Rational design and synthesis of a novel laterally-tetrafluorinated tricyclic mesogen with large negative dielectric anisotropy†
Abstract
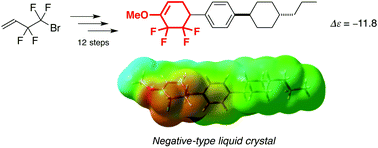
Guided by theoretical calculations, we predicted that 5,5,6,6-tetrafluoro-1-methoxycyclohex-1-ene, a new structural motif, exhibits a large negative dielectric anisotropy, and then developed a synthetic strategy for the preparation of the novel tetrafluorinated tricyclic mesogen in twelve reaction steps starting from a commercially available fluorinated starting material. During the evaluation of its liquid-crystalline characteristics, the tetrafluorinated mesogen was found to display low birefringence and a large negative dielectric anisotropy, confirming that its molecular structure is a reasonable design for the further development of novel mesogenic molecules with large negative dielectric anisotropies.


 Please wait while we load your content...
Please wait while we load your content...