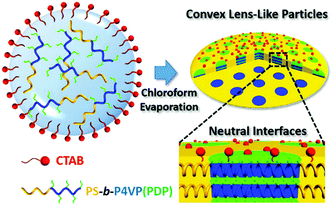
Fabrication of convex lens-shaped polymer particles by tuning the interfacial interaction†
Jiangping
Xu
,
Yi
Yang
,
Ke
Wang
,
Yuqing
Wu
and
Jintao
Zhu
*
Key Laboratory of Materials Chemistry for Energy Conversion and Storage, Ministry of Education, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan 430074, China. E-mail: jtzhu@mail.hust.edu.cn
First published on 5th September 2016
Abstract
In this communication, we report a facile yet effective approach to fabricate polystyrene-b-poly(4-vinyl pyridine) (PS-b-P4VP) convex lens (CL)-like microparticles with hexagonally stacked cylindrical P4VP domains via three-dimensional confined assembly. Addition of a hydrogen bonding agent, 3-n-pentadecylphenol (PDP), not only changes the volume fraction of the P4VP domain, but also alters the interfacial interactions between the blocks and the surfactant. A neutral interface could be created by tuning the content of PDP, resulting in the formation of CL-like microparticles. Selective swelling and then deswelling of these particles offers us a convenient way to synthesize isoporous particles with tunable pore size, potentially useful in ultrafiltration with high selectivity.
Structured block copolymer (BCP) particles have attracted significant attention owing to their various applications in photonics,1,2 sensing,3 template synthesis,4–6 and as catalytic substrates.7 Confined assembly of BCPs in small droplets could be especially useful in fabricating particles with unconventional shapes, internal structures, and surface properties.8–14 Under three-dimensional (3D) confinement, the microphase separation of BCPs gives access to unique shapes and structures, which could be tailored by changing the volume fraction of blocks, particle size, and interfacial interactions between each block and the surrounding medium.10,11,15,16 Among these factors, the interfacial interaction is especially important due to the large surface area/volume ratio of the BCP particles.17,18 Generally, the properties of the surfactants govern the interfacial interaction and thus can shape the morphology of the particles. Manipulation of the interfacial interaction between BCP blocks and the surrounding medium can be achieved by applying mixed surfactants, which have different affinities for each block of the BCP chain.17,19–24
Recently, shaped anisotropic particles have gained increasing interest due to their unique properties in optics24 or cell internalization.25 By precisely tailoring the interfacial interaction, anisotropic particles from BCPs can be readily obtained via an emulsion–solvent evaporation technique. This facile and scalable approach allows control over not only the particle internal structure, but also their overall shape. It is of great importance to control the morphology of BCP colloids by independently and selectively tailoring the interfacial properties at the desired location along the interface. For example, mixtures of surfactants with different affinities to BCP blocks were introduced to control the shape and the internal structure of the particles.12,17,19,20 Moreover, inorganic particles with different surface properties could also be applied as surfactants to tune the interfacial interactions and thus morphology of the particles.22–24,26 Highly specific functional properties of BCP particles are achieved by simultaneously controlling shape anisotropy and the internal structure from nano- to micro-meter length scale. In particular, the convex lens (CL)-like particles19,20 with hexagonally packed cylinders perpendicular to the long axis are of significant interest due to their potential application in concentrating light with enhanced near-field signals.24 Generally, to fabricate such particles, two types of surfactants with different affinities were essential for creating a neutral interface for each block. To the best of our knowledge, these particles with a unique shape could not be obtained by using a single surfactant.
Under 3D confinement, the properties of the interface are mainly governed by the properties of surfactants. On the other hand, the properties of the BCP matrix also significantly affect the interfacial interaction. Herein, we report the manipulation of interfacial interaction by introducing a hydrogen-bonding agent (3-n-pentadecylphenol, PDP) into the polystyrene-b-poly(4-vinyl pyridine) (PS-b-P4VP) matrix. The affinities of the surfactants to BCP chains are tailored by varying the content of PDP, rather than altering the surfactants. Based on this strategy, a neutral interface can be easily generated and consequently the CL-like microparticles can be obtained by using a single surfactant (Scheme 1).
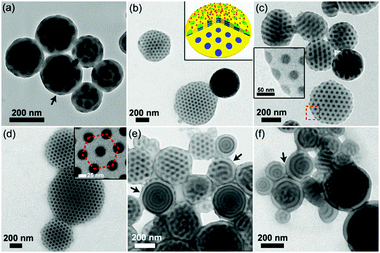
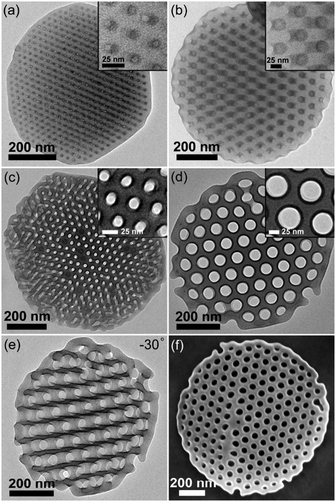
The asymmetric PS51K-b-P4VP18K (subscripts are the Mn of the blocks) was employed to fabricate BCP particles through an emulsion–solvent evaporation method by using cetyltrimethylammonium bromide (CTAB, 3 mg mL−1) as the emulsifier (see experimental details in the ESI†). After evaporation of chloroform from the emulsion droplets, particles with P4VP discrete spherical domains (volume fraction of P4VP: fP4VP = 24.4 vol%) and PS on the particle surface are obtained (Fig. 1a), since CTAB selectively wets PS domains.12,17,24 When the hydrogen bond assisted supramolecules PS51K-b-P4VP18K(PDP)x (x is the molar ratio of PDP to 4VP) are introduced to form the particles, the morphology of the particles changes significantly with the increase of x (Fig. 1b–f). The CL-like microparticles with hexagonally packed P4VP(PDP) cylindrical domains start to appear at x = 0.05 and become the dominant morphology at x = 0.2 (Fig. 1d). The tilted TEM images (Fig. S1, ESI†) indicate that the P4VP(PDP) cylinders persist throughout the entire thickness of the BCP particles. The SEM image (Fig. S2a and b, ESI†) confirms the CL-like shape of the particles, and the Voronoi diagram (Fig. S2c and d, ESI†) indicates that the cylindrical P4VP(PDP) domains are well-oriented and highly ordered.24,27 Both PS and P4VP(PDP) can locate on the surface of the CL-like particles (Fig. 1c), implying a neutral interface for both blocks. PDP has a long alkyl tail, which increases the affinity of the P4VP(PDP) domain to CTAB. Moreover, the hydrophilic phenol head of PDP increases the hydrophilicity of the P4VP(PDP) block. Therefore, P4VP(PDP) segments will migrate to the surface of the particles. This could lead to a balanced interfacial interaction between PS/P4VP(PDP) domains and the surrounding water. In this case, the BCPs will self-assemble into a morphology with low curvature boundary to minimize the entropic penalty associated with bending of the polymer chains. The surface energy varies at different positions on the particles, due to different packing structures of polymer chains at the lateral surface and the end surface of the particles (Scheme S1, ESI†), inducing the transformation of spherical particles into CL-like particles.26 Additionally, the increase of PDP will enlarge the volume fraction of the P4VP domain (x = 0.2, fP4VP(PDP) = 35.8 vol%), resulting in morphological evolution from sphere to cylinder.15 Consequently, CL-like particles with perfectly stacked cylinders are obtained by varying the properties of the BCP matrix, rather than changing the composition of surfactants. In previous studies, CL-like particles can only be obtained by employing two types of surfactants. Our results demonstrate that by carefully adding additives to tailor the properties of the BCP matrix, the CL-like particles can be readily obtained by using a single commercially available surfactant. A further increase of x results in the continuous increase of the affinity of P4VP(PDP) to CTAB and the volume fraction of the P4VP(PDP) domain, triggering the formation of onion-like particles with P4VP(PDP) on the surface (Fig. 1e and f). In this case, CTAB selectively wets P4VP(PDP) instead of PS domains.
