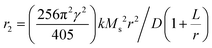Iron carbide nanoparticles: an innovative nanoplatform for biomedical applications
Jing
Yu
ab,
Fan
Chen
a,
Weiliang
Gao
a,
Yanmin
Ju
c,
Xin
Chu
b,
Shenglei
Che
 *a,
Fugeng
Sheng
*d and
Yanglong
Hou
*b
*a,
Fugeng
Sheng
*d and
Yanglong
Hou
*b
aResearch Center of Magnetic and Electronic Materials, College of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: cheshenglei@zjut.edu.cn
bCollege of Engineering, Peking University, Beijing 100871, China. E-mail: hou@pku.edu.cn
cCollege of Life Science, Peking University, Beijing 100871, China
dDepartment of Radiology, Affiliated Hospital of The Academy of Military Medical Sciences, Beijing 100071, China. E-mail: fugeng_sheng@163.com
First published on 21st November 2016
Abstract
Iron carbide nanoparticles (ICNPs) are nano-intermetallic compounds that consist of iron and carbon. Benefiting from the magnetic and chemical activity of iron, and/or mechanical strength and chemical inertness of carbon, they have been widely applied in energetic and biomedical-related fields. Particularly in biomedicine, ICNPs have shown high colloidal stability and good performance in magnetic-dependent diagnosis and therapies such as magnetic resonance imaging (MRI) and magnetic hyperthermia (MH), due to their high magnetization and moderate coercivity. The carbon content protects ICNPs from oxidation and corrosion (ion release), which prolongs their life time and reduces their toxicity in physiological environments, and endows nanoparticles (NPs) with high performance in carbon-relevant theranostics as well. On this basis, ICNPs have great promise in multi-modal imaging or imaging-guided tumor-selective therapy to realize precise diagnoses with mild side effects. This paper aims to cover the state of the art applications of ICNPs in biomedicine, primarily including MRI, MH, magnetic targeting (MT), magnetic separation (MS), photothermal therapy (PTT) and photoacoustic tomography (PAT). The biocompatibility of ICNPs is also addressed.
Introduction
Iron carbide is a kind of intermetallic compound that consists of carbon atoms occupying the interstices between close-packed iron atoms.1 According to the types of interstices that the carbon atoms occupy, they can be further classified to Fe2C, Fe2.2C (trigonal-prismatic interstices), and Fe3C, Fe5C2 and Fe7C3 (octahedral interstices). The presence of carbon atoms provides iron carbides with excellent mechanical strength and chemical inertness, endowing them as crucial components in metallic alloys and hard coatings.2 Meanwhile the chemical activity from iron atoms makes them good candidates for catalysts.3,4 With the development of nanotechnology, iron carbide nanoparticles (ICNPs) have gained tremendous attention due to their unique nanoscale physicochemical properties, and have been widely applied in Fischer–Tropsch syntheses,5–7 oxygen reduction reaction,8–10 electrocatalysis,11,12 batteries,13,14 and supercapacitors.15,16It is noteworthy that similar to most iron-based alloys, ICNPs display appealing magnetic properties, making some magnetic-related applications especially attractive. Magnetic nanoparticles (MNPs) can find applications in various domains including data storage,17 biological18,19 and catalytic fields.20,21 In particular, with the increasing mortality of cancer and cardiovascular diseases, MNPs have been extensively investigated for their biomedical applications, such as magnetic resonance imaging (MRI),22 magnetic hyperthermia (MH),23 magnetic targeting (MT),24 magnetic separation (MS),25 magnetofection,26 biosensors,27 and tissue engineering.28 Iron oxide NPs (IONPs) have been developed to be the most commonly used MNPs in biomedicine due to their tunable magnetic properties as well as their nontoxicity and biodegradability.29,30 However, their further applications are limited by the low saturation magnetization (Ms), since most theranostic effects of MNPs have a positive correlation with the Ms value. Metallic iron NPs have a much higher Ms than IONPs, but they are still unsuitable for medical applications, which can be ascribed to their instability and iron release that can finally result in their toxicity in physiological environments.31,32 ICNPs are certainly promising candidates as they combine a high Ms value with low toxicity. Particularly, Fe5C2 nanoparticles (NPs) have twice the Ms value compared with IONPs, giving rise to significantly improved performance in both MRI and MH.33 The incorporation of carbon in ICNPs elevated their stability. After two months aging in air or by dispersing in an aqueous environment for one month, the ICNPs showed a relatively high stability with the Ms value and morphology retained, leading to reduced toxicity both in vitro and in vivo.33,34 Consequently, ICNPs have emerged as candidates for magnetic-associated biomedical applications in recent years.
In addition to the roles of reinforcement and improving stability, the carbon content also makes ICNPs light responsive. It is widely accepted that carbon-based materials, such as graphene, carbon nanotubes, and graphite shell, can strongly absorb light. By emitting the light absorbed, they are known as probes for fluorescence imaging,35–37 and by converting light energy into other energy such as heat or ultrasound, they can also be used as agents for photothermal therapy (PTT) or photoacoustic tomography (PAT).38,39 Some recent studies have proved that applications of ICNPs in PTT, PAT and light triggered drug delivery are feasible.33,40 Combined with their magnetic properties, ICNPs have shown great promise in biomedical applications to realize multi-modal diagnosis and therapy.
In this mini review, we will summarize the biomedical applications of ICNPs, including MRI, MH, MT, MS, PTT and PAT, with MRI and MH being particularly highlighted. The biocompatibility of ICNPs is also discussed.
Iron carbide nanoparticles for magnetic resonance imaging (MRI)
MRI is regarded as a noninvasive diagnosis tool that generates images with high spatial and temporal resolution. MNPs with unique magnetic properties, which can shorten T2 relaxation times, have been widely employed as negative contrast agents in T2-weighted MRI to improve the signal-to-noise ratio. While conventional T2-weighted MRI contrast agents, most commonly superparamagnetic IONPs, suffer from low transverse relaxivity (r2), which results in the reduction of sensitivity during diagnosis, MNPs with higher r2 values are being pursued to more effectively shorten T2 relaxation times and offer greater MRI contrast enhancement.According to the quantum mechanical outer sphere theory, the r2 value of MNPs is highly dependent on the Ms, which is given by the following equation:41
Iron carbides possess a maximum bulk Ms of 140 emu g−1, which is 52% larger than the value of 92 emu g−1 for IONPs.42 Despite the decrease of the Ms for ICNPs compared with bulk materials, which arises from the small particle size effect and the non-magnetic carbon content, the Ms of ICNPs is still much higher than IONPs. For example, both Fe5C2 and Fe3C NPs show a high Ms at room temperature, with values of 125 emu g−1 and 140 emu g−1, respectively.42,43 Consequently, they can induce greater hypo-intensities on T2-weighted MR imaging compared to the commercially available MRI contrast agent Resovist. The r2 value of Fe5C2 NPs is tested to be 312 mM−1 s−1 on a 3 T clinic MRI scanner, while only 174 mM−1 s−1 can be obtained for Resovist.33 The r2 value for Fe5C2 NPs improves to 464.02 mM−1 s−1 by increasing the magnetic field of the scanner to 7 T, and the value still remains at 283.2 mM−1 s−1 when decreasing the magnet to 0.5 T.34,43 The increased r2 value suggests that these ICNPs have the potential to improve MRI contrast in clinics. By intravenously injecting Fe5C2 NPs into the BALB/c mice, a significantly darker signal in liver regions can be produced in comparison with Fe3O4 NPs, holding great promise of ICNPs for liver MRI.34 After conjugation with targeting motifs, Fe5C2 NPs are effective in serving as MRI contrast agents for some specific tumor types. For instance, the coupling of c(RGDyK) is useful for U87MG tumor imaging, while the linkage of ZHER2:342 can work well for ovarian cancer diagnosis (Fig. 1a and b).33,43
 | ||
| Fig. 1 (a) U87MG tumor bearing mice were intravenously injected with RGD-Fe5C2 NPs. Scans were performed before, and 4 and 24 h after the administration. Tumors are circled by yellow dotted lines, and black areas are highlighted by red arrows. Reproduced with permission from ref. 43. Copyright 2013 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) T2-weighted MR images of SK-OV-3 tumor-bearing mice before and after intravenous injection of Fe5C2-ZHER2:342 for different time points. The tumor sites are circled by a yellow dashed line. The lower row of each figure is the pseudo-color image for the tumor site. Reproduced with permission from ref. 33. Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Quantification of liver contrast changes pre-, 1 h, and 4 h after the injection with Fe5C2 of 5 nm, 14 nm and 22 nm by MRI. (d) r2 relaxivity rates of 22 nm Fe5C2 NPs coated with phospholipids, ZDS, and casein, measured with agarose gel samples containing different concentrations of particles. Reproduced with permission from ref. 44. Copyright 2015 Theranostics Publishing Team. | ||
It is well known that both the size and morphology of MNPs can significantly affect their Ms values. Consequently, further improvement of r2 values can be pursued by synthesis of ICNPs with tunable size and specific morphology. Recently, Xie et al. synthesized Fe5C2 NPs with various sizes by a one-pot method, in which the NPs were achieved by adding Fe(CO)5 to an Ar-purged mixture of octadecylamine and cetyltrimethylammonium bromide (CTAB), followed by heating the mixture up to the boiling point to induce Fe(CO)5 oxidation and carbonization.44 The size of the NPs was tuned by the amount of the Fe(CO)5 precursor, which finally resulted in NPs with diameters of 5 nm, 14 nm, and 22 nm. The impact of size on the performance for MRI was then investigated, which showed that larger Fe5C2 NPs have higher r2 relaxivities (ranging from 342 mM−1 s−1 to 460 mM−1 s−1), due to the surface canting effect. As a result, after intravenously injecting these NPs into nude mice, a more significant drop in signal intensity was observed in the livers of mice treated with 22 nm-sized Fe5C2 NPs, compared to those administered with 5 nm- or 14 nm-sized NPs (Fig. 1c).
Fabricating ICNPs with a certain composition or structure is another way to improve the Ms value. For example, ICNPs composed of 43% Fe2.2C and 43% Fe5C2 showed an Ms of 132 emu g−1, which is higher than pure Fe5C2 NPs (125 emu g−1). The core/shell structure with a bcc-iron(0) core and iron carbide coating (20% Fe5C2 and 23% Fe2.2C) can improve the Ms value to 195 emu g−1, which might originate from the exchange-coupling effect.45 This value can be further increased to 202 emu g−1 by Fe/Fe3C NPs, being among the highest reported colloidally stable NPs. The ultra-high Ms value of these ICNPs means they hold great potential as MRI contrast agents with high r2 relaxivity.46
The improvement of the r2 value can also be realized by varying the surface modification method. It is reported that casein, a family of phosphoproteins that consists of κ-casein, has a unique elongated “hair-like” structure. When ICNPs were modified by casein, a protein layer was formed, presenting long, hydrophilic channels that enable water molecules to come in and interact with the inner water layer that is close to the particle surface. Accordingly, a striking r2 value was achieved by coating casein on the Fe5C2 NPs, which reached 973 mM−1 s−1, and was twice that of Fe5C2 coated with PEGylated phospholipid or zwitterion-dopamine-sulfonate, and among the highest T2 contrast agents (Fig. 1d).44
Overall, ICNPs have high internal magnetization. Precisely tuning the component, size, and structure, as well as controlled surface modification of the NPs, can further improve their Ms value. Together with the high stability and low toxicity, ICNPs are promising candidates for succedaneums for IONPs in MRI.
Iron carbide nanoparticles for magnetic hyperthermia (MH)
MH is a cancer therapy that is based on heat production by exposing MNPs to an alternating current (AC) magnetic field, and the difference in heat tolerance between healthy tissue and tumor cells. It is of growing interest since a recent study in phase II clinical trials on patients suffering from glioblastoma multiforme showed an improvement in patient survival from 6.2 to 13.4 months by using MH.47 The capacity of MNPs to generate heat is commonly characterized by its specific absorption rate (SAR), but the low SAR produced by clinically used MH agents is a barrier that limits the further applications of MH. It might be because heat dissipated by MNPs under an AC magnetic field is determined by two mechanisms, i.e., hysteresis and relaxation losses, while traditionally MH agents in the clinic are IONPs, which are superparamagnetic and can only excite the relaxation process. In addition, it is reported that an SAR of ca. 1 kW g−1 is required for clinical therapeutic applications, and therefore, MH applied nowadays usually requires local injection of a large quantity of MNPs.48 To reduce the quantity of agents injected, MNPs with a higher SAR are desired. Using ferromagnetic NPs instead of superparamagnetic NPs may be a promising way, in which the hysteresis loss is the dominant factor for heat dissipation.As a rough measure for maximum hysteresis loss of ferromagnetic NPs, the product of the remanent magnetization (Mr) and coercivity (Hc) can be taken. Therefore, NPs with an improved Hc, such as iron, cobalt and FePt NPs, are of particular interest to deliver higher SARs. But still, their clinical application is hindered, due to the fact that the maximum hysteresis loss occurs only if the external field exceeds the coercivity field. When the field is below that strength, the loss is negligibly small. Unfortunately, the tissue tolerance of inductive heating limits the safe range of magnetic field amplitudes (H) and frequencies (f) that can be employed for MH therapy, where the product of H and f should be less than 4.85 × 108 A m−1 s−1. In most reports, the magnetic field is measured in the range of 10–30 kA m−1, which is lower than the Hc of most ferromagnetic NPs.49–51 Developing MNPs with softer ferromagnetic properties is desirable by considering both the higher temperature rise and better tissue tolerance.
ICNPs have emerged as good candidates for MH agents recently in view of their moderate Hc. Sajitha investigated the magnetic properties of Fe3C NPs with different diameters, suggesting that their coercivities are size-dependent, varying from 240 Oe to 360 Oe at room temperature.52 Fe5C2 NPs and Fe2C were also shown to be soft magnetic NPs, with a Hc of about 200 Oe and 420 Oe respectively, both of which are within the safety limit of applied magnetic fields.34,45 The reduced Hc also maintained the colloidal stability of ICNPs, and this means physiological aggregation is avoided. The absence of a toxic element such as cobalt or platinum further improved the biocompatibility of the ICNPs. In addition, in a system composed of soft MNPs with a low Hc, relaxation is a non-negligible mechanism that needs to be considered for thermal energy dissipation. It is accepted that increasing either the effective anisotropy (Keff) or Ms can result in an improvement in the relaxation, whether Néel or Brown, and thus, higher Keff or Ms is preferred.53–55 Compared with clinically proven MH agents maghemite and magnetite, which have Keff values of 4.7 kJ m−3 and 9 kJ m−3 respectively, ICNPs showed a much higher Keff, with a value for Fe2.2C of 117 kJ m−3.45,56 As discussed previously, ICNPs also have a greater Ms than IONPs. Moreover, ICNPs are intermetallic in nature, with an electrical conductivity of 12.7 × 103 Ω−1 cm−1 at 373 K, which results in additional heat generation due to eddy current losses under an AC magnetic field compared with IONPs.57 All these properties make ICNPs good candidates with elevated SAR values and improved biocompatibility for better application in MH.
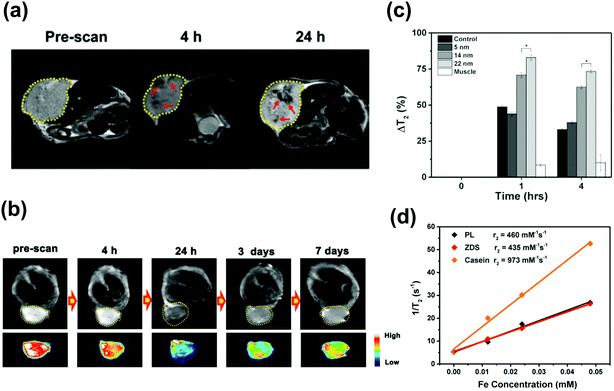
Chaudret et al. synthesized Fe2.2C NPs with diameters of 13.1 nm by a two-step method, in which the “seed” iron(0) NPs were synthesized first, and then carbonized with Fe(CO)5 under H2 protection. The obtained NPs had an Ms of 132 emu g−1, Hc of 420 Oe and Keff of 6.8 × 104 J m−3, which achieved an improved SAR value of ca. 260 W g−1 even under a lower magnetic frequency (96 kHz and 60 mT) compared with Fe3O4 NPs (81 W g−1 at 160 KHz, 60 mT).45,58 However, the heat released by the Fe2.2C NPs was extremely low (5.8 W g−1) when decreasing the external magnetic field (20 mT), due to the anisotropy axis of the NPs being randomly oriented in space (Fig. 2a). This problem can be solved by softening ICNPs through decreasing the anisotropy or Hc to make the NPs still in the ferromagnetic regime at a reduced magnetic field, with the anisotropy axes aligned along the magnetic field direction.54 Since the coercive field is strongly dependent on the carbon content of the ICNPs (Fig. 2b), and the amount of carbon within iron carbides can be modulated by varying the amount of Fe(CO)5 and the reaction atmosphere (by replacing H2 by Ar), the authors optimize the NPs to be core/shell structured with an iron core and the shell composed of iron carbide. The optimized NPs exhibit an improved Ms of 195 emu g−1 and a decreased Hc of 250 Oe, and an abrupt increase of SAR was observed when breaking the critical field (17 mT) (Fig. 2a), which is 3 times larger than the values reported for the best chemically synthesized NPs, showing promise to be used under clinical conditions.45 A similar study was reported by Bahadur et al. by fabricating Fe3O4/Fe3C core/shell NPs, with an Ms of 90 emu g−1 and Hc of 60 Oe. Under a magnetic field of 32.5 mT and frequency of 250 kHz, the SAR was measured to be 375 W g−1.57 The high heat generated by ICNPs proved they are promising for MH.
 | ||
| Fig. 2 (a) SAR measurements at fexc = 54 kHz. (b) Dependence of the coercive fields deduced from hyperthermia measurements vs. the iron content determined from XRD data.45 Reproduced with permission from ref. 45. Copyright © 2012, American Chemical Society. | ||
For traditional IONP MH agents, some extrinsic characteristics of NPs such as morphology, composition, and size distribution are also key in controlling their heat generation, apart from the above-mentioned intrinsic properties.59,60 ICNPs are a kind of novel MNPs that might have similar characteristics to IONPs. As a result, the synthesis of mono-disperse ICNPs with controlled size, morphology and composition, and investigating their influences on heat dissipation, are of great importance. However, up to now, only several reports have concerned the controlled preparation of ICNPs, and there is still a lack of relevant reports on their effects on hyperthermia. More efforts in the future are needed on these aspects.
Other biomedical applications of iron carbide nanoparticles
Except for MRI and MH, MT is another application for ICNPs due to their benign magnetic properties. After being exposed to an external magnetic field, ICNPs can be magnetized, driven magnetically, and concentrated at the desired site, which reduces the dosage required, eliminates associated side effects, and fixes particles at a local site to keep them away from the reticuloendothelial system (RES) as well. For example, Hou et al. pointed out that after injecting Fe5C2 NPs into tumor-bearing mice through the tail vein, a much higher level of NPs accumulated in the tumor by MT. By loading the anti-cancer drug doxorubicin (DOX) onto NPs, a remarkable red fluorescence was observed at the tumor site in the magnetic-targeting group (Fig. 3a), and a reduced premature release in normal organs was also achieved, suggesting that ICNPs are promising for targeted drug delivery.40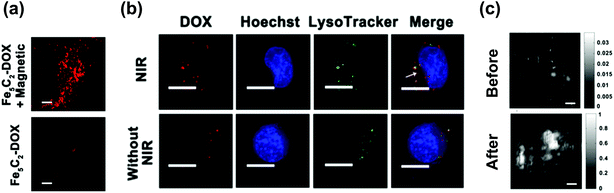 | ||
| Fig. 3 (a) Fluorescence images of DOX in tumors by a frozen section. The scale bars are 200 μm. (b) Fluorescence images of SK-OV-3 cells treated with Fe5C2-DOX NPs for 2 h followed by irradiation/non-irradiation (808 nm, 0.8 W cm−2, 5 min). Cells were re-incubated for 30 min after treating. Nuclei were stained with Hoechst 33342. The scale bars are 20 μm. Lysosomes were stained with LysoTracker Green, and the arrowed part is cytosol. Reproduced with permission from ref. 40. Copyright © 2016, American Chemical Society. (c) PAT results of the tumor site before and after intravenously injecting with Fe5C2 NPs for 24 h. The scale bars are 1 mm. The grey bars are the photoacoustic signal intensity. Reproduced with permission from ref. 33. Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
ICNPs were also proposed for MS by binding the target compounds on NPs first and then re-collecting them by an external magnetic field. Particularly in biomedicine, this process can be applied for blood purification of toxins, urea, germs or other deleterious materials.61 García-Rosales et al. reported the efficient removal of highly toxic As(V) by ICNPs, with the maximum As(V) uptake of 1.4 mg g−1.62 Compared with iron NPs, ICNPs showed a better adsorption capacity, with the rate constant k2 and the amount of solute adsorbed at equilibrium qe 5 and 4.2 times higher, respectively. Similarly, Fe/Fe3C NPs were applied to immobilize Se(IV), which is an essential trace element, but is toxic if taken in excess. Se(IV) sorption is rapid, with the equilibrium reached in approximately 30 min. Interestingly, the presence of organic ligands such as lysine and dodecanoate, which are of relevance to clinical conditions, has no visible effect on the sorption kinetics. Compared with Fe3O4 NPs, Fe/Fe3C NPs are superior, due to their ability to immobilize Se(IV) rapidly and irreversibly, indicating that ICNPs are good candidates for magnetic purification.46
Apart from the magnetic component iron, carbon is also incorporated in ICNPs, which on the one hand tunes the magnetic properties of the NPs, and on the other hand, gives NPs carbon-related properties. In addition, most reported protocols for the synthesis of ICNPs resulted in a carbon layer coating on the NPs during the carburization, promoting the functions of carbon.2,42,63,64 Studies on NPs with similar structures have shown the feasibility of carbon-based theranostics. For example, core/shell-structured FeCo/graphite NPs have been proved to show high optical absorbance in the near infrared (NIR) region, and can work effectively for PTT.65 Accordingly, Hou's group synthesized Fe5C2 NPs with a thin carbon shell coating, and investigated their carbon-relevant properties.33 The NPs exhibited an appreciable absorbance in the NIR region, and a significant temperature increment could be seen when excited under NIR laser radiation. This photothermal conversion is able to elevate the temperature of tumor centers from 26 °C to 47 °C within 2 min on nude mice with an intravenous injection of NPs, and can completely eliminate the tumor within 45 days without relapse. In addition, heat produced by NPs is also capable of improving the drug delivery for synergetic therapy.40 After engulfing the DOX-loaded ICNPs into lysosomes through endocytosis, the escaping of DOX from lysosomes to the cytosol is significantly enhanced after NIR irradiation, which promotes the transporting of DOX to the nucleus to effectively induce cell apoptosis (Fig. 3b). The combination of PTT with enhanced drug release can more efficiently inhibit tumors under NIR irradiation. Furthermore, by transforming the heat energy into ultrasound through adiabatic expansion, PAT is expected for the tumor diagnosis. A strongly enhanced photoacoustic signal in the tumor was observed 24 h post intravenous injection of Fe5C2 NPs (Fig. 3c), being a supplementary method to MRI for fault-free tumor diagnosis.33
Biocompatibility of iron carbide nanoparticles
The applications of NPs in biomedicine should meet a number of specific requirements, generally including biocompatibility, chemical activity, resistance to aggregation in biological media, dimensional homogeneity and the ability to functionalize their surface with different types of ligands.66 As discussed previously, ICNPs have high colloidal stability which reduces the particle aggregation. Of note is that for iron-containing NPs, it is pivotal to explore their free iron release due to the association between the release of free iron and oxidative DNA damage, which could further lead to mutagenic and/or carcinogenic responses.67 The incorporation of carbon content, even a carbon shell/layer, in ICNPs gives the NPs better chemical inertness, which decreases the iron release.57 As a result, ICNPs are expected to have higher biocompatibility than iron NPs or IONPs.A series of cytotoxicity assays of ICNPs, in the forms of Fe5C2 NPs, Fe3C NPs or Fe7C3 NPs, were carried out on RAW 264.7 (macrophage) cells, NIH 3T3 (mouse fibroblast) cells, L929 (mouse fibroblast) cells, SK-BR-3 (human breast adenocarcinoma) cells, MCF-7 (human breast adenocarcinoma) cells, SK-OV-3 (human ovarian carcinoma) cells, HeLa (cervical cancer) cells and U87MG (human glioblastoma) cells through tetrazolium-based colorimetric assays (MTT assays) and/or live/dead cell discrimination.33,34,43,57 No significant cytotoxicity was observed for all ICNPs within all cell lines, indicating the good biocompatibility of the ICNPs. However, some toxic effects of NPs may affect cellular physiology (such as DNA replication, cell division, etc.) in more subtle ways without causing cell death, which means there is a need for more efficient assays. Khabashesku et al. studied the cytotoxicity of Fe7C3 NPs based on proliferation assays and long-term live cell imaging.66 The results suggested that the presence of Fe7C3 NPs has no significant effect on the cell morphology. Engulfed NPs do not interfere with the process of mitotic division, and cells can successfully enter and complete mitosis at rates comparable to those of non-treated ones. Even cells heavily loaded with Fe7C3 NPs 24 h post incubation are capable of DNA synthesis, without statistically significant changes of the DNA synthesis precursor EdU. Assayed by microscopic analysis of the nuclear morphology, almost no difference in the cellular proliferation rate and cell viability were observed during 10 days of culturing cells with NPs, demonstrating that Fe7C3 NPs not only have low cytotoxicity, but also do not affect the cytophysiological parameters of in vitro cultured cells.
In addition to in vitro assays, more comprehensive studies were investigated in vivo to judge whether ICNPs are suitable for biomedical applications. Hou et al. recorded the daily activity of mice for 45 days after the administration of Fe5C2 NPs, and the results showed no abnormality in the animals' body weight, eating and drinking. Also, no significant abnormality in the major organs was observed by investigating the viscera index and H&E staining.33 Likewise, Schumacher et al. examined the body weight and oxygen partial pressure of mice 1 week after intravenously injecting them with Fe3C NPs, finding that the overall condition and pulmonary function remained unaffected.68 No detectable injury of the abdominal organs was induced, with plasma biomarkers, albumin and plasma glucose levels, creatinine, blood cell integrity and coagulation, and inflammatory plasma markers remaining comparable to the controls, suggesting ICNPs are physiologically tolerable in the short-term.
However, it is still premature to introduce ICNPs into clinical applications for the risks of the latency period of materials to the initial exposure, which can last up to 20–40 years.69 Risk evaluation based on long-term exposure scenarios should be investigated. Schumacher's group examined the long-term effects of Fe3C NPs on mice after repeated dose administration over 1 year, which covered a significant fraction of the biological lifespan of mice.68 The results showed that the exposure to Fe3C NPs did not induce any negative side effects, with normal body weights and clinical features. The mice under investigation displayed no signs of organ injury, and the pulmonary function, blood counts and plasma parameters remained comparable to the controls. No areas of necrosis, fibrosis or neoplastic lesions were found, neither macroscopically nor histologically. Even no significant immunological (inflammation or fibrosis) response was detected, and the glucose metabolism of the animals also remained unaffected. This reveals that long-term exposure to comparatively high doses of Fe3C NPs is well tolerated.
The high biocompatibility of ICNPs are to some extent ascribed to the carbon-based protection. It is shown that carbon-encapsulated Fe3C NPs are rather stable after incubation in acidic solutions in vitro, with the iron concentration in the PBS solutions remaining below the limit of detection after 1 h incubation. Even under relatively harsh acidic conditions (0.1 M HCl), the amount of NPs disintegrated was only 0.14 wt%, compared to 1.90 wt% of Fe3O4 NPs dissolved.70 However, due to the differences among the contents (Fe2C, Fe2.2C, Fe3C, Fe5C2 or Fe7C3) and structures (single particle or core/shell structure, amorphous carbon or graphite coating), the chemical activities of different ICNPs are varied. For example, compared to carbon-coated Fe3C, the study by Xie’s group demonstrated that Fe5C2 NPs, even with a carbon shell, still lack stability, with most of the NPs partially or completely degraded in a pH 5.0 buffer solution within 72 h, which may produce an adverse long-term effect within a biological system, probably due to the amorphous structure of carbon.2,43 This leads to the differences of biocompatibility among the ICNPs, and there is a need to systematically explore the impact of ICNPs in physiological environments in more detail for optimizing ICNPs in biomedicine.
In addition to the acute toxicity assessment, another requirement launched by the FDA is the clearance or degradation of NPs. Schumacher et al. found that similar to the observations on IONPs, PEGylated ICNPs predominantly accumulated in tissues with high phagocyte activity, i.e. liver and lung, 24 h post intravenous injection, with the highest concentration in lung tissue, while organs with less phagocytes, such as the kidneys and brain, were histologically free of ICNPs.68 However, the pulmonary tissue trapping of NPs was unexpected in clinical trials. Fortunately, there is a trend of a decrease in the particle concentrations of ICNPs over time in lung and liver tissue, suggesting that ICNPs are either degraded or excreted. However, the degradation/excretion rate was slow, and the particles were still detectable in mice 1 year after injection. The high stability of ICNPs in vivo may increase their risk in clinical trials, especially their long-term effects. Moreover, it seems that ICNPs could be extracted from the body, but their metabolic pathways remained unclear, and how long it takes for these NPs to be totally cleared or degraded is also unknown. Both of these problems need further clarification.
Conclusions and perspectives
ICNPs are a series of nano-intermetals that consist of iron and carbon atoms with different element ratios and atomic occupations. The composition of iron endows ICNPs with high magnetization and moderate coercivity, giving them promise in magnetic-dependent biomedical applications, including MRI, MH, MT and MS. ICNPs exhibit better performance compared with clinically used IONPs due to their improved magnetic properties. As another crucial ingredient for ICNPs, carbon is able to regulate the magnetic properties on the one hand, and can give the NPs high stability on the other hand. As a result, ICNPs are capable of holding their high performance for a longer time and showing higher compatibility both in vitro and in vivo. The carbon content also renders ICNPs NIR-absorbable, which is beneficial for their application in PTT and PAT. The combination of MRI and PAT makes ICNPs feasible for multi-modal imaging for precise diagnosis, and together with MH, PTT and MT, these NPs have great potential for imaging-guided targeted therapy with mild side effects.Despite the considerable achievements, the clinical application of ICNPs is still premature. Firstly, synthetic protocols developed nowadays can only prepare several kinds of pure ICNPs, and their microstructures were difficult to be subtly tuned. As the performance and toxicity of ICNPs are dispersion-, composition- and nanostructure-dependent, the synthesis of mono-disperse ICNPs with tunable compositions, sizes, morphology and structures needs more attention. The second inconvenience is the incompleteness/non-uniformity during carbonization, which leads to the presence of pure metals with possible high toxicity.66 Controlling the nucleation, growth and carbonization process during the synthesis of ICNPs is still an unsolved problem. The third problem comes from the surface modification of ICNPs, which may affect their in vivo behavior as well as their performance as contrast agents. More insightful investigations into the surface modification of ICNPs is required. In addition, more efforts are needed on the comparison of the imaging/therapeutic effect, as well as the biocompatibility among ICNPs with different compositions and structures to optimize the NPs applied, and also the intrinsic mechanisms. Ultimately, to meet the FDA's demands for intravenously injected. NPs, which should be cleared in a reasonable amount of time, the biodistribution and degradation/metabolic pathways of ICNPs need to be further investigated. More importantly, learning from the lessons of IONP-based Ferridex and Resovist, which were approved for clinical use but have been abandoned as MRI contrast agents since 2009 due to a lack of sales and poor performance in clinical trials,71 ICNPs with compelling advantages in both MRI and MH in vitro still have a long way to go to verify their feasibility in clinical applications. The performance of ICNPs in clinical trials remains to be studied. Anyhow, despite the challenges confronted, it is believed that with interdisciplinary collaborations from physics, chemistry, biology, pharmacy, and clinical medicine, ICNPs hold great promise in biomedicine, and even could be applied in clinical trials in the near future.
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (NSFC) (No. 51602285, 21575161, 81421004, 51672010), the Key Laboratory of Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Chinese Academy of Sciences (No. NSKF201607).References
- D. B. Cao, F. Q. Zhang, Y. W. Li, J. G. Wang and H. J. Jiao, J. Phys. Chem. B, 2005, 109, 10922–10935 CrossRef CAS PubMed.
- C. Yang, H. B. Zhao, Y. L. Hou and D. Ma, J. Am. Chem. Soc., 2012, 134, 15814–15821 CrossRef CAS PubMed.
- H. Park, D. H. Youn, J. Y. Kim, W. Y. Kim, Y. H. Choi, Y. H. Lee, S. H. Choi and J. S. Lee, ChemCatChem, 2015, 7, 3488–3494 CrossRef CAS.
- Y. Zhang, W. J. Jiang, L. Guo, X. Zhang, J. S. Hu, Z. D. Wei and L. J. Wan, ACS Appl. Mater. Interfaces, 2015, 7, 11508–11515 Search PubMed.
- E. de Smit, I. Swart, J. F. Creemer, G. H. Hoveling, M. K. Gilles, T. Tyliszczak, P. J. Kooyman, H. W. Zandbergen, C. Morin, B. M. Weckhuysen and F. M. F. de Groot, Nature, 2008, 456, 222–239 CrossRef CAS PubMed.
- J. Blanchard and N. Abatzoglou, Catal. Today, 2014, 237, 150–156 CrossRef CAS.
- K. Xu, B. Sun, J. Lin, W. Wen, Y. Pei, S. Yan, M. Qiao, X. Zhang and B. Zong, Nat. Commun., 2014, 5, 5783–5791 CrossRef CAS PubMed.
- M. L. Xiao, J. B. Zhu, L. G. Feng, C. P. Liu and W. Xing, Adv. Mater., 2015, 27, 2521–2527 CrossRef CAS PubMed.
- M. B. Zakaria, RSC Adv., 2016, 6, 10341–10351 RSC.
- V. Vij, J. N. Tiwari, W. G. Lee, T. Yoon and K. S. Kim, Sci. Rep., 2016, 6, 20132 CrossRef CAS PubMed.
- H. Huang, X. Feng, C. C. Du, S. Y. Wu and W. B. Song, J. Mater. Chem. A, 2015, 3, 4976–4982 RSC.
- Z. Y. Wu, X. X. Xu, B. C. Hu, H. W. Liang, Y. Lin, L. F. Chen and S. H. Yu, Angew. Chem., Int. Ed., 2015, 54, 8179–8183 CrossRef CAS PubMed.
- Y. L. Tan, K. Zhu, D. Li, F. Bai, Y. J. Wei and P. Zhang, Chem. Eng. J., 2014, 258, 93–100 CrossRef CAS.
- K. Ujimine and A. Tsutsumi, J. Power Sources, 2006, 160, 1431–1435 CrossRef CAS.
- M. L. Yan, Y. D. Yao, J. Q. Wen, W. D. Fu, L. Long, M. Wang, X. M. Liao, G. F. Yin, Z. B. Huang and X. C. Chen, J. Alloys Compd., 2015, 641, 170–175 CrossRef CAS.
- E. C. Vermisoglou, E. Devlin, T. Giannakopoulou, G. Romanos, N. Boukos, V. Psycharis, C. Lei, C. Lekakou, D. Petridis and C. Trapalis, J. Alloys Compd., 2014, 590, 102–109 CrossRef CAS.
- S. H. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989–1992 CrossRef CAS PubMed.
- E. Duguet, S. Mornet, S. Vasseur, F. Grasset, P. Veverka, G. Goglio, A. Demourgues, J. Portier and E. Pollert, Prog. Solid State Chem., 2006, 34, 237–247 CrossRef.
- D. Ho, X. Sun and S. Sun, Acc. Chem. Res., 2011, 44, 875–882 CrossRef CAS PubMed.
- A. H. Lu, W. Schmidt, N. Matoussevitch, H. Bonnemann, B. Spliethoff, B. Tesche, E. Bill, W. Kiefer and F. Schuth, Angew. Chem., Int. Ed., 2004, 43, 4303–4306 CrossRef CAS PubMed.
- S. C. Tsang, V. Caps, I. Paraskevas, D. Chadwick and D. Thompsett, Angew. Chem., Int. Ed., 2004, 43, 5645–5649 CrossRef CAS PubMed.
- G. Lamanna, M. Kueny-Stotz, H. Mamlouk-Chaouachi, C. Ghobril, B. Basly, A. Bertin, I. Miladi, C. Billotey, G. Pourroy, S. Begin-Colin and D. Felder-Flesch, Biomaterials, 2011, 32, 8562–8573 CrossRef CAS PubMed.
- D. Ho, X. L. Sun and S. H. Sun, Acc. Chem. Res., 2011, 44, 875–882 CrossRef CAS PubMed.
- J. Yu, D. Y. Huang, M. Z. Yousaf, Y. L. Hou and S. Gao, Chin. Phys. B, 2013, 22, 23–35 Search PubMed.
- R. Gao, S. Zhao, Y. Hao, L. Zhang, X. Cui, D. Liu, M. Zhang and Y. Tang, J. Sep. Sci., 2015, 38, 3914–3920 CrossRef CAS PubMed.
- J. Kim, J. E. Lee, S. H. Lee, J. H. Yu, J. H. Lee, T. G. Park and T. Hyeon, Adv. Mater., 2008, 20, 478–483 CrossRef CAS.
- M. Martin, P. Salazar, R. Villalonga, S. Campuzano, J. M. Pingarron and J. L. Gonzalez-Mora, J. Mater. Chem. B, 2014, 2, 739–746 RSC.
- J. Yu, F. Liu, M. Z. Yousaf and Y. L. Hou, Prog. Biochem. Biophys., 2013, 40, 903–917 CAS.
- R. Hao, J. Yu, Z. G. Ge, L. Y. Zhao, F. G. Sheng, L. L. Xu, G. J. Li and Y. L. Hou, Nanoscale, 2013, 5, 11954–11963 RSC.
- D. Ling, N. Lee and T. Hyeon, Acc. Chem. Res., 2015, 48, 1276–1285 CrossRef CAS PubMed.
- S. Peng, C. Wang, J. Xie and S. H. Sun, J. Am. Chem. Soc., 2006, 128, 10676–10677 CrossRef CAS PubMed.
- H. Markides, M. Rotherham and A. J. El Haj, J. Nanomater., 2012, 5, 4873–4881 Search PubMed.
- J. Yu, C. Yang, J. D. S. Li, Y. C. Ding, L. Zhang, M. Z. Yousaf, J. Lin, R. Pang, L. B. Wei, L. L. Xu, F. G. Sheng, C. H. Li, G. J. Li, L. Y. Zhao and Y. L. Hou, Adv. Mater., 2014, 26, 4114–4120 CrossRef CAS PubMed.
- G. Huang, J. Hu, H. Zhang, Z. Zhou, X. Chi and J. Gao, Nanoscale, 2014, 6, 726–730 RSC.
- M. Hazani, R. Naaman, F. Hennrich and M. M. Kappes, Nano Lett., 2003, 3, 153–155 CrossRef CAS.
- P. Huang, J. Lin, X. S. Wang, Z. Wang, C. L. Zhang, M. He, K. Wang, F. Chen, Z. M. Li, G. X. Shen, D. X. Cui and X. Y. Chen, Adv. Mater., 2012, 24, 5104–5110 CrossRef CAS PubMed.
- Q. Liu, B. D. Guo, Z. Y. Rao, B. H. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed.
- Y. W. Chen, T. Y. Liu, P. J. Chen, P. H. Chang and S. Y. Chen, Small, 2016, 12, 1458–1468 CrossRef CAS PubMed.
- J. Yu, X. Chu and Y. L. Hou, Chem. Commun., 2014, 50, 11614–11630 RSC.
- J. Yu, Y. M. Ju, L. Y. Zhao, X. Chu, W. L. Yang, Y. L. Tian, F. G. Sheng, J. Lin, F. Liu, Y. H. Dong and Y. L. Hou, ACS Nano, 2016, 10, 159–169 CrossRef CAS PubMed.
- A. Roch, R. N. Muller and P. Gillis, J. Chem. Phys., 1999, 110, 5403–5411 CrossRef CAS.
- I. K. Herrmann, R. N. Grass, D. Mazunin and W. J. Stark, Chem. Mater., 2009, 21, 3275–3281 CrossRef CAS.
- W. Tang, Z. P. Zhen, C. Yang, L. N. Wang, T. Cowger, H. M. Chen, T. Todd, K. Hekmatyar, Q. Zhao, Y. L. Hou and J. Xie, Small, 2014, 10, 1245–1249 CrossRef CAS PubMed.
- T. A. Cowger, W. Tang, Z. P. Zhen, K. Hu, D. E. Rink, T. J. Todd, G. D. Wang, W. Z. Zhang, H. M. Chen and J. Xie, Theranostics, 2015, 5, 1225–1232 CrossRef CAS PubMed.
- A. Meffre, B. Mehdaoui, V. Kelsen, P. F. Fazzini, J. Carrey, S. Lachaize, M. Respaud and B. Chaudret, Nano Lett., 2012, 12, 4722–4728 CrossRef CAS PubMed.
- R. L. D. Loyo, S. I. Nikitenko, A. C. Scheinost and M. Simonoff, Environ. Sci. Technol., 2008, 42, 2451–2456 CrossRef PubMed.
- K. Maier-Hauff, F. Ulrich, D. Nestler, H. Niehoff, P. Wust, B. Thiesen, H. Orawa, V. Budach and A. Jordan, J. Neuro-Oncol., 2011, 103, 317–324 CrossRef PubMed.
- P. Wust, U. Gneveckow, M. Johannsen, D. Bohmer, T. Henkel, F. Kahmann, J. Sehouli, R. Felix, J. Ricke and A. Jordan, Int. J. Hyperthermia, 2006, 22, 673–685 CrossRef CAS PubMed.
- S. Dutz and R. Hergt, Nanotechnology, 2014, 25, 452001 CrossRef PubMed.
- R. D. Rutledge, W. H. Morris, M. S. Wellons, Z. Gai, J. Shen, J. Bentley, J. E. Wittig and C. M. Lukehart, J. Am. Chem. Soc., 2006, 128, 14210–14211 CrossRef CAS PubMed.
- T. Ibusuki, S. Kojima, O. Kitakami and Y. Shimada, IEEE Trans. Magn., 2001, 37, 2223–2225 CrossRef CAS.
- E. P. Sajitha, V. Prasad, S. V. Subramanyam, A. K. Mishra, S. Sarkar and C. Bansal, J. Phys.: Condens. Matter, 2007, 19, 046214 CrossRef.
- E. A. Perigo, G. Hemery, O. Sandre, D. Ortega, E. Garaio, F. Plazaola and F. J. Teran, Appl. Phys. Rev., 2015, 2, 041302 Search PubMed.
- B. Mehdaoui, A. Meffre, L.-M. Lacroix, J. Carrey, S. Lachaize, M. Respaud, M. Gougeon and B. Chaudret, J. Appl. Phys., 2010, 107, 09A324 CrossRef.
- A. H. Habib, C. L. Ondeck, P. Chaudhary, M. R. Bockstaller and M. E. McHenry, J. Appl. Phys., 2008, 103, 07A307 CrossRef.
- S. Maenosono and S. Saita, IEEE Trans. Magn., 2006, 42, 1638–1642 CrossRef CAS.
- M. Sharma, S. Mantri and D. Bahadur, J. Magn. Magn. Mater., 2012, 324, 3975–3980 CrossRef CAS.
- C. Blanco-Andujar, D. Ortega, P. Southern, Q. A. Pankhurst and N. T. K. Thanh, Nanoscale, 2015, 7, 1768–1775 RSC.
- C. Blanco-Andujar, A. Walter, G. Cotin, C. Bordeianu, D. Mertz, D. Felder-Flesch and S. Begin-Colin, Nanomedicine, 2016, 11, 1889–1910 CrossRef CAS PubMed.
- F. Gazeau, M. Levy and C. Wilhelm, Nanomedicine, 2008, 3, 831–844 CrossRef CAS PubMed.
- I. K. Herrmann, A. Schlegel, R. Graf, C. M. Schumacher, N. Senn, M. Hasler, S. Gschwind, A.-M. Hirt, D. Gunther, P.-A. Clavien, W. J. Stark and B. Beck-Schimmer, Nanoscale, 2013, 5, 8718–8723 RSC.
- O. E. Gutierrez-Muñiz, G. García-Rosales, E. Ordoñez-Regil, M. T. Olguin and A. Cabral-Prieto, J. Environ. Manage., 2013, 114, 1–7 CrossRef PubMed.
- X. Wang, P. Zhang, J. Gao, X. Chen and H. Yang, Dyes Pigm., 2015, 112, 305–310 CrossRef CAS.
- V. Davydov, A. Rakhmanina, H. Allouchi, C. Autret, P. Limelette and V. Agafonov, Fullerenes, Nanotubes, Carbon Nanostruct., 2012, 20, 451–454 CrossRef CAS.
- W. S. Seo, J. H. Lee, X. M. Sun, Y. Suzuki, D. Mann, Z. Liu, M. Terashima, P. C. Yang, M. V. McConnell, D. G. Nishimura and H. J. Dai, Nat. Mater., 2006, 5, 971–976 CrossRef CAS PubMed.
- V. Davydov, A. Rakhmanina, I. Kireev, I. Alieva, O. Zhironkina, O. Strelkova, V. Dianova, T. D. Samani, K. Mireles, L. H. Yahia, R. Uzbekov, V. Agafonov and V. Khabashesku, J. Mater. Chem. B, 2014, 2, 4250–4261 RSC.
- X. H. Wu, S. Kannan, V. M. S. Ramanujam and M. F. Khan, J. Toxicol. Environ. Health, Part A, 2005, 68, 657–666 CrossRef CAS PubMed.
- I. K. Herrmann, B. Beck-Schimmer, C. M. Schumacher, S. Gschwind, A. Kaech, U. Ziegler, P. A. Clavien, D. Gunther, W. J. Stark, R. Graf and A. A. Schlegel, Nanomedicine, 2016, 11, 783–796 CrossRef CAS PubMed.
- G. Frost, Br. J. Cancer, 2013, 109, 1965–1973 CrossRef CAS PubMed.
- C. M. Schumacher, I. K. Herrmann, S. B. Bubenhofer, S. Gschwind, A. M. Hirt, B. Beck-Schimmer, D. Gunther and W. J. Stark, Adv. Funct. Mater., 2013, 23, 4888–4896 CrossRef CAS.
- Y.-X. J. Wang, Quant. Imaging Med. Surg., 2011, 1, 35–40 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |