Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dialkylgallium alkoxides – a tool for facile and stereoselective synthesis of PLA–drug conjugates†
M.
Cybularczyk-Cecotka
ab,
R.
Zaremba
ab,
A.
Hurko
ac,
A.
Plichta
c,
M.
Dranka
c and
P.
Horeglad
 *a
*a
aCentre of New Technologies, University of Warsaw, Banacha 2c, 02-097 Warsaw, Poland. E-mail: phoreglad@uw.edu.pl
bFaculty of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland
cFaculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664, Warsaw, Poland
First published on 10th November 2017
Abstract
Herein, a method for the synthesis of PLA-(β-blocker) conjugates with a tunable stereostructure of the PLA fragment is demonstrated using stereoselective [R2Ga(μ-β-blocker)]2 catalysts and [R2Ga(μ-OR)]2/H-(β-blocker) catalytic systems for the ring-opening polymerisation (ROP) and immortal ring-opening polymerisation (iROP) of racemic lactide (rac-LA), respectively.
The growing interest in polylactide (PLA) – a biodegradable and biocompatible polymer – is due to its numerous applications,1 ranging from packaging to medical materials including PLA–drug conjugates and PLA-based drug delivery systems.2 While PLA–drug conjugates could be synthesized, among others, by the polymerisation of lactide using non-toxic Zn3 and Mg4 complexes, a catalyst was crucial for the modification of the PLA structure and properties of the PLA–drug conjugates.4a However, although the tacticity of PLA can affect its physicochemical properties,5 including the degradation rate of PLA–drug conjugates,6 both the synthesis of PLAs of different stereostructures, including PLA copolymers built of blocks of different tacticity,7 and their use in order to tailor the drug release properties of PLA–drug conjugates or drug delivery systems based on PLA,6,8 are in their infancy. We have shown that dialkylgallium catalysts [R2Ga(μ-OR)]2, which constitute a rare example of gallium catalysts for the polymerisation of lactide or other cyclic esters,9 can catalyse the polymerisation of rac-LA to PLA in a controlled and stereoselective manner,10 leading to a non-cytotoxic PLA.6 Dialkylgallium alkoxides exhibit unique features with regard to the stereoselective polymerisation of rac-lactide (rac-LA). In this case the addition of a Lewis base (LB), such as pyridines or THF, to non-selective [R2Ga(μ-OR)]2 complexes resulted in the formation of heteroselective [R2Ga(μ-OR)]2/LB catalytic systems offering tuneable heteroselectivity in the range of 0.5 < Pr ≤ 0.85 (Pr = probability of racemo linkages in PLA).10,11 On the other hand, the addition of N-heterocyclic carbenes or organosuperbases to [R2GaOR]2 led to isoselective species, resulting in a facile stereoselectivity switch,12,13 which allowed for the synthesis of stereodiblock PLA copolymers.13 Therefore [R2Ga(μ-OR)]2, which exhibits low reactivity towards different functional groups,14 should be considered as an interesting catalyst for the synthesis of PLA–drug conjugates, additionally offering easy modification of the tacticity/stereostructure of PLA. We hereby demonstrate that PLA-(β-blocker) conjugates with a tunable stereostructure of the PLA can be synthesized by the polymerisation of rac-LA with [R2Ga(β-blocker)]2 (Scheme 1a) or stereoselective [R2Ga(β-blocker)]2/LB catalysts. Importantly, we also show that dialkylgallium alkoxides can be applied for the stereoselective immortal ring-opening polymerisation (iROP) of rac-LA. In this case [R2Ga(μ-OR)]2/H-(β-blocker) (Scheme 1b) and [R2Ga(μ-OR)]2/H-(β-blocker)/LB catalytic systems offer a facile and stereoselective synthesis of PLA-(β-blocker) conjugates.15
 | ||
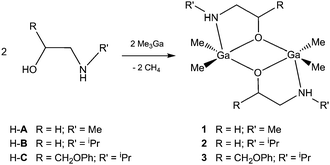
| Scheme 1 Synthesis of PLA-(β-blocker) conjugates with [Me2Ga(β-blocker)]2 catalytic centres using ROP (a) and iROP (b) of rac-LA. H-β-blocker represents a skeleton characteristic for β-blockers,16 and β-blocker represents a respective alkoxide anion with a deprotonated OH group. OR represents an alkoxide group. | ||
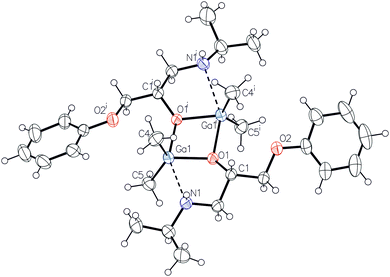
In order to confirm the structure of [R2Ga(β-blocker)]2 active species in the ROP of lactide, we investigated the synthesis, structure and activity of [Me2Ga(μ-OCHRCH2NHR′)]2 (R = H, R′ = Me (1); R = H, R′ = iPr (2); R = CH2OPh, R′ = iPr (3)) in the ROP of rac-LA, where HOCHRCH2NHR’ mimics the main skeleton of β-blockers16 (Scheme 2). For 1–3, which were isolated as colourless crystals, the X-ray analysis revealed the presence of dimers in the solid state (Fig. 1, see the ESI† for the structure of 1 and 2). Although, the dimeric structure of 1–3 is typical for [Me2Ga(O,X)]2 where (O,X) represents a monoanionic alkoxide bidentate ligand with Lewis base functionality,14 the lack of reactivity of the secondary amine group of A–C towards Ga–Me is noteworthy prior to further synthesis of PLA-(A–C) as well as PLA–atenolol conjugates (see below). Noteworthily, a weak Ga–N bond to the fifth coordinate site of gallium was observed for 1–3, while the Ga–N bond distances increased in the order 1 (2.333(4) Å) < 2 (2.359(1) Å) < 3 (2.548(1) Å) with growing steric hindrances on the R and R′ substituents.
As the structures of 1–3 were similar, both in the solid state and solution (see the ESI†), to [Me2Ga(μ-OCH(Me)CO2Me)]2 which mimics active centres in the ROP of lactide,10,11 we expected that PLA–drug conjugates could be synthesized due to insertion of lactide into the Ga–Oalkoxide group of 1–3, moreover, in a stereoselective fashion. In this case, the weak Ga–N chelate bond, as well as Ga–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C resulting from the interaction of the growing PLA chain with gallium,10 should not affect the insertion of lactide into the Ga–Oalkoxide bond of 1–3 or any other [Me2Ga(β-blocker)]2, which is advantageous in comparison with e.g. analogous aluminium complexes, which form considerably stronger chelate bonds with both Lewis base functionalities of alkoxide ligands or growing PLA chains.17 Furthermore, the weakest Ga–N bond for 3, in which the structure of C mimics a whole main skeleton of β-blockers, indicates that the insertion of lactide into the Ga–Oalkoxide bond of any [R2Ga(β-blocker)]2, should be facilitated in comparison with model 1 or 2 complexes. Finally, the propagating species in the rac-LA polymerisation with 1–3 should be similar to propagating species in the case of [Me2Ga(μ-OCH(Me)CO2Me)]2,11,13a and therefore allow for the stereoselective polymerisation. Our reasoning was confirmed by the activity and stereoselectivity of compound 2 in the ROP of rac-LA (Table 1).
C resulting from the interaction of the growing PLA chain with gallium,10 should not affect the insertion of lactide into the Ga–Oalkoxide bond of 1–3 or any other [Me2Ga(β-blocker)]2, which is advantageous in comparison with e.g. analogous aluminium complexes, which form considerably stronger chelate bonds with both Lewis base functionalities of alkoxide ligands or growing PLA chains.17 Furthermore, the weakest Ga–N bond for 3, in which the structure of C mimics a whole main skeleton of β-blockers, indicates that the insertion of lactide into the Ga–Oalkoxide bond of any [R2Ga(β-blocker)]2, should be facilitated in comparison with model 1 or 2 complexes. Finally, the propagating species in the rac-LA polymerisation with 1–3 should be similar to propagating species in the case of [Me2Ga(μ-OCH(Me)CO2Me)]2,11,13a and therefore allow for the stereoselective polymerisation. Our reasoning was confirmed by the activity and stereoselectivity of compound 2 in the ROP of rac-LA (Table 1).
| No. | Cat. | T [°C] | Time [h] | Conv. [%] | 10−3Mnb | 10−3Mnc | Đ | P r | P r |
|---|---|---|---|---|---|---|---|---|---|
| a In CH2Cl2. b Expected Mn [g mol−1] value calculated according to LA/Ga ratio and conversion. c M n [g mol−1] determined by gel permeation chromatography (GPC) in CH2Cl2, using conventional calibration. d Probability of racemo linkages in PLA calculated on the basis of homonuclear decoupled 1H NMR spectra according to Chamberlain et al.18 e Probability of racemo linkages in PLA calculated on the basis of 13C NMR.19 f DBU – 1,8-diazabicyclo[5.4.0]undec-7-ene. g Possibility of transesterification reactions revealed by MALDI-TOF (see the ESI). h Reference polymerisations of 50 equiv. of rac-LA with 3 equiv. of pyridine or DMAP revealed essentially no PLA formation. | |||||||||
| 1 | 2 | 70 | 24 | 85 | 6.1 | 8.1 | 1.13 | 0.50 | — |
| 2 | 2 | 40 | 144 | 75 | 5.4 | 6.7 | 1.12 | 0.50 | — |
| 3h |
2/piridine(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
40 | 144 | 67 | 4.8 | 7.4 | 1.12 | 0.60 | 0.59 |
| 4g,h |
2/DMAP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
40 | 120 | 92 | 6.6 | 11.0 | 1.15 | 0.84 | 0.86 |
| 5a |
2/DBUf(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
−20 | 18 | 94 | 6.8 | 7.3 | 1.38 | 0.22 | 0.22 |
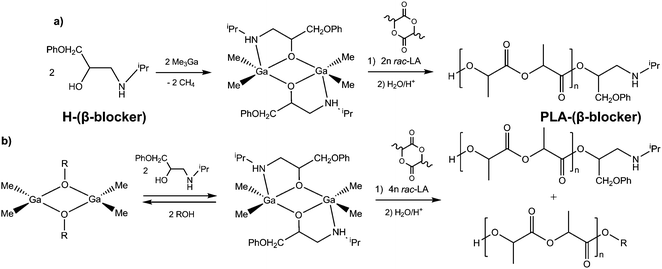
Compound 2 catalysed polymerisation of rac-LA at 40 °C and 70 °C showing similar activities and stereoselectivities to dialkylgallium alkoxides already reported by us.10,11,13a Importantly, the interaction of a secondary amine group of OCH2CH2NHiPr, typical for β-blockers, with gallium neither had an adverse effect on the activity of the investigated catalysts nor affected the insertion of rac-LA into the Ga–Oalkoxide bond. The latter led to the essentially exclusive formation of HO–PLA–OCH2CH2NHiPr, which was clearly evidenced by MALDI-TOF spectroscopy (see the ESI†). Importantly, the heteroselective polymerisation of rac-LA with 2/pyridine and 2/DMAP catalytic systems leading to the heterotactically enriched PLA up to Pr of 0.85 (Table 1, entries 3 and 4), as well as isoselective polymerisation using 2/DBU (Pr = 0.22, Table 1, entry 5), indicated the facile modification of the tacticity of PLA, in the range of Pr between 0.22 and 0.85, for HO–PLA–OCH2CH2NHiPr and potentially HO–PLA–(β-blocker) conjugates. Although the approach presented on Scheme 1a and discussed above indicates the possibility of the synthesis of PLA-(β-blocker) conjugates, it would not be the most convenient one, e.g. due to possible reactivity of drugs towards Me3Ga. Therefore we focused on the possibility of the synthesis of [R2Ga(β-blocker)]2 catalytic centres using the immortal ring-opening polymerisation (iROP) of lactide with [Me2Ga(μ-OR)]2/H-(β-blocker) catalytic systems (Scheme 1b).
