Dinuclear rhenium pyridazine complexes containing bridging chalcogenide anions: synthesis, characterization and computational study†
Abstract
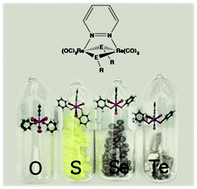
The synthesis of a series of neutral dinuclear rhenium complexes of the general formula [Re2(μ-ER)2(CO)6(μ-pydz)] (pydz = pyridazine; E = S, Se or Te; R = methyl or phenyl; the TeMe is not included) has been carried out via new, either one-pot or two-step, procedures. The one-pot synthesis consists of the oxidative addition of RE–ER across the Re–Re bond of [Re2(CO)10], in the presence of 1 equivalent of pyridazine, and affords the corresponding dinuclear complexes in high yields (ca. 85%). Furthermore, a general two-step procedure has been carried out, which involves the synthesis of heterocubane-like [Re4(μ3-ER)4(CO)12] molecules and their reaction with pyridazine, quantitatively affording the corresponding dinuclear species through a symmetric [2+2] fragmentation pathway. The molecular structure of the complexes has been elucidated by single crystal XRD analysis, and TD-DFT calculations predicted the existence of conformers differing in the orientation of the chalcogen substituents with respect to the pyridazine ligand. The relative stabilities and the activation barriers for the interconversion have been calculated, observing a regular trend that has been rationalized depending on the hybridization of the chalcogen atom. Variable temperature NMR studies experimentally confirmed the theoretical prediction, showing, in solution, two conformers with different relative amounts and different interconversion rates between them, depending on the chalcogen nature. From the electrochemical point of view the S, Se and Te complexes display a bi-electronic reversible oxidation peak, differently from the two mono-electronic irreversible oxidation peaks previously observed for the O derivatives. Moreover, a progressive narrowing of the HOMO–LUMO gap on going from O to Te, arising from the increase of the HOMO level, has been observed. This is in line with the decreasing electron-withdrawing strength of the chalcogenide bridging ligand, so that the energy gap for the telluride derivative is 1.64 eV, the smallest value in the whole family of the di-rhenium pyridazine complexes. The spectroscopic HOMO–LUMO gap parallels this trend, with a significant red-shift of the metal-to-ligand charge transfer absorption, making the telluride complex highly promising as a photosensitizer in the field of solar energy conversion. In agreement with the narrow HOMO–LUMO gap, no photoluminescence has been observed upon optical excitation.


 Please wait while we load your content...
Please wait while we load your content...