In our previous report, we demonstrated the morphological transition of the PS51K-b-P4VP18K(PDP)x particles stabilized by poly(vinyl alcohol) (PVA).15 Since PVA has almost the same affinities to PS and P4VP, it can generate a neutral interface for PS and P4VP segments. Consequently, both blocks can simultaneously locate on the surface of the particles. However, the addition of PDP will increase the attractive interaction between P4VP(PDP) and the surrounding water, as PDP has a hydrophilic phenol head. No CL-like particles can be obtained in that case since the interfaces are selective for P4VP(PDP) instead of being neutral to both blocks.
In a similar report by Kim et al., they found that CL-like particles could not be formed by PS27K-b-P4VP7K(PDP)0.2 in CTAB (5 mg mL−1), although the volume fraction of P4VP(PDP) (fP4VP(PDP) = 29.1 vol%) is located in the cylindrical regime.24 Presumably, high concentrations of CTAB would retard the formation of CL-like particles. More CTAB will induce more P4VP(PDP) chains to the interface, resulting in the breaking of the neutral interface for both PS and P4VP(PDP). Thus, P4VP(PDP) will locate on the surface of the particles in this case. In order to prove this assumption, we fabricate PS51K-b-P4VP18K(PDP)0.2 particles with CTAB of 5 mg mL−1 concentration. Indeed, no CL-like particles can be observed in this case (Fig. S3, ESI†).
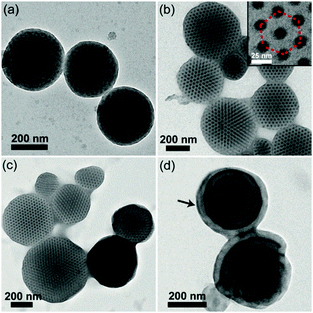
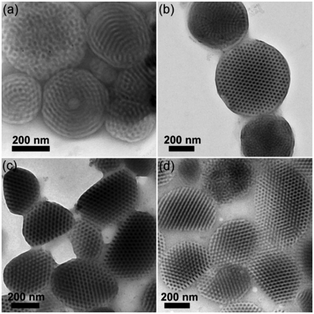
In order to demonstrate the generality of our strategy, triblock copolymer-based supramolecules P4VP4.5K-b-PS38K-b-P4VP4.5K(PDP)x are employed to fabricate CL-like particles by using CTAB as a surfactant (3 mg mL−1). As shown in Fig. 2, CL-like particles can be observed at x = 0.25–0.50. Onion-like particles with P4VP(PDP) on the surface are obtained at x = 1.0. However, no CL-like particles can be observed if PVA was used as an emulsifier when x is increased from 0 to 1.0 (Fig. S4, ESI†). The results coincide with those of the PS51K-b-P4VP18K(PDP)x system. We presume that the fraction of CTAB in the surfactant solution would play an important role in the formation of CL-like particles. Thus, mixed surfactants of CTAB and PVA with different weight ratios (w) are employed for fabricating BCP particles. As shown in Fig. 3, when w = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, no CL-like particles can be observed (Fig. 3a), whereas when w is higher than 1
9, no CL-like particles can be observed (Fig. 3a), whereas when w is higher than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (for instance, 1
9 (for instance, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 1
7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 1
5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), CL-like particles are formed. A solvent–absorption annealing approach (details are given in the ESI†) can be applied to shape the morphology of the BCP particles after their formation.12,16,18,28 The particles with a twisted cylindrical structure (Fig. 3a) are employed as the initial state during the annealing process. After removal of the mixed surfactants with w = 1
3), CL-like particles are formed. A solvent–absorption annealing approach (details are given in the ESI†) can be applied to shape the morphology of the BCP particles after their formation.12,16,18,28 The particles with a twisted cylindrical structure (Fig. 3a) are employed as the initial state during the annealing process. After removal of the mixed surfactants with w = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, the particles are redispersed in mixed surfactants with w = 1
9, the particles are redispersed in mixed surfactants with w = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or 1
5 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The spherical particles with twisted cylinders transform into CL-like particles after being annealed in chloroform for 8 h (Fig. S5, ESI†). The interfacial interaction between polymers and surfactants dominates the shape and the internal structure of the particles. Therefore, CL-like particles can be obtained through either of these two approaches, e.g., an emulsion–solvent evaporation method or a solvent–absorption annealing method, provided the neutral interfacial interaction is established.12
3. The spherical particles with twisted cylinders transform into CL-like particles after being annealed in chloroform for 8 h (Fig. S5, ESI†). The interfacial interaction between polymers and surfactants dominates the shape and the internal structure of the particles. Therefore, CL-like particles can be obtained through either of these two approaches, e.g., an emulsion–solvent evaporation method or a solvent–absorption annealing method, provided the neutral interfacial interaction is established.12
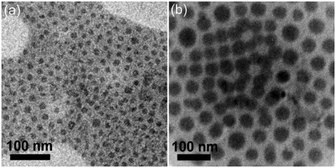
The CL-like particles can be transformed into mesoporous particles (Fig. 4) since the P4VP(PDP) domains can be swelled and the PDP can be removed using a selective solvent.15,29 The pore size is dominated by two factors: (1) the size of P4VP(PDP) domains and (2) the swelling temperature. The diameters of P4VP(PDP) domains within the CL-like particles shown in Fig. 2c and Fig. 1d are 14.0 ± 0.5 nm and 30.2 ± 1.3 nm, respectively. After swelling at 25 °C for 24 h, the diameters of the pores turn out to be 6.0 ± 0.8 nm and 16.0 ± 2.0 nm, respectively (Fig. 4a and b). Heating will increase the stretching of P4VP chains in ethanol, inducing the enlargement of the pores. As shown in Fig. 4c and d, after swelling at 60 °C for 20 min, the diameters of the pores increase to 15.5 ± 1.0 nm and 50.4 ± 1.9 nm, even larger than the initial diameter of the P4VP(PDP) cylinder. Since heating will soften the PS matrix, the stretching of P4VP chains will expand the PS chains, leading to larger pores. A similar phenomenon has been reported in our previous study.16 Interestingly, the size of the mesopores is nearly monodispersed. Thus, the strategy presented here can be used for preparing particles with isoporosity and tunable pore size, which could be potentially applied in ultrafiltration.30–33
The 3D confined assembly–disassembly strategy was recently developed to fabricate nano-objects with designable morphologies.9,12,34,35 Here, the CL-like particles with hexagonally stacked P4VP(PDP) cylinders are employed for the disassembly process, and isolated nanofibers with monodispersed diameter are expected. The P4VP(PDP) domains are crosslinked by 1,4-diiodobutane (DIB) before dispersing the particles in chloroform to dissolve the PS matrix. However, only spherical particles with crosslinked P4VP cores are obtained after disassembly of the CL-like particles (Fig. 5). Inside the P4VP(PDP) domains, microphase separation between the P4VP chains and PDP chains occurs (Scheme 1).36 Therefore, the crosslinking of 4VP by DIB is suppressed by the PDP sub-domains, leading to the incomplete crosslinking of the P4VP(PDP) cylinders. As a result, the disassembly process in chloroform will make the cylinders break up into small pieces, resulting in spherical particles instead of cylindrical nanofibers. In order to confirm this assumption, P4VP4.5K-b-PS27K-b-P4VP4.5K (without PDP, fP4VP = 23.3 vol%) was introduced to fabricate particles with cylindrical structures. After crosslinking by DIB and disassembling in chloroform, nanofibers can be obtained (Fig. S6, ESI†). In this case, the P4VP domains are completely crosslinked and thus the internal structure can be preserved after disassembly.
Conclusions
In summary, we have demonstrated a facile yet efficient approach to fabricate anisotropic CL-like particles with hexagonally stacked cylindrical domains by employing a single surfactant to construct a neutral interface for both blocks. Additives are introduced into the BCP matrix to tailor the interfacial interaction between the polymer chains and the surrounding medium. More interestingly, by selectively swelling the P4VP(PDP) domains, isoporous particles with uniform channels are obtained, which can be potentially applied in separation membranes with high selectivity.Acknowledgements
We gratefully acknowledge funding of this work provided by the National Basic Research Program of China (973 Program, 2012CB821500), National Natural Science Foundation of China (51473059 and 51525302), and Natural Science Foundation of Hubei Scientific Committee (2015BHE001). We also thank the HUST Analytical and Testing Center for allowing us to use its facilities.Notes and references
- S.-H. Kim, S. Y. Lee, S.-M. Yang and G.-R. Yi, NPG Asia Mater., 2011, 3, 25–33 CrossRef CAS.
- M. Bockstaller, R. Kolb and E. L. Thomas, Adv. Mater., 2001, 13, 1783–1786 CrossRef CAS.
- A. Setaro, S. Lettieri, P. Maddalena and L. De Stefano, Appl. Phys. Lett., 2007, 91, 051921 CrossRef.
- M. P. Kim, D. J. Kang, D.-W. Jung, A. G. Kannan, K.-H. Kim, K. H. Ku, S. G. Jang, W.-S. Chae, G.-R. Yi and B. J. Kim, ACS Nano, 2012, 6, 2750–2757 CrossRef CAS PubMed.
- L. A. Connal, N. A. Lynd, M. J. Robb, K. A. See, S. G. Jang, J. M. Spruell and C. J. Hawker, Chem. Mater., 2012, 24, 4036–4042 CrossRef CAS PubMed.
- K. H. Ku, M. P. Kim, K. Paek, J. M. Shin, S. Chung, S. G. Jang, W. S. Chae, G. R. Yi and B. J. Kim, Small, 2013, 9, 2667–2672 CrossRef CAS PubMed.
- Z. Lu, G. Liu, H. Phillips, J. M. Hill, J. Chang and R. A. Kydd, Nano Lett., 2001, 1, 683–687 CrossRef CAS.
- P. Chi, Z. Wang, B. Li and A.-C. Shi, Langmuir, 2011, 27, 11683–11689 CrossRef CAS PubMed.
- R. Deng, F. Liang, W. Li, S. Liu, R. Liang, M. Cai, Z. Yang and J. Zhu, Small, 2013, 9, 4099–4103 CrossRef CAS PubMed.
- Z. Jin and H. Fan, Soft Matter, 2014, 10, 9212–9219 RSC.
- A.-C. Shi and B. Li, Soft Matter, 2013, 9, 1398–1413 RSC.
- J. Xu, K. Wang, J. Li, H. Zhou, X. Xie and J. Zhu, Macromolecules, 2015, 48, 2628–2636 CrossRef CAS.
- H. Yabu, K. Motoyoshi, T. Higuchi and M. Shimomura, Phys. Chem. Chem. Phys., 2010, 12, 11944–11947 RSC.
- J. M. Shin, M. P. Kim, H. Yang, K. H. Ku, S. G. Jang, K. H. Youm, G.-R. Yi and B. J. Kim, Chem. Mater., 2015, 27, 6314–6321 CrossRef CAS.
- R. Deng, S. Liu, J. Li, Y. Liao, J. Tao and J. Zhu, Adv. Mater., 2012, 24, 1889–1893 CrossRef CAS PubMed.
- J. Xu, K. Wang, R. Liang, Y. Yang, H. Zhou, X. Xie and J. Zhu, Langmuir, 2015, 31, 12354–12361 CrossRef CAS PubMed.
- D. Klinger, C. X. Wang, L. A. Connal, D. J. Audus, S. G. Jang, S. Kraemer, K. L. Killops, G. H. Fredrickson, E. J. Kramer and C. J. Hawker, Angew. Chem., Int. Ed., 2014, 53, 7018–7022 CrossRef CAS PubMed.
- R. Deng, F. Liang, W. Li, Z. Yang and J. Zhu, Macromolecules, 2013, 46, 7012–7017 CrossRef CAS.
- B. V. K. J. Schmidt, J. Elbert, D. Scheid, C. J. Hawker, D. Klinger and M. Gallei, ACS Macro Lett., 2015, 4, 731–735 CrossRef CAS.
- S.-J. Jeon, G.-R. Yi and S.-M. Yang, Adv. Mater., 2008, 20, 4103–4108 CrossRef CAS.
- K. H. Ku, H. Yang, S. G. Jang, J. Bang and B. J. Kim, J. Polym. Sci. Polym. Chem., 2016, 54, 228–237 CrossRef CAS.
- K. H. Ku, H. Yang, J. M. Shin and B. J. Kim, J. Polym. Sci. Polym. Chem., 2015, 53, 188–192 CrossRef CAS.
- S. G. Jang, D. J. Audus, D. Klinger, D. V. Krogstad, B. J. Kim, A. Cameron, S.-W. Kim, K. T. Delaney, S.-M. Hur, K. L. Killops, G. H. Fredrickson, E. J. Kramer and C. J. Hawker, J. Am. Chem. Soc., 2013, 135, 6649–6657 CrossRef CAS PubMed.
- K. H. Ku, J. M. Shin, M. P. Kim, C.-H. Lee, M.-K. Seo, G.-R. Yi, S. G. Jang and B. J. Kim, J. Am. Chem. Soc., 2014, 136, 9982–9989 CrossRef CAS PubMed.
- J. A. Champion and S. Mitragotri, Proc. Natl. Acad. Sci. U.S.A., 2006, 103, 4930–4934 CrossRef CAS PubMed.
- H. Yang, K. H. Ku, J. M. Shin, J. Lee, C. H. Park, H.-H. Cho, S. G. Jang and B. J. Kim, Chem. Mater., 2016, 28, 830–837 CrossRef CAS.
- A. W. Bosse, C. J. Garcia-Cervera and G. H. Fredrickson, Macromolecules, 2007, 40, 9570–9581 CrossRef CAS.
- L. Li, K. Matsunaga, J. Zhu, T. Higuchi, H. Yabu, M. Shimomura, H. Jinnai, R. C. Hayward and T. P. Russell, Macromolecules, 2010, 43, 7807–7812 CrossRef CAS.
- S. Mei and Z. Jin, Small, 2013, 9, 322–329 CrossRef CAS PubMed.
- H. Yu, X. Qiu, N. Moreno, Z. Ma, V. M. Calo, S. P. Nunes and K.-V. Peinemann, Angew. Chem., Int. Ed., 2015, 54, 13937–13941 CrossRef CAS PubMed.
- H. Yu, X. Qiu, S. P. Nunes and K.-V. Peinemann, Angew. Chem., Int. Ed., 2014, 53, 10072–10076 CrossRef CAS PubMed.
- Y. Wang, U. Goesele and M. Steinhart, Nano Lett., 2008, 8, 3548–3553 CrossRef CAS PubMed.
- Y. Wang and F. B. Li, Adv. Mater., 2011, 23, 2134–2148 CrossRef CAS PubMed.
- Y. Ruan, L. Gao, D. Yao, K. Zhang, B. Zhang, Y. Chen and C.-Y. Liu, ACS Macro Lett., 2015, 4, 1067–1071 CrossRef CAS.
- J. Xu, Y. Wu, K. Wang, L. Shen, X. Xie and J. Zhu, Soft Matter, 2016, 12, 3683–3687 RSC.
- J. Ruokolainen, R. Makinen, M. Torkkeli, T. Makela, R. Serimaa, G. ten Brinke and O. Ikkala, Science, 1998, 280, 557–560 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional figures of the BCP particles. See DOI: 10.1039/c6qm00072j |
| This journal is © the Partner Organisations 2017 |