Although metal complexes have been shown to polymerise rac-LA in an immortal and stereoselective fashion in the presence of alcohols,20 [Me2Ga(μ-OR)]2/R’OH have not been demonstrated so far to catalyse the iROP of heterocyclic monomers. In order to demonstrate the possibility of the synthesis of PLA-(β-blocker) conjugates using immortal ring-opening polymerisation (iROP) of rac-LA with [Me2Ga(μ-OR)]2/H-(β-blocker), we focused on [Me2Ga(μ-OCH(Me)CO2Me)]2/(H-A) or (H-B) as well as [Me2Ga(μ-OC6H4OMe)]2/(H-B), (H-C) or H-atenolol (β-blocker) catalytic systems. Notably, dialkylgallium alkoxides and aryloxides are not prone to the reaction with alcohols leading to the evolution of alkane and formation of dialkoxide or trialkoxide gallium species.14 On the other hand, the exchange of alkoxide groups between dialkylgallium alkoxides and alcohol added should lead in the case of [Me2Ga(μ-OCH(Me)CO2Me)]2/(H-A) or (H-B) as well as [Me2Ga(μ-OC6H4OMe)]2/(H-B), (H-C) or H-atenolol to the presence of both [Me2Ga(μ-OCH(Me)CO2Me)]2 and [Me2Ga(μ-OC6H4OMe)]2 complexes, as well as [Me2Ga(μ-A)]2 (1), [Me2Ga(μ-B)]2 (2), [Me2Ga(μ-C)]2 (3) or [Me2Ga(μ-atenolol)]2 species (Scheme 1b).11 The formation of the latter catalytic centres resulted in the formation of PLA-A, PLA-B, PLA-C, as well as PLA–atenolol conjugates (Table 2).
| No. | ROH/LB | T [°C] | Time [h] | Conv. [%] | 10−3 Mnb | 10−3 Mnc | Đ | P r | P r |
|---|---|---|---|---|---|---|---|---|---|
a Pyr – pyridine, γ-pic – γ-picoline.
b Expected Mn [g mol−1] value calculated according to LA/Ga ratio and conversion.
c
M
n [g mol−1] determined by gel permeation chromatography (GPC) in CH2Cl2, using conventional calibration.
d Probability of racemo linkages in PLA calculated on the basis of homonuclear decoupled 1H NMR spectra according to Chamberlain et al.18
e Probability of racemo linkages in PLA calculated on the basis of 13C NMR.19
f
rac-LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga = 20 (no LB)/80 (DMAP Ga = 20 (no LB)/80 (DMAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga = 3 Ga = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
g Reference polymerisations of 50 equiv. of rac-LA with H-B/pyr (1 1).
g Reference polymerisations of 50 equiv. of rac-LA with H-B/pyr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and H-B/DMAP (1 3) and H-B/DMAP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) revealed essentially no PLA formation and the conversion of 6% (atactic PLA), respectively. 3) revealed essentially no PLA formation and the conversion of 6% (atactic PLA), respectively.
