Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Triazolinedione-“clicked” poly(phosphoester)s: systematic adjustment of thermal properties†
Greta
Becker
ab,
Laetitia
Vlaminck
c,
Maria M.
Velencoso
 a,
Filip E.
Du Prez
a,
Filip E.
Du Prez
 *c and
Frederik R.
Wurm
*c and
Frederik R.
Wurm
 *a
*a
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: wurm@mpip-mainz.mpg.de
bGraduate School Materials Science in Mainz, Staudinger Weg 9, 55128 Mainz, Germany
cDepartment of Organic and Macromolecular Chemistry, Polymer Chemistry Research Group, Ghent University, Krijgslaan 281 S4-bis, 9000 Ghent, Belgium. E-mail: Filip.Duprez@Ugent.be
First published on 21st June 2017
Abstract
The thermal properties of halogen-free flame retardant poly(phosphoester)s from acyclic diene metathesis polycondensation have been optimized by a systematic post-modification using 1,2,4-triazoline-3,5-dione derivatives. The straightforward modification not only increased their glass transition temperatures significantly but also improved the thermal stability with respect to their char yields.
Poly(phosphoester)s (PPEs) can be prepared via both ring-opening polymerization and polycondensation.1,2 We recently applied ADMET polycondensation to prepare a variety of PPEs.3–6 Currently, PPEs are studied in biomedical applications for their biocompatibility,7 biodegradability and adjustable hydrophilicity.7,8 Furthermore, PPEs are also currently under discussion as potent flame retardant additives, as many halogenated compounds are banned from the market.3 PPEs show higher resistance to leaching and migration, compared to low molecular weight additives.9 Indeed, phosphorus is an efficient char promoter forming an accumulated protective layer on the materials surface, which limits the release of fuel to the flame.10,11 Additionally, PPEs typically show low toxicity or almost smoke-free burning.12,13 Recently, the combination of phosphorus-containing flame retardants with nitrogen compounds has attracted much attention. The combination of both elements physically and chemically can dramatically improve the action of the phosphorus species in the condensed phase upon combustion.14,15 Nitrogen compounds are believed to act by the release of inert gases or by the promotion of char formation as a result of a condensation reaction. Besides phosphorus and nitrogen, the introduction of aromatic structures is also often mentioned to promote the charring.16
As a systematic study on the thermal properties and char yield, with structural variation of the main- and side chains of PPEs, is missing in the current literature, the main goal of this research was the preparation of modified PPEs via ADMET polycondensation, as a result of the variation of both the backbone and side chains. Furthermore, we used the efficient, recently revisited “click chemistry” of 1,2,4-triazoline-3,5-diones (TAD)17,18 to modify the double bonds along the PPE backbone to further adjust the material properties. This TAD-ene chemistry can not only be applied as a reliable and convenient post-modification reaction of unsaturated polymers but will also introduce additional nitrogen atoms into the PPEs, which is expected to have a positive influence on their flame retardant performance. TADs are one of the most reactive (di)enophiles and show quantitative conversions at room temperature with isolated alkenes or conjugated dienes, respectively in an Alder-ene or Diels–Alder reaction. TAD has recently been applied in a wide range of applications, such as self-healing,19 surface chemistry,20–22 and post-modification of unsaturated ADMET polymers.23
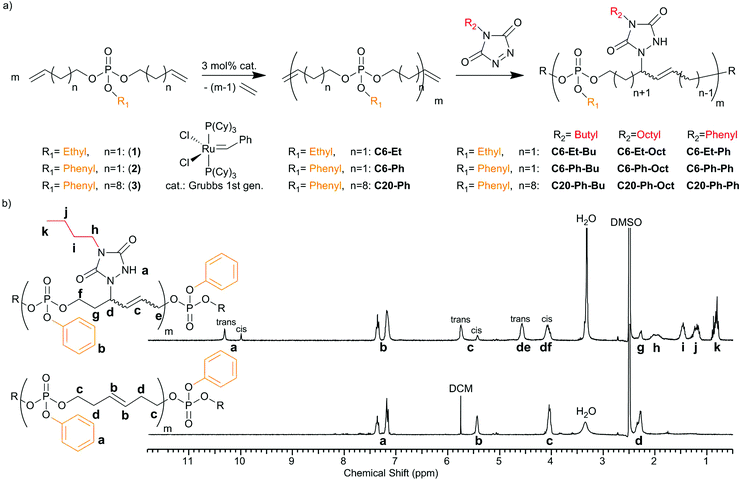
This is the first report on the post-modification of PPEs via their internal double bonds for tuning their thermal properties. Supposing that TAD-modification can increase the glass transition temperature (Tg), this would undoubtedly broaden the range of applications for PPEs, which typically exhibit low Tg's between −40 and −60 °C.3 Finally, the introduced nitrogen atoms and additional aromatic structures will alter the degradation behavior of the polymers during combustion, making it a useful and straightforward modification for the design of novel PPE-based flame retardants. In this context, di(but-3-en-1-yl)ethyl phosphate (1), di(but-3-en-1-yl)phenyl phosphate (2) and di(undec-10-en-1-yl)phenyl phosphate (3) (Fig. 1a) have been used as the respective monomers for ADMET polycondensation,4,5 yielding PPEs with different alkyl spacers between the phosphate centers along the backbone (6 or 20 methylene groups) and different pendant groups (ethoxy or phenoxy; C6-Et, C6-Ph and C20-Ph). Successful polycondensation of the monomers can be determined from the 1H NMR spectra by the decrease in signals for terminal double bonds at 5.10 and 5.80 ppm and the appearance of a new signal at ca. 5.40–5.50 ppm for the internal double bonds formed during the metathesis reaction (Fig. 1b bottom and S1–S6†). The obtained polymers are viscous oils with molecular weights of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 and 7100 g mol−1 for C6-Et and C6-Ph polymers, respectively, determined via end-group analysis of the ratio between signals for terminal and internal double bonds. Molecular weight dispersities show values of 1.46 (C6-Et) and 1.84 (C6-Ph), and up to 2.21 (C20-Ph).
700 g mol−1 and 7100 g mol−1 for C6-Et and C6-Ph polymers, respectively, determined via end-group analysis of the ratio between signals for terminal and internal double bonds. Molecular weight dispersities show values of 1.46 (C6-Et) and 1.84 (C6-Ph), and up to 2.21 (C20-Ph).
Subsequently, all polymers were post-modified via TAD click chemistry. The double bonds reacted fast in solution at room temperature with three TAD derivatives, Ph-TAD, Bu-TAD and Oct-TAD (Fig. 1a).
1H NMR spectroscopy reveals full modification in all cases, as the original resonance attributed to the unsaturations (b at 5.47 ppm, Fig. 1b bottom) is completely replaced by two new resonances as a result of the successful reaction (c at 5.44 and 5.76 ppm, Fig. 1b top). In the case of the modified short-chain polymers (C6-Et and C6-Ph), a split-up of the P–O neighbouring methylene group for cis and trans isomers can additionally be observed, due to the allylic position of the phosphate groups in the backbone. In the case of the modified C20-Ph polymers, the split-up for the P–O neighbouring methylene group is not observed, because the internal double bonds are too distant. Additional proof of full modification is given via31P {H}NMR, which is a sensitive probe to assess the modification of the polymers. Also in this case, the spectra reveal full modification of all polymers and show a clear upfield shift (for C6-Et polymers from −1.00 to −1.39 ppm, exemplarily shown in Fig. 2b and S10–S12†). After modification, a broader signal emerges due to an increased stiffness of the backbone as a result of the allylic position of P–O and the double bond. As SEC analysis for all modified polymers shows similar dispersities after TAD-modification, as well as unmodified shape of the SEC-curves, it can be concluded that no degradation takes place after the straightforward chemical procedure (shown in Fig. 2a and S13, and Table S2†).
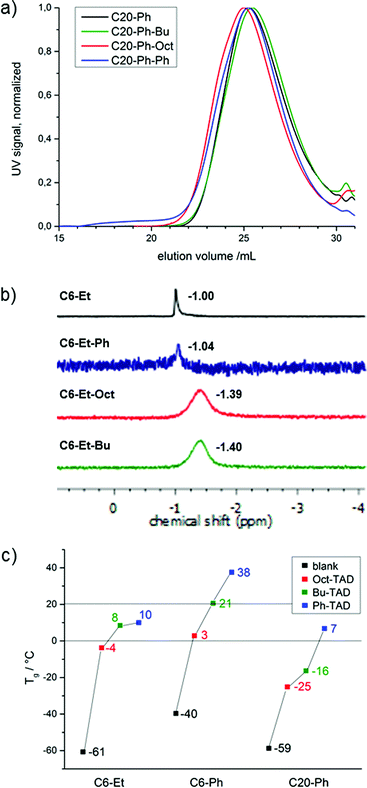 | ||
| Fig. 2 (a) SEC traces of C20-Ph, C20-Ph-Bu, C20-Ph-Oct and C20-Ph-Ph in THF, UV signal; (b) 31P-NMR spectra of C6-Et, C6-Et-Bu, C6-Et-Oct and C6-Et-Ph; (c) Tg's of all PPEs. | ||
As mentioned earlier, aliphatic PPEs typically exhibit Tg's below 0 °C.4 The polymers used in this study exhibit Tg's of −40 °C (C6-Ph), −59 °C (C20-Ph) and −61 °C (C6-Et) (Fig. 2c). All modified polymers show an increased Tg with ΔTg ranging from 34 to 77 °C compared to unmodified polymers, which could be expected from the observations with previously reported polymers after TAD modification.23 The highest increase of Tg was found for the modification with the rigid Ph-TAD, resulting in Tg's of 38 °C (C6-Ph), 10 °C (C6-Et) and 7 °C (C20-Ph). For the two alkyl-containing TADs, the more flexible Oct-TAD shows the lowest effect on the Tg's: 3 °C (C6-Ph), −4 °C (C6-Et) and 25 °C (C20-Ph).
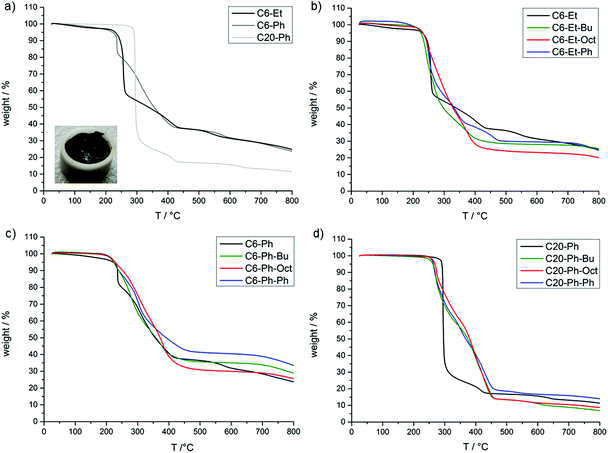
The thermal stability and degradation of the PPE and the TAD-modified polymers were investigated via thermogravimetric analysis (TGA). Fig. 3 shows the TGA thermograms of the polymers (detailed TGA data are summarized in Table S3†) and shows a large difference in the thermal stabilities and the char yields of the non-modified PPEs with varying backbone lengths. Note that C6-Et and C6-Ph show onset degradation temperatures (at 95 wt%) at 231 and 222 °C, respectively, which are considerably lower in comparison to C20-Ph (291 °C). Additionally, the char yields at 700 °C for C6-Et (29 wt%) and C6-Ph (28 wt%) are higher than that for C20-Ph (13 wt%). Both effects can be explained by the increased amount of phosphorus in C6-Et and C6-Ph (15 and 12 wt%) compared to C20-Ph (7 wt%). It is known that the P–O–C bond decomposes at ca. 180 °C and is less stable than the C–C-bonds.24–26 This lower bonding strength stimulates the degradation of the phosphate to phosphoric acids, which can form stable phosphorus–carbon structures, enhancing the charring of the polymer in the condensed phase and therefore increasing the residual mass.3,24,26,27
Also, the length of the alkyl chain in the backbone of the PPE was found to have a more significant effect on the thermal stability than the nature of the substituent in the side chain (Et or Ph). Thus, although C6-Ph shows a less pronounced decomposition than C6-Et between 250–350 °C, probably because of a charring effect of the aromatic side group, the degradation profile for both polymers is very similar at temperatures above 400 °C.
For all TAD-modified C6-Et and C6-Ph polymers (Fig. 3), a similar degradation profile in comparison to their unmodified polymers can be observed. They show a similar residual mass at 700 °C (Table S3†) and a similar onset degradation temperature as the unmodified one. From previous studies of TAD derivatives,23 it is known that the urazole moiety degrades around 300 °C (Tonset = 250 °C) by scission of the carbon–nitrogen linkage to the polymer chain, followed by further degradation to provide aniline and phenyl isocyanate in the gas phase. This indicates that the degradation of the PPE backbone takes place at lower temperatures than the degradation of the TAD moieties, which can explain the limited effect of these nitrogen containing moieties on the thermal degradation of the PPEs. Only the pending Ph-urazole moiety is found to have a significant effect on the thermal degradation of the C6-Ph polymer, as the residual mass at 700 °C increases from 28 wt% (C6-Ph) to 39 wt% (C6-Ph-Ph).
For the modified C20-Ph polymers (Fig. 3d), the results are the most striking. All modified polymers show an earlier degradation temperature between 262–268 °C instead of 291 °C (C20-Ph), probably attributed to the cleavage of urazole moieties from the polymers. Furthermore, while C20-Ph degrades in one step with a weight loss of 55 wt% at 295 °C, the modified polymers degrade over an improved temperature range of around 200 °C and show a weight loss of only 27 wt% at 295 °C. Therefore, in this case, the simultaneous degradation of the PPE and TAD groups shows a notable improvement in the thermal stability from 300 °C to 450 °C. However, contrary to what might be expected, there is no notable increase in the char yield, except again for C20-Ph-Ph, which leaves a final residue of 16 wt% compared to the 13 wt% of C20-Ph. This modified polymer shows a similar pattern like C6-Ph-Ph. The increased degradation temperature of the modified C20-Ph polymers is situated in the range of the ones of epoxy resins, which makes them interesting as flame-retardant additives for such resins.
Conclusions
ADMET-derived PPEs with a variation of chain-lengths between the phosphate centers (C6 or C20) and side chains (aliphatic or aromatic) have been quantitatively post-modified in an ultrafast way with both aliphatic and aromatic TADs. The post-modification improves the polymer properties and PPEs with Tg's above room temperature were obtained. Additionally, the modification led to an increase in decomposition temperature, because of enhanced charring behaviour, especially for the long-chain polymers. Moreover, the modification results in a rise of the residual mass after heating to 600 °C for short-chain polymers with aromatic side chains. With these interesting findings, we strongly believe that the functionalized PPEs can find applications as flame retardant additives for materials in numerous applications.Acknowledgements
G. Becker is a recipient of a fellowship through funding of the Excellence Initiative (DFG/GSC 266) in the Graduate School of Excellence “MAINZ” (Material Sciences in Mainz). The authors thank the Deutsche Forschungsgemeinschaft for funding. M. M. Velencoso thanks the Marie Skłodowska-Curie fellowship 705054-NOFLAME (Flame Retardant Nanocontainers) project. L. Vlaminck acknowledges the Special Research Fund (BOF) of Ghent University for funding of her PhD project. Open Access funding provided by the Max Planck Society.References
- F. Zhang, J. A. Smolen, S. Zhang, R. Li, P. N. Shah, S. Cho, H. Wang, J. E. Raymond, C. L. Cannon and K. L. Wooley, Nanoscale, 2015, 7, 2265–2270 RSC.
- K. N. Bauer, H. T. Tee, M. M. Velencoso and F. R. Wurm, Prog. Polym. Sci. DOI:10.1016/j.progpolymsci.2017.05.004.
- K. Täuber, F. Marsico, F. R. Wurm and B. Schartel, Polym. Chem., 2014, 5, 7042–7053 RSC.
- F. Marsico, M. Wagner, K. Landfester and F. R. Wurm, Macromolecules, 2012, 45, 8511–8518 CrossRef CAS.
- K. N. Bauer, H. T. Tee, I. Lieberwirth and F. R. Wurm, Macromolecules, 2016, 49, 3761–3768 CrossRef CAS.
- M. Steinmann, J. Markwart and F. R. Wurm, Macromolecules, 2014, 47, 8506–8513 CrossRef CAS.
- Z. Zhao, J. Wang, H.-Q. Mao and K. W. Leong, Adv. Drug Delivery Rev., 2003, 55, 483–499 CrossRef CAS PubMed.
- T. Steinbach and F. R. Wurm, Angew. Chem., Int. Ed., 2015, 54, 6098–6108 CrossRef CAS PubMed.
- S. Bourbigot and S. Duquesne, J. Mater. Chem., 2007, 17, 2283–2300 RSC.
- P. Joseph and S. Tretsiakova-Mcnally, Polym. Adv. Technol., 2011, 22, 395–406 CrossRef CAS.
- D. K. Chattopadhyay and D. C. Webster, Prog. Polym. Sci., 2009, 34, 1068–1133 CrossRef CAS.
- H.-Y. Ma, L.-F. Tong, Z.-B. Xu and Z.-P. Fang, Adv. Funct. Mater., 2008, 18, 414–421 CrossRef CAS.
- A. M. Borreguero, P. Sharma, C. Spiteri, M. M. Velencoso, M. S. Carmona, J. E. Moses and J. F. Rodríguez, React. Funct. Polym., 2013, 73, 1207–1212 CrossRef CAS.
- T.-M. Nguyen, S. Chang, B. Condon, R. Slopek, E. Graves and M. Yoshioka-Tarver, Ind. Eng. Chem. Res., 2013, 52, 4715–4724 CrossRef CAS.
- S. Gaan, G. Sun, K. Hutches and M. H. Engelhard, Polym. Degrad. Stab., 2008, 93, 99–108 CrossRef CAS.
- L. Costes, F. Laoutid, M. Aguedo, A. Richel, S. Brohez, C. Delvosalle and P. Dubois, Eur. Polym. J., 2016, 84, 652–667 CrossRef CAS.
- H. A. Houck, K. De Bruycker, S. Billiet, B. Dhanis, H. Goossens, S. Catak, V. Van Speybroeck, J. M. Winne and F. E. Du Prez, Chem. Sci., 2017, 8, 3098–3108 RSC.
- K. De Bruycker, S. Billiet, H. A. Houck, S. Chattopadhyay, J. M. Winne and F. E. Du Prez, Chem. Rev., 2016, 116, 3919–3974 CrossRef CAS PubMed.
- S. Billiet, K. De Bruycker, F. Driessen, H. Goossens, V. Van Speybroeck, J. M. Winne and F. E. Du Prez, Nat. Chem., 2014, 6, 815–821 CrossRef CAS PubMed.
- O. Roling, K. De Bruycker, B. Vonhören, L. Stricker, M. Körsgen, H. F. Arlinghaus, B. J. Ravoo and F. E. Du Prez, Angew. Chem., Int. Ed., 2015, 54, 13126–13129 CrossRef CAS PubMed.
- S. van der Heijden, L. Daelemans, K. De Bruycker, R. Simal, I. De Baere, W. Van Paepegem, H. Rahier and K. De Clerck, Compos. Struct., 2017, 159, 12–20 CrossRef.
- K. De Bruycker, M. Delahaye, P. Cools, J. Winne and F. E. D. Prez, Macromol. Rapid Commun., 2017 DOI:10.1002/marc.201700122.
- L. Vlaminck, K. De Bruycker, O. Turunc and F. E. Du Prez, Polym. Chem., 2016, 7, 5655–5663 RSC.
- D. Sun and Y. Yao, Polym. Degrad. Stab., 2011, 96, 1720–1724 CrossRef CAS.
- M. M. Velencoso, M. J. Ramos, R. Klein, A. De Lucas and J. F. Rodriguez, Polym. Degrad. Stab., 2014, 101, 40–51 CrossRef CAS.
- W. Xing, L. Song, P. Lv, G. Jie, X. Wang, X. Lv and Y. Hu, Mater. Chem. Phys., 2010, 123, 481–486 CrossRef CAS.
- H. Wang, Q. Wang, Z. Huang and W. Shi, Polym. Degrad. Stab., 2007, 92, 1788–1794 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses protocols, NMR spectra, SEC and TGA diagrams and curves. See DOI: 10.1039/c7py00813a |
| This journal is © The Royal Society of Chemistry 2017 |