Monolithic hierarchical gold sponges for efficient and stable catalysis in a continuous-flow microreactor†
You
Yu
 *a,
Wenqing
Xiao
a,
Tongtong
Zhou
a,
Ping
Zhang
a,
Casey
Yan
bc and
Zijian
Zheng
*a,
Wenqing
Xiao
a,
Tongtong
Zhou
a,
Ping
Zhang
a,
Casey
Yan
bc and
Zijian
Zheng
 *bc
*bc
aKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069, China. E-mail: yuyou@nwu.edu.cn
bNanotechnology Center, Institute of Textiles and Clothing, the Hong Kong Polytechnic University, Hong Kong, China
cAdvanced Research Centre for Fashion and Textiles, the Hong Kong Polytechnic University Shenzhen Research Institute, Shenzhen, China. E-mail: tczzheng@polyu.edu.hk
First published on 23rd August 2016
Abstract
Monolithic hierarchical gold sponges are prepared by the polymer-assisted metal deposition method under air- and moisture-compatible conditions. These sponges feature excellent catalytic properties, robustness and chemical recyclability for the reduction reaction of 4-nitrophenol, and are also available for the scale-up catalysis of the same reaction in a continuous-flow microreactor.
Metal-based materials with large surface area-to-volume ratios have received considerable attention due to their potential applications in the fields of energy storage and conversion,1 optical sensors,2 catalysis chemistry3 and so on.4 In particular, metal particles and wires at the nano-/micro-scale have been demonstrated as promising candidates for catalysing many chemical reactions.5 However, these small particles and wires irreversibly aggregate in solution, thereby leading to a gradual decrease in catalytic activity as time goes on. To address these issues, one main strategy is to decorate these small metal catalysts with polymers,6 graphene sheets7 and others,8 or immobilize them on matrices of metal–organic frameworks,9 particles,10 fibres,11 and aerogels.12 The stability of these catalysts is therefore significantly improved and the catalytic reactivity is maintained at high levels throughout the reaction. Nevertheless, the fabrication as well as the separation process of these modified metal catalysts usually requires sophisticated and multiple steps,13 which are always tedious and undesirable in scale-up applications.
To get rid of any tedious fabrication and separation processes, another strategy is to fabricate monolithic metals with nano- and micro-pores, namely metal sponges that feature porous structures to provide superior catalytic properties (>90%) and kinetic constants (>0.5 × 10−3 s−1). These porous structures can not only make the metal more chemically reactive compared to their bulks,14 but can also avoid complicated preparation and time-consuming separation processes. With excellent catalytic properties toward chemical reactions, metal sponges are free standing (or self-supported) and highly conductive, showing broad applications in the field of materials science, such as promising electrode candidates for hydrogen revolution,15 water splitting,4d,16 and fabricating enzyme-free detectors,17 and energy conversion18 and storage devices.19 However, it should be noted that the fabrication of metal sponges often adopts either expensive bottom-up approaches in a vacuum20 or top-down removal strategies that require a high-temperature and/or corrosive environment,21 which does not allow ambient and mild fabrication at low cost. Even though vacuum-free electrochemical deposition can realize relatively low-cost metal deposition,22 it requires conductive substrates which significantly limit the wider use of non-conductive polymeric materials. Moreover, the as-deposited metal coatings often crack easily and detach from the substrate surface during catalysis due to weak physical adhesion between the metal and the substrate.23 As a consequence, the stability and catalytic properties of these metals are always poor.
To tackle the adhesion problem between the metal and the substrate, recently, it was found that long-chain functional polymers are helpful for immobilizing stable and robust metal nanoparticles (or thin films) on different non-conductive substrates.24 For example, by using the surface-initiated polymerization method, polymer brushes were covalently grafted from the surface of mesoporous silica films or nanoporous anodic aluminium oxide membranes.25 Subsequent metal ion loading and reduction could yield high-performance and durable metal thin films on the substrate surface. The as-prepared metal thin films showed high catalytic efficiency and stability for typical catalytic reactions. Still, polymer brushes are grafted via surface-initiated-atom transfer radical polymerization (SI-ATRP) which must be performed in an inert atmosphere for several hours. Therefore, it is difficult to scale-up the whole fabrication process. To date, how to fabricate highly efficient, reactive and stable metal sponges with scale-up potential in a facile, ambient and time-saving manner still remains a significant challenge.
Herein, we report a solution-processed, air- and moisture-compatible approach to prepare free-standing monolithic gold sponges by the polymer-assisted metal deposition method (PAMD). Functional polymers are grafted onto the supporting polyurethane (PU) sponges in a dip-coating fashion, followed by electroless deposition to form a uniform Au layer with excellent robustness and chemical stability. The Au sponge possesses an ultrahigh catalytic efficiency (∼95% conversion in several minutes) for the reduction reaction of 4-nitrophenol (4-NP) even after 100 times of repeated use. We also demonstrate a continuous-flow system made of the Au sponge, which allows separation-free, continuous catalytic reduction of flow-in liquid chemicals for a long time.
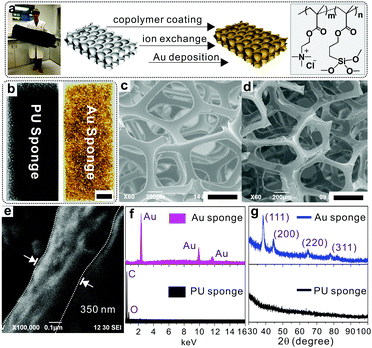
The fabrication process is illustrated in Fig. 1a, which includes three steps, namely co-polymer coating, ion exchange and gold deposition. The copolymer, i.e., poly[2-(methacryloyloxy)ethyl trimethylammonium chloride-co-3-(trimethoxysilyl)propyl methacrylate] (p(METAC-co-MPTS)), used in this study contains two kinds of functional groups: one is MPTS which is used to anchor the polymers onto the PU via a self-condensation reaction, while the other is METAC which acts as the reservoir of precursors for the subsequent electroless deposition of Au. In a typical fabrication, pristine PU sponges were immersed in an ethanol solution of the co-polymer and incubated in an ammonia atmosphere, which resulted in a uniform wrapping of p(METAC-co-MPTS) on the surface of the PU sponges. After that, the PdCl42− precursors for electroless deposition were loaded via an ion exchange process, where the Cl− in METAC was replaced by PdCl42− due to the high affinity of PdCl42− to the residue of quaternary ammonium in METAC. Au sponges were finally obtained by sequentially immersing the PdCl42−-loaded PU in electroless deposition solutions of copper then gold. As shown in Fig. 1b, the PU sponge was successfully coated with Au. The corresponding SEM images in Fig. 1c–e indicate that a 350 nm-thick Au film was uniformly and densely coated on the PU matrices. In addition, the successful fabrication of Au sponges was monitored by energy-dispersive X-ray spectroscopy (EDS). As shown in Fig. 1f and Fig. S1a (ESI†), after co-polymer coating, two new peaks, respectively, for Si (from MPTS) and Cl (from METAC) indicate that the co-polymer was successfully immobilized onto the PU surface. After ion exchange with PdCl42−, a series of peaks belonging to PdCl42− moieties were observed. Finally, three new peaks corresponding to gold signals were generated simultaneously and confirmed the successful deposition of Au. X-ray diffraction (XRD) was also used to further confirm the presence of gold (Fig. 1g and Fig. S1b, ESI†) with characteristic peaks ranging from 30° to 100°. Remarkably, the as-prepared Au sponges feature high conductivity (300 S cm−1) and comparable porosity with the pristine PU sponges. The reason for this can be ascribed to the fact that PAMD is a full solution-processed method to realize metal deposition under air- and moisture-compatible conditions. The polymers and small molecules used in each step can freely penetrate into the microstructures of the sponges by diffusion. Theoretically, it is feasible to fabricate metal sponges of different pore sizes.
Importantly, the whole fabrication process is time-saving and only takes ∼60 min to complete. Therefore, it shows great potential for fabricating Au sponges in a large scale.
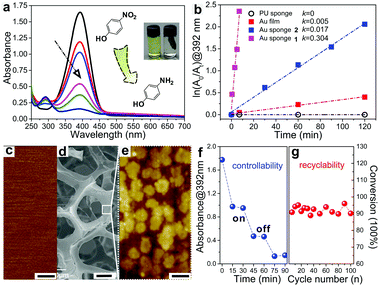
As a proof-of-concept, the as-fabricated Au sponges were employed to catalyse the reduction of 4-NP using sodium borohydride as a reducing agent. The product of the reaction, 4-aminophenol (4-AP), is one important chemical in drug and dyeing agent manufacturing and also in the photograph developing sector. By monitoring the decreased characteristic absorbance of 4-NP (392 nm) or the increased characteristic absorbance of 4-AP (292 nm) using UV-vis spectroscopy, the reduction progress can be traced.6c,26 Here, the as-fabricated Au sponges were immersed in an aqueous solution of 4-NP and sodium borohydride. The UV-vis spectra show that the intensity of the characteristic absorbance of 4-NP at 392 nm gradually decreased as the reduction proceeded (Fig. 2a). At the same time, a new peak was simultaneously generated at 292 nm, indicating that the reduction of 4-NP to 4-AP could be catalysed by the Au sponges as predicted.
To further understand the catalytic mechanism of 4-NP using the Au sponge as a catalyst, the reduction of 4-NP was systematically studied. ln(A0/At) at 392 nm was plotted against reaction times and the reduction of 4-NP to 4-AP was found to follow a first-order kinetics when excessive sodium borohydride was used (Fig. 2b). Therefore, the obtained rate constants (k) were utilized as the criterion to evaluate the catalytic properties in the following studies. When the reduction was carried out using pristine PU sponges as a catalyst, no decrease in absorbance at 392 nm was observed upon stirring for a long time (120 min), showing that there was no reduction of 4-NP (k = 0 s−1). However, when the as-prepared Au sponge 1 was immersed in the same solution, the characteristic absorbance peak of 4-NP disappeared within a few minutes as shown in Fig. 2a and b (k = 0.304 s−1). This result demonstrated that Au sponges played a key role in this catalytic reaction and further supported our prediction mentioned above. We also evaluated the effect of different sizes of the Au sponges on the catalytic rate of 4-NP. When a smaller Au sponge 2 (13.3% in volume ratio to 1) was employed, the reduction rate of 4-NP significantly decreased and the value of k was only 5.6% to that of Au sponge 1 (Fig. 2b). It is easy to understand the reason for that: the smaller sponge possesses a smaller surface area which decreases both the transmission rate of electrons and the possibility of Au–H bond formation between Au sponges and sodium borohydride.26,27 If the surface area of the sponge decreases further, the rate will sharply decrease. To confirm that we evaporated gold films on a flat substrate to obtain a smooth gold surface with a roughness of ∼1.2 nm to catalyse the same reaction (Fig. 2c). The reaction rate was found to be only 1.5% of that of Au sponge 1.
Table 1 lists the catalytic efficiencies for reducing 4-NP to 4-AP using various gold-based catalytic systems. The as-prepared Au sponges show ultrahigh efficiency (∼95% conversion in several minutes) and a kinetic constant of 5.0 × 10−3 s−1, which are among the best performing systems. The outstanding catalytic performance of the Au sponges is mainly attributed to the following reasons. First, the surface of the Au film on the sponge deposited by ELD is rough (roughness ∼28.3 nm), where there are lots of clusters of gold nanoparticles with a size of 100–400 nm aggregated on the surface of Au sponges (Fig. 2e). Second, the structures of the Au sponges are interconnected with each other, featuring numerous micropores that increase the specific surface area (Fig. 2d). Such a hierarchical structure of Au made them more chemically reactive than that of the corresponding macroscopic metals (i.e., flat gold films), promoting the reaction rates (Fig. S2, ESI†).4c,26,27 Compared with these listed strategies, Au sponges demonstrated here can be readily prepared under very mild conditions, and the sophisticated processes for fabricating and separating catalysts are not required. In addition, the on/off catalytic reaction can be easily controlled by immersing the Au sponges in or pulling them out from the solutions (Fig. 2f). More importantly, the as-prepared Au sponges are stable throughout the reaction and can provide excellent catalytic efficiency of more than 90% even after performing the catalytic reaction 100 times (Fig. 2g).
| Entry | k app (×10−3 (s−1)) | Structures | Separation | m Au/[4-NP] |
|---|---|---|---|---|
| 1 | 0.7–14 | Au NPs with ligands26 | Not mentioned | Not mentioned |
| 2 | 1.3–8.5 | Au clusters18b | Not mentioned | 30 μg/1.67 mM |
| 3 | 6.1 | Au NPs@GO sheets7b | Centrifugation | 4 μg/1.54 mM |
| 4 | 0.68–12 | Au NPs@fibrous silica microspheres10d | Centrifugation | 0.5 mg/0.06 mM |
| 5 | 14 | Au NPs@Fe3O4@SiO210e | Magnetic force | Not mentioned/0.08 mM |
| 6 | 8.0 | AuNPs@fiber11b | Decantation | 0.5 mg/3 mM |
| 7 | 6.3 | Au NPs@porous films6c | Filtration | 1.8 mg/1.67 mM |
| 8 | 1.1 | Au NPs@hydrogel12b | Not mentioned | 12 μg/0.6 mM |
| 9 | 4.0 | Au foam@diatom23d | Filtration | 0.3 mg/8 μM |
| 10 | 5.0 | Monolithic Au sponges (this work) | Pulling out | 3 mg/1.67 mM |
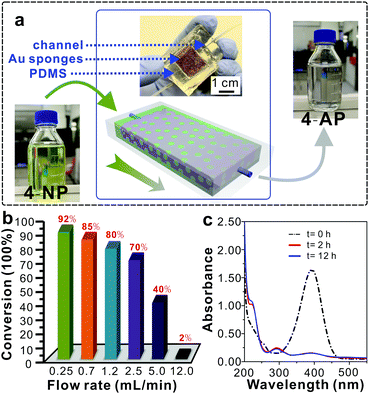
Taking advantage of the outstanding catalytic efficiency, excellent chemical stability and recyclability of the Au sponges, we designed a continuous-flow catalytic system to realize the scale-up reduction of 4-NP (Fig. 3a). To this end, a Au sponge with a thickness of 4 mm and a width by length of 5 × 60 mm2 was embedded in a fluidic channel where a total of 500 mL of 4-NP solution slowly passed through pores within the sponge and was continuously reduced to colourless 4-AP (Fig. 3a). In this system, it was found that the flow rate had a profound influence on the reduction efficiency of 4-NP in which the efficiency increased with decreased flow rate (Fig. 3b). The specific conversion was up to 92% at a flow rate of 0.24 mL min−1, which was very close to that in the intermittent way (shown in Fig. 2). Moreover, this continuous-flow process was monitored by UV-vis spectroscopy, and it was found that there was no obvious difference detected between the two samples that were obtained after 2 h and 12 h of reduction (Fig. 3c). This result indicates that the Au sponges featured excellent stability and high efficiency for the reduction reaction of 4-NP in the continuous-flow reaction system.
In conclusion, we have prepared free-standing monolithic Au sponges via the PAMD method under air- and moisture-compatible conditions. The as-prepared Au sponges are very stable, and feature high catalytic efficiency and excellent recyclability for the reduction of 4-NP. Moreover, they possess the ability to scale-up catalytic reactions in a continuous-flow fashion. By using commercial porous supports and the PMAD method, this kind of Au sponge can be fabricated without using expensive vacuum equipment and sophisticated chemistry, showing great potential applications in industrial fields. Not limited to PU matrices, Au can be stabilized onto arbitrary substrates with the assistance of functional polymer layers, especially for substrates that cannot withstand high temperature, organic solvent, and extreme acid (and/or alkaline) conditions. Furthermore, many other noble metal sponges can also be prepared by this approach. It is anticipated that this novel strategy and the as-prepared Au sponges can be used in sensors, water-purifiers, chemical catalysis and other related fields.
We acknowledge the Natural Science Foundation of Shaanxi Province (2016JQ5035), NFFTBS (No. J1210057), the Research Grant Council of Hong Kong (PolyU5036/13P, Poly5030/12P), and the NSFC (21125316, 21434009, 21604069) for the financial support of this work.
Notes and references
- (a) L. Wen, Z. Wang, Y. Mi, R. Xu, S.-H. Yu and Y. Lei, Small, 2015, 11, 3408 CrossRef CAS PubMed; (b) N. P. Dasgupta, J. Sun, C. Liu, S. Brittman, S. C. Andrews, J. Lim, H. Gao, R. Yan and P. Yang, Adv. Mater., 2014, 26, 2137 CrossRef CAS PubMed.
- (a) P.-C. Hsu, D. Kong, S. Wang, H. Wang, A. J. Welch, H. Wu and Y. Cui, J. Am. Chem. Soc., 2014, 136, 10593 CrossRef CAS PubMed; (b) S. Ye, A. R. Rathmell, Z. Chen, I. E. Stewart and B. J. Wiley, Adv. Mater., 2014, 26, 6670 CrossRef CAS PubMed; (c) G. Zheng, L. Polavarapu, L. M. Liz-Marzan, I. Pastoriza-Santos and J. Perez-Juste, Chem. Commun., 2015, 51, 4572 RSC.
- (a) J. Biener, M. M. Biener, R. J. Madix and C. M. Friend, ACS Catal., 2015, 5, 6263 CrossRef CAS; (b) H. E. Ho, Y. Ishikawa, N. Asao, Y. Yamamoto and T. Jin, Chem. Commun., 2015, 51, 12764 RSC.
- (a) S. Bhanushali, P. Ghosh, A. Ganesh and W. Cheng, Small, 2015, 11, 1232 CrossRef CAS PubMed; (b) S. Gong, D. T. H. Lai, B. Su, K. J. Si, Z. Ma, L. W. Yap, P. Guo and W. Cheng, Adv. Electron. Mater., 2015, 1, 1400063 CrossRef; (c) C.-H. Kuo, W. Li, L. Pahalagedara, A. M. El-Sawy, D. Kriz, N. Genz, C. Guild, T. Ressler, S. L. Suib and J. He, Angew. Chem., Int. Ed., 2015, 54, 2345 CrossRef CAS PubMed; (d) B. Liu, H. Yao, W. Song, L. Jin, I. M. Mosa, J. F. Rusling, S. L. Suib and J. He, J. Am. Chem. Soc., 2016, 138, 4718 CrossRef CAS PubMed.
- (a) J. Zhang, L. Zhong, Y. Sun, A. Li, J. Huang, F. Meng, B. K. Chandran, S. Li, L. Jiang and X. Chen, Adv. Mater., 2016, 20, 2978 CrossRef PubMed; (b) X. Zhang and Z. Su, Adv. Mater., 2012, 24, 4574 CrossRef CAS PubMed; (c) Y. Zhu, L. Fan, B. Yang and J. Du, ACS Nano, 2014, 8, 5022 CrossRef CAS PubMed.
- (a) A. Zinchenko, Y. Miwa, L. I. Lopatina, V. G. Sergeyev and S. Murata, ACS Appl. Mater. Interfaces, 2014, 6, 3226 CrossRef CAS PubMed; (b) M. Chen, H. Kang, Y. Gong, J. Guo, H. Zhang and R. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 21717 CrossRef CAS PubMed; (c) Y. Ye, M. Jin and D. Wan, J. Mater. Chem. A, 2015, 3, 13519 RSC.
- (a) Y. Fang and E. Wang, Nanoscale, 2013, 5, 1843 RSC; (b) T. Wu, L. Zhang, J. Gao, Y. Liu, C. Gao and J. Yan, J. Mater. Chem. A, 2013, 1, 7384 RSC; (c) H. Liu, J. Wang, Z. Feng, Y. Lin, L. Zhang and D. Su, Small, 2015, 11, 5059 CrossRef CAS PubMed.
- (a) L. Peng, J. Zhang, S. Yang, B. Han, X. Sang, C. Liu, X. Ma and G. Yang, Chem. Commun., 2015, 51, 4398 RSC; (b) A. Shivhare, S. J. Ambrose, H. Zhang, R. W. Purves and R. W. J. Scott, Chem. Commun., 2013, 49, 276 RSC.
- (a) A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2012, 41, 5262 RSC; (b) H. Li, B. Meng, S.-H. Chai, H. Liu and S. Dai, Chem. Sci., 2016, 7, 905 RSC.
- (a) Q. Ji, J. P. Hill and K. Ariga, J. Mater. Chem. A, 2013, 1, 3600 RSC; (b) Z. Jin, F. Wang, F. Wang, J. Wang, J. C. Yu and J. Wang, Adv. Funct. Mater., 2013, 23, 2137 CrossRef CAS; (c) S. Wang, Q. Zhao, H. Wei, J.-Q. Wang, M. Cho, H. S. Cho, O. Terasaki and Y. Wan, J. Am. Chem. Soc., 2013, 135, 11849 CrossRef CAS PubMed; (d) H. Yang, S. Li, X. Zhang, X. Wang and J. Ma, J. Mater. Chem. A, 2014, 2, 12060 RSC; (e) J. Zheng, Y. Dong, W. Wang, Y. Ma, J. Hu, X. Chen and X. Chen, Nanoscale, 2013, 5, 4894 RSC.
- (a) Z. Zhang, C. Shao, Y. Sun, J. Mu, M. Zhang, P. Zhang, Z. Guo, P. Liang, C. Wang and Y. Liu, J. Mater. Chem., 2012, 22, 1387 RSC; (b) M.-L. Wang, T.-T. Jiang, Y. Lu, H.-J. Liu and Y. Chen, J. Mater. Chem. A, 2013, 1, 5923 RSC.
- (a) G. Nyström, M. P. Fernández-Ronco, S. Bolisetty, M. Mazzotti and R. Mezzenga, Adv. Mater., 2016, 28, 472 CrossRef PubMed; (b) G. Marcelo, M. López-González, F. Mendicuti, M. P. Tarazona and M. Valiente, Macromolecules, 2014, 47, 6028 CrossRef CAS.
- (a) E.-M. Felix, M. Antoni, I. Pause, S. Schaefer, U. Kunz, N. Weidler, F. Muench and W. Ensinger, Green Chem., 2016, 18, 558 RSC; (b) A. M. Khalil, V. Georgiadou, M. Guerrouache, S. Mahouche-Chergui, C. Dendrinou-Samara, M. M. Chehimi and B. Carbonnier, Polymer, 2015, 77, 218 CrossRef CAS.
- (a) B. C. Tappan, S. A. Steiner and E. P. Luther, Angew. Chem., Int. Ed., 2010, 49, 4544 CrossRef CAS PubMed; (b) A. Wittstock and M. Bäumer, Acc. Chem. Res., 2014, 47, 731 CrossRef CAS PubMed; (c) V. Zielasek, B. Jürgens, C. Schulz, J. Biener, M. M. Biener, A. V. Hamza and M. Bäumer, Angew. Chem., Int. Ed., 2006, 45, 8241 CrossRef CAS PubMed.
- (a) B. You, N. Jiang, M. Sheng, M. W. Bhushan and Y. Sun, ACS Catal., 2016, 6, 714 CrossRef CAS; (b) X. Wang, Y. V. Kolen'ko, X.-Q. Bao, K. Kovnir and L. Liu, Angew. Chem., Int. Ed., 2015, 54, 8188 CrossRef CAS PubMed.
- (a) M. Gong, W. Zhou, M. J. Kenney, R. Kapusta, S. Cowley, Y. Wu, B. Lu, M.-C. Lin, D.-Y. Wang, J. Yang, B.-J. Hwang and H. Dai, Angew. Chem., Int. Ed., 2015, 54, 11989 CrossRef CAS PubMed; (b) S. Chen and S.-Z. Qiao, ACS Nano, 2013, 7, 10190 CrossRef CAS PubMed.
- (a) J. Huang, H. Li, Y. Zhu, Q. Cheng, X. Yang and C. Li, J. Mater. Chem. A, 2015, 3, 8734 RSC; (b) A. Wittstock, J. Biener and M. Baumer, Phys. Chem. Chem. Phys., 2010, 12, 12919 RSC.
- (a) Y. Li, Y. He and W. Yang, J. Power Sources, 2015, 278, 569 CrossRef CAS; (b) H. Choi, O.-H. Kim, M. Kim, H. Choe, Y.-H. Cho and Y.-E. Sung, ACS Appl. Mater. Interfaces, 2014, 6, 7665 CrossRef CAS PubMed.
- (a) Y. Yang, L. Li, G. Ruan, H. Fei, C. Xiang, X. Fan and J. M. Tour, ACS Nano, 2014, 8, 9622 CrossRef CAS PubMed; (b) H. Chen, L. Hu, M. Chen, Y. Yan and L. Wu, Adv. Funct. Mater., 2014, 24, 934 CrossRef CAS; (c) X. Lang, A. Hirata, T. Fujita and M. Chen, Nat. Nanotechnol., 2011, 6, 232 CrossRef CAS PubMed; (d) L. Liu, Y. Yu, C. Yan, K. Li and Z. Zheng, Nat. Commun., 2015, 6, 7260 CrossRef CAS PubMed.
- S. Sattayasamitsathit, A. M. O'Mahony, X. Xiao, S. M. Brozik, C. M. Washburn, D. R. Wheeler, W. Gao, S. Minteer, J. Cha, D. B. Burckel, R. Polsky and J. Wang, J. Mater. Chem., 2012, 22, 11950 RSC.
- (a) X. Guo, W. Ye, H. Sun, Q. Zhang and J. Yang, Nanoscale, 2013, 5, 12582 RSC; (b) I. McCue, E. Benn, B. Gaskey and J. Erlebacher, Annu. Rev. Mater. Res., 2016, 46, 263 CrossRef CAS; (c) Z. Qi and J. Weissmüller, ACS Nano, 2013, 7, 5948 CrossRef CAS PubMed.
- F. S. Karoonian, M. Etesami, M. A. Hasnat and N. Mohamed, Catal. Commun., 2012, 21, 14 CrossRef CAS.
- (a) J.-H. Kim, K. M. Twaddle, J. Hu and H. Byun, ACS Appl. Mater. Interfaces, 2014, 6, 11514 CrossRef CAS PubMed; (b) B. Le Droumaguet, R. Poupart and D. Grande, Polym. Chem., 2015, 6, 8105 RSC; (c) M. Mohan, N. Mohan and D. K. Chand, J. Mater. Chem. A, 2015, 3, 21167 RSC; (d) Y. Yu, J. Addai-Mensah and D. Losic, Langmuir, 2010, 26, 14068 CrossRef CAS PubMed.
- (a) T. Du, B. Li, X. Wang, B. Yu, X. Pei, W. T. S. Huck and F. Zhou, Angew. Chem., Int. Ed., 2016, 55, 4260 CrossRef CAS PubMed; (b) Y. Yu, X. Xiao, Y. Zhang, K. Li, C. Yan, X. Wei, L. Chen, H. Zhen, H. Zhou, S. Zhang and Z. Zheng, Adv. Mater., 2016, 28, 4926 CrossRef CAS PubMed; (c) Y. Yu, C. Yan and Z. Zheng, Adv. Mater., 2014, 26, 5508 CrossRef CAS PubMed; (d) Y. Yu, Y. Zhang, K. Li, C. Yan and Z. Zheng, Small, 2015, 11, 3444 CrossRef CAS PubMed.
- (a) A. Calvo, M. C. Fuertes, B. Yameen, F. J. Williams, O. Azzaroni and G. J. A. A. Soler-Illia, Langmuir, 2010, 26, 5559 CrossRef CAS PubMed; (b) J. Liu, S. Ma, Q. Wei, L. Jia, B. Yu, D. Wang and F. Zhou, Nanoscale, 2013, 5, 11894 RSC.
- R. Ciganda, N. Li, C. Deraedt, S. Gatard, P. Zhao, L. Salmon, R. Hernandez, J. Ruiz and D. Astruc, Chem. Commun., 2014, 50, 10126 RSC.
- M. Eo, J. Baek, H. D. Song, S. Lee and J. Yi, Chem. Commun., 2013, 49, 5204 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supporting figures. See DOI: 10.1039/c6qm00115g |
| This journal is © the Partner Organisations 2017 |