|
|||||||||
| 1 | H-B(I) | 70 | 24 | 90 | 3.2 | 4.4 | 1.20 | 0.50 | — |
| 2 | H-B(I) | 40 | 144 | 62 | 2.2 | 3.3 | 1.20 | 0.50 | — |
| 3a,g | H-B(I)/pyr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
40 | 144 | 72 | 2.6 | 3.9 | 1.20 | 0.58 | 0.58 |
| 4g | H-B(I)/DMAP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
40 | 120 | 91 | 3.3 | 5.0 | 1.24 | 0.83 | 0.85 |
| 5 | H-B(II) | 70 | 24 | 81 | 2.9 | 5.2 | 1.18 | 0.53 | — |
| 6a | H-B(II)/pyr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
40 | 144 | 96 | 3.5 | 3.9 | 1.43 | 0.79 | 0.85 |
| 7a | H-B(II)/γ-pic (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
40 | 144 | 85 | 3.1 | 3.2 | 1.53 | 0.70 | 0.75 |
| 8a | H-B(II)/γ-pic (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
40 | 144 | 92 | 3.3 | 3.6 | 1.46 | 0.83 | 0.87 |
| 9 | H-C(II) | 40 | 240 | 94 | 3.4 | 4.4 | 1.40 | 0.61 | 0.66 |
| 10a | H-C(II)/pyr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
40 | 240 | 93 | 3.3 | 4.1 | 1.46 | 0.61 | 0.68 |
| 11 | H-atenolol(II) | 70 | 24 | 99 | — | — | — | — | — |
| 12 | H-atenolol(III) | 40 | 720 | 93 | 13.4 | 19.9 | 1.71 | 0.56 | 0.57 |
| 13 | H-atenolol(III)f | 70/40 | 24/288 | 87 | 12.5 | 19.2 | 1.67 | 0.67 | 0.69 |
[Me2Ga(μ-OCH(Me)CO2Me)]2/(H-B) polymerised rac-LA at 40 °C and 70 °C, which led to the formation of both HO–PLA-(μ-OCH(Me)CO2Me) and HO–PLA-B chains, as evidenced by MALDI-TOF spectroscopy (see the ESI†). Moreover, the presence of PLA chains of similar Mn, as shown by GPC, indicated the presence of equilibrium and quick exchange of alkoxide groups OCH(Me)CO2Me and H-B at the gallium centre under polymerisation conditions. However, as the molecular weight of the end groups of the resulting HO–PLA-(μ-OCH(Me)CO2Me) (104.1 Da) and HO–PLA-B (103.2 Da) chains were almost the same, we confirmed the formation of both HO–PLA-(μ-OCH(Me)CO2Me) and HO–PLA-(A–B) using [Me2Ga(μ-OCH(Me)CO2Me)]2/(H-A) (see Fig. S69 and S70, ESI†). On the other hand, the polymerisation of rac-LA with [Me2Ga(μ-OC6H4OMe)]2/(H-B) led to the formation of essentially only HO–PLA-B chains indicating essentially no insertion of rac-LA into the Ga–Oaryloxide bond of [Me2Ga(μ-OC6H4OMe)]2 under the investigated conditions. The activity of [Me2Ga(μ-OC6H4OMe)]2/(H-B) was almost the same as in the case of [Me2Ga(μ-OCH(Me)CO2Me)]2/(H-B) which was in line with the formation of [Me2Ga(μ-B)]2 species. The formation of dimeric catalytic species allowed for the heteroselective polymerisation of rac-LA using both [Me2Ga(μ-OC6H4OMe)]2/(H-B)/pyridines and [Me2Ga(μ-OCH(Me)CO2Me)]2/(H-B)/pyridines (Table 2, entries 3, 4, 6–8), which is in agreement with the mechanism of heteroselective polymerisation of rac-LA with dialkylgallium alkoxides recently suggested by us.11 The latter suggests an increase in heteroselectivity observed in the case of [Me2Ga(μ-OC6H4OMe)]2/(H-B), without the addition of an external Lewis base, which could be associated with the interaction of dialkylgallium alkoxides with HOC6H4OMe, released upon formation of [Me2Ga(μ-B)]2. Importantly, the results discussed above clearly show that heteroselective polymerisation of rac-LA with dialkylgallium alkoxides in the presence of a Lewis base works also under iROP conditions with [Me2Ga(μ-OR)]2/R’OH/LB catalytic systems. Although [Me2Ga(μ-OCH(Me)CO2Me)]2/(H-B)/DBU (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) led to predominantly isotactic PLA (Pr = 0.22), both GPC and MALDI-TOF were in this case inconclusive for the controlled and immortal nature of rac-LA polymerisation (see the ESI†).
2) led to predominantly isotactic PLA (Pr = 0.22), both GPC and MALDI-TOF were in this case inconclusive for the controlled and immortal nature of rac-LA polymerisation (see the ESI†).
We confirmed also the possibility of the synthesis of PLA-(β-blocker) conjugates using [Me2Ga(μ-OC6H4OMe)]2/(H-C) (Table 2, entries 9 and 10) as well as [Me2Ga(μ-OC6H4OMe)]2/H-atenolol (Table 2, entries 11–13). In both cases the formation of essentially only HO–PLA-C or HO–PLA–atenolol (Fig. 2) under applied polymerisation conditions was evidenced by MALDI-TOF (see the ESI†). Finally, both controlled and heteroselective iROP of rac-LA, as well as facile modification of the stereostructure of PLA, was demonstrated by the synthesis of the HO-(atactic-PLA)-b-(heterotactically enriched-PLA)–atenolol conjugate (Table 2, entry 13).
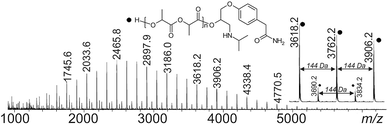 | ||
Fig. 2 MALDI-TOF spectrum of PLA obtained with [Me2Ga(μ-OC6H4OMe)]2/H-atenolol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 70 °C (Table 2, entry 11). The distribution refers to PLA with OH and atenolol end groups, with K+. 2) at 70 °C (Table 2, entry 11). The distribution refers to PLA with OH and atenolol end groups, with K+. | ||
We showed that dialkylgallium alkoxides can be applied for the facile synthesis of PLA–drug conjugates, using as an example the synthesis of HO–PLA-(β-blocker) conjugates in the presence of [R2Ga(μ-β-blocker)]2 catalytic centres. Moreover, the stereostructure of the PLA fragment of HO–PLA-(β-blocker) can be easily tuned by the simple modification of the catalytic system. Importantly, the use of [R2Ga(μ-OR)]2/H-(β-blocker) for the synthesis of HO–PLA-(β-blocker) demonstrated for the first time that dialkylgallium alkoxides/aryloxides can be used in the immortal ROP of lactide. The latter opens new synthetic pathways of both PLA–drug conjugates and PLA copolymers of novel architectures. Both are currently being investigated in our research group.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Foundation for Polish Science, IMPULS competition within SKILLS project, Grant No. 150/UD/SKILLS/2015, cofinanced by the EU European Social Fund.Notes and references
- Poly (Lactic acid), ed. R. Auras, L.-T. Lim, S. E. M. Selke and H. Tsui, John Wiley & Sons, Inc., New Jersey, 2010 Search PubMed.
- (a) H. P. Merkle, Eur. J. Pharm. Biopharm., 2015, 97, 293 CrossRef CAS PubMed; (b) B. Tyler, D. Gullotti, A. Mangraviti, T. Utsuki and H. Brem, Adv. Drug Delivery Rev., 2016, 107, 163 CrossRef CAS PubMed.
- (a) R. Tong and J. Cheng, Angew. Chem., Int. Ed., 2008, 47, 4830 CrossRef CAS PubMed; (b) R. Tong and J. Cheng, Bioconjugate Chem., 2010, 21, 111 CrossRef CAS PubMed; (c) R. Tong and J. Cheng, Macromolecules, 2012, 45, 2225 CrossRef CAS PubMed.
- (a) T. Han, J. Utko, L. B. Jerzykiewicz and P. Sobota, Dalton Trans., 2011, 40, 12660 RSC; (b) T. Han, R. Petrus, D. Bykowski, L. Jerzykiewicz and P. Sobota, Organometallics, 2015, 34, 4871 CrossRef CAS.
- (a) J. M. Becker, R. J. Pounder and A. P. Dove, Macromol. Rapid Commun., 2010, 31, 1923 CrossRef CAS PubMed; (b) M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486 RSC.
- E. Oledzka, P. Horeglad, Z. Gruszczyńska, A. Plichta, G. Nałęcz-Jawecki and M. Sobczak, Molecules, 2014, 19, 19460 CrossRef PubMed.
- W. Zhao, Y. Wang, X. Liu, X. Chen, D. Cui and E. Y.-X. Chen, Chem. Commun., 2012, 48, 6375 RSC.
- C. Ma, P. Pan, G. Shan, Y. Bao, M. Fujita and M. Maeda, Langmuir, 2015, 31, 1527 CrossRef CAS PubMed.
- For the review on this topic see: S. Dagorne, M. Normand, E. Kirillov and J.-F. Carpentier, Coord. Chem. Rev., 2013, 257, 1869 CrossRef CAS.
- P. Horeglad, P. Kruk and J. Pécaut, Organometallics, 2010, 29, 3729 CrossRef CAS.
- P. Horeglad, M. Cybularczyk, A. Litwińska, A. M. Dąbrowska, M. Dranka, G. Z. Żukowska, M. Urbańczyk and M. Michalak, Polym. Chem., 2016, 7, 2022 RSC.
- P. Horeglad, G. Szczepaniak, M. Dranka and J. Zachara, Chem. Commun., 2012, 48, 1171 RSC.
- (a) P. Horeglad, A. Litwińska, G. Z. Żukowska, D. Kubicki, G. Szczepaniak, M. Dranka and J. Zachara, Appl. Organomet. Chem., 2013, 27, 328 CrossRef CAS; (b) P. Horeglad, M. Cybularczyk, B. Trzaskowski, G. Z. Żukowska, M. Dranka and J. Zachara, Organometallics, 2015, 34, 3480 CrossRef CAS.
- C. J. Carmalt and S. J. King, Coord. Chem. Rev., 2006, 250, 682 CrossRef CAS.
- P. Horeglad and M. Cybularczyk, Polish Pat., 10P38641PL00, 2017 Search PubMed.
- Current Cardiovascular Drugs, ed. W. H. Frishman, A. Cheng-Lai and J. Nawarskas, Springer, 2005 Search PubMed.
- (a) J. Lewiński, P. Horeglad, K. Wójcik and I. Justyniak, Organometallics, 2005, 24, 4588 CrossRef; (b) S. Dagorne, F. Le Bideau, R. Welter, S. Bellemin-Laponnaz and A. Maisse-François, Chem. – Eur. J., 2007, 13, 3202 CrossRef CAS PubMed.
- B. M. Chamberlain, M. Cheng, D. R. Moore, T. M. Ovitt, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 3229 CrossRef CAS PubMed.
- J. E. Kasperczyk, Macromolecules, 1995, 28, 3937 CrossRef CAS.
- Review: (a) N. Ajellal, J.-F. Carpentier, C. Guillaume, S. M. Guillaume, M. Helou, V. Poirier, Y. Sarazin and A. Trifonov, Dalton Trans., 2010, 39, 8363 RSC Ga complexes: ; (b) F. Hild, N. Neehaul, F. Bier, M. Wirsum, C. Gourlaouen and S. Dagorne, Organometallics, 2013, 32, 587 CrossRef CAS; (c) N. Maudoux, E. Tan, Y. Hu, T. Roisnel, V. Dorcet, J.-F. Carpentier and Y. Sarazin, Main Group Met. Chem., 2016, 39, 131 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data of 1–3; 1H and 13C NMR for 1–3; details of rac-LA polymerisation, including 1H and 13C NMR and MALDI-TOF spectra of PLA. CCDC 1569393–1569395. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7nj03089d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |