same shape, warranting good OER activity in the latter as well. This is confirmed by the fact that an OER current density of 10 mA cm−2 is achievable with both catalysts, with the overpotential when using Ir/C being merely 34 mV lower (432 vs. 398 mV).
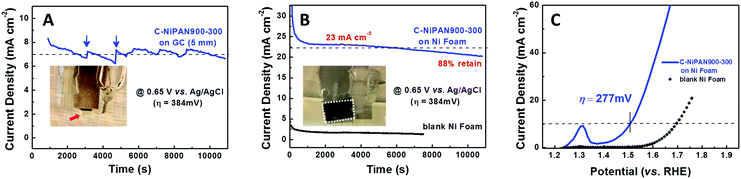
To further assess the OER activity of C-NiPAN900-300, its OER kinetics was studied, with C-NiPAN900 and Ir/C being the control and benchmark catalyst, respectively. Its Tafel slope is comparable to that of Ir/C (123 vs. 121 mV dec−1), but notably lower than that of C-NiPAN900 (179 mV dec−1) (Fig. 3B). The small Tafel slope (123 mV dec−1) and low overpotential (e.g. 432 mV @ 10 mA cm−2) are desirable characteristics which place C-NiPAN900-300 among the top transition metal-based OER catalysts reported recently,17 and on a par with noble metal based OER catalysts18 (Table S1, ESI†). Besides high OER activity, C-NiPAN900-300 exhibited good stability in aqueous alkaline electrolytes. In chronoamperometric (i–t) tests on a C-NiPAN900-300 loaded glassy carbon (GC) electrode in 0.1 M KOH at a fixed overpotential of 384 mV, a stable current density of 7 mA cm−2 was observed over a notable period of 3 h (Fig. 4A). The good stability is also further indicated from the SEM images taken on the electrode after i–t tests (Fig. S3, ESI†), where little change is visible for the small NP decorated CNFs. The observed kinks in the current curve here were due to oxygen bubble evolution and elimination occurring at the electrode surface. During O2 bubble growth (Fig. 4A, inset: red arrow), the access of the electrolyte to some electrochemically active sites on the catalyst surface was blocked, which led to a decrease in the current density. With the “grown-up” bubbles detaching from the electrode surface, the previously blocked active sites were fully revived such that the current density was recovered instantly (Fig. 4A, blue arrow).
To further improve the current density, a more conductive Ni foam with a 3D porous structure was used as the support in the air-cathode with a C-NiPAN900-300 loading of ∼5 mg cm−2. The i–t experiments were performed at the same overpotential (384 mV) but in 1 M KOH for higher electrolyte conductivity (Fig. 4B). It is worth noting that the electrode was deliberately kept vertical during the tests in order to facilitate the release of small O2 bubbles at the earlier stage, which minimizes the disturbance caused by the repeated build-up and release of notably large O2 bubbles. As anticipated, the current density was increased two-fold (7 → 23 mA cm−2), with a fading rate of about 12% over a similar period (3 h) of testing. In sharp contrast, a blank Ni foam tested under the same conditions only gave a current density as small as ∼2.5 mA cm−2, suggesting that the origin of the high current density is the high OER activity of C-NiPAN900-300 in the air-electrode. This was further verified in the experiments using OER inactive carbon cloth as the conductive support, in which a high OER current was observed with C-NiPAN900-300 being loaded, while almost no current was detected in its absence (Fig. S4A, ESI†). Meanwhile, the stable OER current was sustained over a similar length of time (Fig. S4B, ESI†), showing little dependence on the nature of the conductive substrate. On the C-NiPAN900-300/Ni foam air-electrode, the potential required to achieve a current density of 10 mA cm−2 is about 1.507 V (vs. RHE) (Fig. 4C), which corresponds to an overpotential of 277 mV. This value is notably lower than that of many reported highly efficient Ni-based OER catalysts19 in a 1 M KOH or NaOH electrolyte (Table S2, ESI†), further confirming the superior OER activity of C-NiPAN900-300. The incorporation of C-NiPAN900-300 in the form of a free-standing mat onto the Ni foam to produce a binder-free electrode for further improved performances, however, remains a challenge. Foremost, one has to deal with the CNF mat/Ni foam interface to lower the contact resistance, requiring seamless integration which is difficult to achieve in the binder-free mode. Moreover, high mechanical strength is needed for the C-NiPAN900-300 mat to survive manipulations in the integration process with the Ni foam. This is part of our on-going research.
The good stability of C-NiPAN900-300 is further validated using an accelerated degradation test (ADT) through continuous OER scans at 20 mV s−1 (Fig. S5A, ESI†). A difference of as small as 13 mV was observed over 500 scans at a current density of 10 mA cm−2. Interestingly, a similar ADT on C-NiPAN900 leads to a clear improvement in the OER activity over the initial tens of scans (Fig. S5B & C, ESI†), rather than the commonly observed degradation in activity. After about 100 LSV scans, C-NiPAN900 reached its best OER performance which is very close to that of the thermally oxidized product, i.e. C-NiPAN900-300 (Fig. S5D, ESI†). Such an enhancement in the OER activity likely originated from the Ni electrochemical oxidation over consecutive LSV scans.8a,b,20 In these repeating scans metallic Ni NPs on CNFs were oxidized electrochemically, leading to a gradual material conversion to Ni–NiOx/CNF hybrid nanofibers with a composition similar to that of C-NiPAN900-300 (obtained via mild thermal oxidization in air). The small difference in both OER activity and stability between the two electrodes obtained from different approaches suggests that C-NiPAN900 is a robust “pre-electrode”, which can be easily activated for high OER activity via a simple thermal or electrochemical oxidation. The scalable C-NiPAN900 fibres with finely tunable physical parameters have enabled a smart design of hybrid electrodes for a desired catalytic performance, with C-NiPAN900-300 as an excellent example.
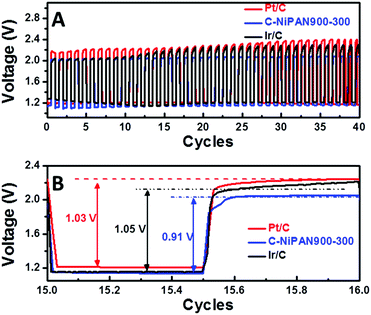
With metal oxide NPs anchored on nitrogen-doped CNFs, C-NiPAN900-300 is expected to exhibit good ORR activity as well. This was confirmed in the cyclic voltammetry (CV) scans (Fig. S6, ESI†), where a pronounced peak with a sharp slope emerged as the electrolyte was switched from the O2-depleted to the O2-rich state. Being active towards both OER and ORR, it was a natural choice to assess C-NiPAN900-300 as a bifunctional air-cathode in ZnABs using home-built cells.11 Similarly, Pt/C and Ir/C were taken as benchmark catalysts to prepare air cathodes following the same procedure, and the resultant batteries were tested under similar conditions. From the charge and discharge polarization curves (Fig. S7, ESI†) one can see that the batteries using C-NiPAN900-300 or Ir/C in the air cathode delivered similar discharge voltage at the same current densities, which however, is slightly lower than that of the one with Pt/C as the catalyst. Nevertheless, the lower charge potential of C-NiPAN900-300 and Ir/C compensates for the shortage in the discharge, making them comparably as good as Pt/C in the overall performance. As the 3 batteries were cycled at a current density of 5 mA cm−2, the advantage of the battery using C-NiPAN900-300 as the catalyst in the air-cathode is evidently clear (Fig. 5). The charge voltage was ∼2.0 V (Fig. 5A, blue line) which is similar to the one with the Ir/C catalyst (Fig. 5A, black line, 2.0 V), but notably lower, and hence better, than the ZnABs with Pt/C as the catalyst (Fig. 5A, red line, 2.2 V). Moreover, the C-NiPAN900-300-based ZnAB displays a more stable charge voltage compared to the ZnABs with Pt/C or Ir/C, as seen in the first 40 cycles of discharge and charge tests (Fig. 5A). The superior performance in terms of both lower charge potential and higher stability can be traced to the excellent OER activity of C-NiPAN900-300 which was revealed through electrochemical studies (Fig. 3). In the discharge process, the C-NiPAN900-300-based ZnAB even showed an increase in voltage, which is in sharp contrast to the other two with some voltage degradation. Therefore, despite a slightly lower start discharge voltage (1.14 vs. 1.21 or 1.16 V for Pt/C or Ir/C), the voltage gap (the energy loss in the charge–discharge reactions) for the C-NiPAN900-300-based ZnAB was about 120–140 mV smaller than that of the latter two (Fig. 5B). If the cycling performances of the three ZnABs are compared in 40 cycles of charge–discharge tests (Fig. 5A), one can easily spot the C-NiPAN900-300-based ZnAB as the more stable one with a noticeably higher energy efficiency (or a smaller voltage gap). Such higher energy efficiency and the notably improved cycling stability are highly desirable for rechargeable ZnABs. The superior performance, together with the advantages of easy fabrication on a large scale and at a low-cost, makes C-NiPAN900-300 an appealing catalyst for future ZnABs and other OER catalyst-critical applications.
Conclusions
In summary, we have developed an efficient composite OER catalyst of highly conductive CNFs decorated with Ni/NiOx NPs (C-NiPAN900-300), by electrospinning a nickel-salt containing polymer solution with subsequent thermal treatments. Thanks to its high OER activity and excellent stability, C-NiPAN900-300 equips the resultant ZnAB with multiple merits, such as a lower and more stable charge voltage, a smaller charge–discharge voltage gap, as well as a notably improved cycling stability as compared to its counterparts using Pt/C or Ir/C as the catalyst. The post-carbonization thermal treatment on the “pre-electrode” of C-NiPAN900, interestingly, can be replaced by consecutive LSV scans which produce essentially the same catalyst with a similar OER performance. This work not only introduces an attractive low-cost, scalable and high-performance oxygen electrocatalyst for practical ZnAB applications, but also further demonstrates the high applicability and effectiveness of the strategy of coupling the electrospinning technique with simple thermal treatments in the development of high performance OER catalysts. Such a strategy is anticipated to be universal for the production of various catalysts with far-reaching benefits to advanced technologies for sustainable development.Acknowledgements
This research was conducted under the project of IMRE/12-2P0504, funded by the Advanced Energy Storage Research Programme and supported by the Science and Engineering Research Council (SERC) of A*STAR (Agency for Science, Technology and Research).Notes and references
- (a) M. Görlin, P. Chernev, J. Ferreira de Araújo, T. Reier, S. Dresp, B. Paul, R. Krähnert, H. Dau and P. Strasser, J. Am. Chem. Soc., 2016, 138, 5603–5614 CrossRef PubMed; (b) C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed; (c) J. Wang, H. X. Zhong, Z. L. Wang, F. L. Meng and X. B. Zhang, ACS Nano, 2016, 10, 2342–2348 CrossRef CAS PubMed.
- (a) J.-S. Lee, S. T. Kim, R. Cao, N.-S. Choi, M. Liu, K. T. Lee and J. Cho, Adv. Energy Mater., 2011, 1, 34–50 CrossRef CAS; (b) F. Cheng and J. Chen, Chem. Soc. Rev., 2012, 41, 2172–2192 RSC.
- (a) M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed; (b) Y. Lee, J. Suntivich, K. J. May, E. E. Perry and Y. Shao-Horn, J. Phys. Chem. Lett., 2012, 3, 399–404 CrossRef CAS PubMed.
- (a) C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed; (b) W. T. Hong, M. Risch, K. A. Stoerzinger, A. Grimaud, J. Suntivich and Y. Shao-Horn, Energy Environ. Sci., 2015, 8, 1404–1427 RSC; (c) S. Liu, L. Hu, X. Xu, A. A. Al-Ghamdi and X. Fang, Small, 2015, 11, 4267–4283 CrossRef CAS PubMed.
- L.-A. Stern and X. Hu, Faraday Discuss., 2014, 176, 363–379 RSC.
- X. Yu, Y. Feng, B. Guan, X. W. D. Lou and U. Paik, Energy Environ. Sci., 2016, 9, 1246–1250 CAS.
- (a) O. Diaz-Morales, I. Ledezma-Yanez, M. T. M. Koper and F. Calle-Vallejo, ACS Catal., 2015, 5, 5380–5387 CrossRef CAS; (b) M. Gao, W. Sheng, Z. Zhuang, Q. Fang, S. Gu, J. Jiang and Y. Yan, J. Am. Chem. Soc., 2014, 136, 7077–7084 CrossRef CAS PubMed.
- (a) M. Alsabet, M. Grden and G. Jerkiewicz, Electrocatalysis, 2011, 2, 317–330 CrossRef CAS; (b) G.-Q. Han, Y.-R. Liu, W.-H. Hu, B. Dong, X. Li, X. Shang, Y.-M. Chai, Y.-Q. Liu and C.-G. Liu, Appl. Surf. Sci., 2015, 359, 172–176 CrossRef CAS; (c) K. Fominykh, P. Chernev, I. Zaharieva, J. Sicklinger, G. Stefanic, M. Döblinger, A. Müller, A. Pokharel, S. Böcklein, C. Scheu, T. Bein and D. Fattakhova-Rohlfing, ACS Nano, 2015, 9, 5180–5188 CrossRef CAS PubMed.
- (a) X. Zhou, Z. Xia, Z. Zhang, Y. Ma and Y. Qu, J. Mater. Chem. A, 2014, 2, 11799–11806 RSC; (b) Y. Feng, H. Zhang, Y. Zhang, X. Li and Y. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 9203–9210 CrossRef CAS PubMed; (c) D. U. Lee, B. J. Kim and Z. Chen, J. Mater. Chem. A, 2013, 1, 4754–4762 RSC; (d) J. Wang, H. X. Zhong, Y.-L. Qin and X. B. Zhang, Angew. Chem., Int. Ed., 2013, 52, 5248–5253 CrossRef CAS PubMed; (e) H. X. Zhong, J. Wang, F. L. Meng and X. B. Zhang, Angew. Chem., Int. Ed., 2016, 55, 9937–9941 CrossRef CAS PubMed; (f) J. Wang, K. Li, H. X. Zhong, D. Xu, Z. L. Wang, Z. Jiang, Z. J. Wu and X. B. Zhang, Angew. Chem., Int. Ed., 2015, 54, 10530–10534 CrossRef CAS PubMed.
- (a) Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed; (b) M. Gong, W. Zhou, M.-C. Tsai, J. Zhou, M. Guan, M.-C. Lin, B. Zhang, Y. Hu, D.-Y. Wang, J. Yang, S. J. Pennycook, B.-J. Hwang and H. Dai, Nat. Commun., 2014, 5, 4695 CrossRef CAS PubMed; (c) S. Chen and S. Z. Qiao, ACS Nano, 2013, 7, 10190–10196 CrossRef CAS PubMed.
- B. Li, X. Ge, F. W. T. Goh, T. S. A. Hor, D. Geng, G. Du, Z. Liu, J. Zhang, X. Liu and Y. Zong, Nanoscale, 2015, 7, 1830–1838 RSC.
- T. W. Hansen, A. T. De La Riva, S. R. Challa and A. K. Datye, Acc. Chem. Res., 2013, 46, 1720–1730 CrossRef CAS PubMed.
- H. Long, T. Shi, H. Hu, S. Jiang, S. Xi and Z. Tang, Sci. Rep., 2014, 4, 7413 CrossRef CAS PubMed.
- D. Y. Ma, G. Y. Shi, H. Z. Wang, Q. H. Zhang and Y. G. Li, Nanoscale, 2013, 5, 4808–4815 RSC.
- N. A. M. Barakat, B. Kim and H. Y. Kim, J. Phys. Chem. C, 2009, 113, 531–536 CAS.
- X. Liu, J. Liu and X. Sun, J. Mater. Chem. A, 2015, 3, 13900–13905 CAS.
- (a) S. Chen, J. Duan, J. Ran, M. Jaroniec and S. Z. Qiao, Energy Environ. Sci., 2013, 6, 3693–3699 RSC; (b) Q. Liu, J. Jin and J. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 5002–5008 CrossRef CAS PubMed; (c) J. W. Ren, M. Antonietti and T. P. Fellinger, Adv. Energy Mater., 2015, 5, 1401660 CrossRef; (d) S. Dou, L. Tao, J. Huo, S. Wang and L. Dai, Energy Environ. Sci., 2016, 9, 1320–1326 RSC.
- (a) T. Y. Ma, S. Dai, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2014, 136, 13925–13931 CrossRef CAS PubMed; (b) R. M. Yadav, J. Wu, R. Kochandra, L. Ma, C. S. Tiwary, L. Ge, G. Ye, R. Vajtai, J. Lou and P. M. Ajayan, ACS Appl. Mater. Interfaces, 2015, 7, 11991–12000 CrossRef CAS PubMed; (c) Y. Zhao, R. Nakamura, K. Kamiya, S. Nakanishi and K. Hashimoto, Nat. Commun., 2013, 4, 2390 Search PubMed.
- (a) L. Kuai, J. Geng, C. Chen, E. Kan, Y. Liu, Q. Wang and B. Geng, Angew. Chem., Int. Ed., 2014, 53, 7547–7551 CrossRef CAS PubMed; (b) K. Xu, P. Chen, X. Li, Y. Tong, H. Ding, X. Wu, W. Chu, Z. Peng, C. Wu and Y. Xie, J. Am. Chem. Soc., 2015, 137, 4119–4125 CrossRef CAS PubMed; (c) M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2013, 135, 8452–8455 CrossRef CAS PubMed; (d) F. Song and X. Hu, Nat. Commun., 2014, 5, 4477 CAS; (e) F. Song and X. Hu, J. Am. Chem. Soc., 2014, 136, 16481–16484 CrossRef CAS PubMed; (f) Y. Yang, H. Fei, G. Ruan, C. Xiang and J. M. Tour, ACS Nano, 2014, 8, 9518–9523 CrossRef CAS PubMed.
- M. Alsabet, M. Grden and G. Jerkiewicz, Electrocatalysis, 2014, 5, 136–147 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD, LSV curves of ADT tests of the C-NiPAN900 and C-NiPAN900-300 catalysts, CV curves of C-NiPAN900-300, and OER performance comparison of the catalysts reported and those in this work. DOI: 10.1039/c6qm00151c |
| This journal is © the Partner Organisations 2017 |