Design and engineering of high-performance photocatalytic systems based on metal oxide–graphene–noble metal nanocomposites
Narendra
Singh
 a,
Jai
Prakash
a,
Jai
Prakash
 *ab and
Raju Kumar
Gupta
*ab and
Raju Kumar
Gupta
 *ac
*ac
aDepartment of Chemical Engineering and Center for Nanosciences, Indian Institute of Technology Kanpur, Kanpur-208016, UP, India. E-mail: jaip@iitk.ac.in; jai.gupta1983@gmail.com; guptark@iitk.ac.in; Fax: +91 5122590104; Tel: +91 5122596972
bDepartment of Physics, University of the Free State, Bloemfontein-9300, South Africa
cCenter for Environmental Science and Engineering, Indian Institute of Technology Kanpur, Kanpur-208016, UP, India
First published on 14th August 2017
Abstract
The present mini-review deals with recent developments in designing and engineering high-performance photocatalytic systems based on functional nanomaterials including molecular graphene, noble metals, and metal oxide nanostructures. Major research is focused on designing photo-functional systems with enhanced optical properties, charge separation, high charge transfer, and photocatalytic efficiency. Regarding these challenging issues, several metal oxide-based photocatalysts have been functionalized in various ways to improve their electrical and optical properties and enhance photocatalytic activity. The functionalization of metal oxide photocatalyst (ZnO/TiO2) nanomaterials by coupling with noble metal and/or graphene nanostructures has attracted considerable interest in the development of high-performance photocatalytic systems in recent years. These multicomponent nanocomposite systems composed of metal oxides, graphene (or its derivatives) and noble metals are highly significant as alternatives to extensively studied bi-component systems (e.g., metal oxide–graphene and metal oxide–noble metal systems) for the design of high-performance photocatalytic systems. The combination of metal oxides with graphene and noble metals provides improved optoelectronic properties due to the excellent electronic and surface plasmon resonance properties of these nanomaterials. This article first briefly explains the fundamentals of the various components and their roles in building efficient photocatalysts. Furthermore, recent developments in designing metal oxide–graphene–noble metal-based high-performance photocatalyst systems and engineering their properties are discussed with an emphasis on the associated mechanisms and their applications in various photocatalytic processes.
Design, System, ApplicationThe present article provides a systematic study on how to design high-performance photocatalytic systems and engineer various structural, optical and physio-chemical properties for enhanced performance. A high-performance photocatalytic system should exhibit enhanced transport properties that can be engineered by assembling functional nanomaterial systems and enhancing their interactions to achieve high photocatalytic efficiencies. This paper focuses on designing and engineering metal oxide–graphene–noble metal-based functional nanocomposite systems for photocatalytic applications. The review includes the specific functions of metal oxide photocatalysts, molecular graphene and its derivatives along with the unique surface plasmon properties of noble metals and their synergic effect when they are assembled together to design a high-performance photocatalytic system. Furthermore, the progress and recent developments in designing these nanomaterials and engineering their properties to enhance the photocatalytic properties are summarized. |
1. Introduction
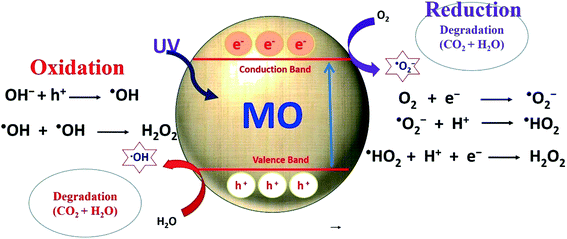
Water pollution is an emerging problem across the world due to rapid population growth and modern industrialization.1 It not only affects public health, but also causes problems for aquatic life and animals. In past years, water pollution has received significant attention from researchers, and various experimental techniques have been developed for wastewater treatment to minimize the harmful effects. These techniques include various advanced oxidation, adsorption, membranes, coagulation/precipitation and photocatalytic processes.2–5 Among these processes for wastewater treatment, photocatalysis is one of the most prominent. This method effectively degrades organic pollutants using low-cost photocatalytic materials that can be modified to utilize abundant solar light to further enhance the degradation process.In the field of photocatalysis, research is being carried out to design and engineer functional nanomaterials to enhance their electrical and optical properties. Enhancing the optoelectronic properties of photocatalysts enhances light absorption, photo-induced charge separation, and charge transfer, resulting in high photocatalytic efficiency.2,6–8 Metal oxides such as titanium dioxide (TiO2) and zinc oxide (ZnO) have shown great promise in the field of photocatalysis. Under the influence of photo-irradiation, these nanomaterials produce photogenerated charge carriers [i.e., electron (e−) and hole (h+) pairs], which show great potential for the reduction and oxidation of organic/inorganic molecules on their surfaces.9–12 These charge carriers react with oxygen or water molecules present on the surfaces of the photocatalysts to produce reactive oxygen species (ROS), as indicated in Fig. 1. The formed ROS further interact with organic molecules and convert them to CO2 and H2O as a result of photocatalytic degradation.13
Unfortunately, the high recombination rates of these photogenerated e− and h+ pairs makes these materials inefficient for photocatalytic activities. In addition, the wide band gaps of these materials also hinders their practical application in the visible-light region of solar energy, which is the greatest source of energy on the earth and sufficiently available. Moreover, when the surfaces of these nanomaterials are covered with the products of catalysis reactions, interactions between incoming target molecules and the surface are restricted by some extent. As a result, photoexcited e− and h+ pairs cannot be transferred for further photocatalytic reactions.14 Therefore, it has been realized that designing multi-component systems by assembling functional nanomaterials with metal oxide photocatalysts would be a better way to solve these issues.
Several other metal oxide nanomaterials and techniques have been designed to improve light absorption and subsequently charge separation, via synthesis of nanocomposite materials using metal nanoparticles (NPs), metal oxides, carbon nanotubes (CNTs), quantum dots (QDs), graphene and its derivatives, etc. Among these nanomaterials, several combinations have been developed by researchers over the years to increase solar light absorption or/and suppress charge recombination. For example, photocatalysts based on CdTe quantum dot/layered double hydroxide (Co-based)/BiVO4,15 BiVO4 photoanodes (via the chemical-thermal process using Zn2+ as a directing agent),16 or MgO/ZnO/In2O3 ternary heterostructures17 have been developed with improved photocatalytic properties. Li et al. synthesized ZnCo layered double hydroxide nanowalls for electrochemical water oxidation (electrochemical process).18 He et al. prepared a TiO2 photoanode through photochemical treatment using phosphate buffer solution. Subsequently, a NiCr double hydroxide shell structure over a TiO2 photoanode was developed via photo-assisted modification and deposition. These core–shell structures showed enhanced photoelectrochemical water oxidation efficiency.19 He et al. prepared a BiVO4 photoanode modified with a CoAl layered double hydroxide. The oxidation efficiency and incident photon-to-current efficiency were two times greater than those of pristine BiVO4.20 Wang et al. synthesized composites of mixed metal oxides and graphitic carbon nitride and used them for water oxidation reaction and the generation of H2O2 through oxygen reduction.21 Similarly, Xiang et al. reported semiconductor (TiO2, ZrO2, and SnO2)−chromophore [Ru(bpy)2(dpbpy)]2+(P2)-polyoxometalate catalyst photoanodes for water splitting using dye-sensitized photoelectrochemical cells.22 TiO2/WO3-based nanocomposites have been extensively studied in the field of photocatalysis.23–25 WO3 is another widely studied metal oxide photocatalyst; its band gap is lower than that of TiO2, and it shows the potential for visible light photocatalytic activity.26–28 For example, Saleh et al. demonstrated that a WO3-multiwalled CNT-based nanocomposite exhibited sunlight-induced photodegradation of Rhodamine B.26 In the case of metal oxide semiconductors, for the efficient reduction of oxygen by photo-excited electrons and the subsequent oxidation of target molecules by holes, the CB bottom potentials of the metal oxides should be more negative. Although WO3 is a good photocatalyst but it shows poorer performance for the reduction of oxygen at the surface because of its more positive CB level compared to TiO2.26 Furthermore, WO3 also undergoes more photo-corrosion than TiO2 during long-term irradiation.29 Therefore, WO3 shows higher charge-recombination rates and hence worse photocatalytic properties than TiO2.
Among metal oxide photocatalysts, TiO2 and ZnO have been used extensively over the years due to their attractive properties such as low cost, resistance to corrosion, stability and nontoxicity. In this review article, combinations of these metal oxides with functional nanomaterials such as noble metal- and graphene-based nanostructures have been studied for their photocatalytic applications in various processes. The motivation behind these assemblies is their individual optical and electrical properties, which synergistically improve the optoelectronic properties of the nanocomposite materials to provide high-performance photocatalytic systems with enhanced efficiency. Moreover, assembling metal oxides with graphene (or its derivatives) and noble metal NPs provides improved electron transfer properties along with visible light-activated photocatalytic activity and solves the problem of the fast recombination of photoexcited charge carriers.30,31 Wen et al.32 designed such a visible light-activated nanocomposite photocatalyst system by assembling TiO2, Ag NPs and graphene nanostructures. This assembly showed strong absorption in the visible light region and enhanced the photocatalytic efficiency for the degradation of dye molecules.32 The strong visible light absorption and enhanced photocatalytic properties were attributed to the combined effects of the surface plasmon resonance (SPR) properties of the Ag NPs and the strong interaction of graphene assembled on the TiO2 surface. In addition, the formation of Schottky junctions at the Ag/TiO2 interface inhibits the recombination of photogenerated charge carriers. In addition, the high electron transfer ability of graphene enhances the photocatalytic efficiency of the nanocomposite photocatalyst system compared to Ag–TiO2 and graphene–TiO2 systems.32 This reveals that designing photocatalyst systems that combine metal oxides and functional nanomaterials to engineer the electrical, physical and optical properties can produce the high-performance photocatalytic systems (Fig. 2). Huang et al. designed a nanorod photocatalytic system by assembling a layered structure of Ag NPs and graphene on TiO2 nanorods. They explained the mechanism of confined migration of hot electrons by engineering the shapes and sizes of the nanomaterials for high photocatalytic degradation of MB.14
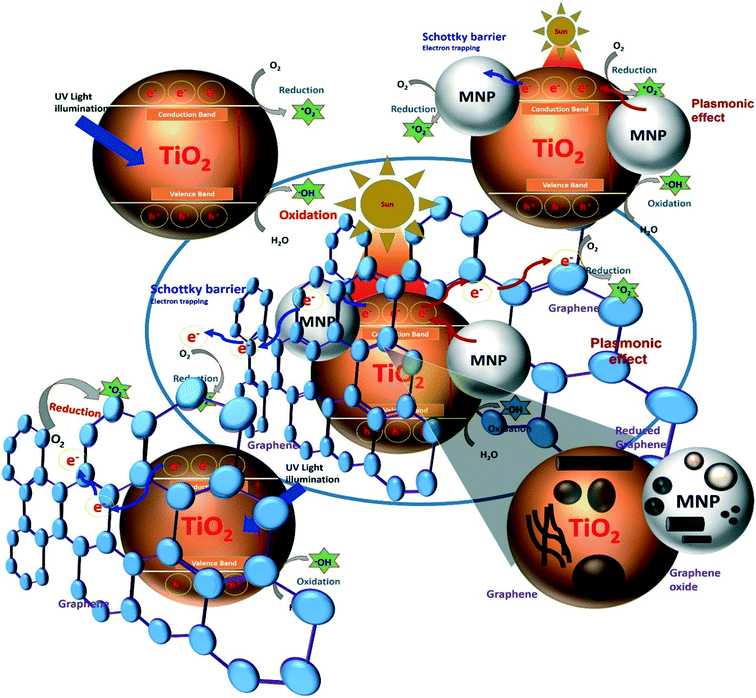 | ||
| Fig. 2 Design and engineering of high-performance photocatalytic systems based on metal oxide–graphene–noble metal nanocomposites (MNP = metal nanoparticles). | ||
Similarly, Roy et al.33 developed a highly efficient nanocomposite photocatalyst composed of graphene nanosheets, Au nanorods and ZnO NPs for the photocatalytic reduction of nitrobenzene to aniline. The photocatalytic activities of the reported system were around five times greater than those of commercially available TiO2 and ZnO nanostructures. Fig. 2 shows that how a high-performance photocatalytic system can be designed by assembling metal oxide photocatalysts (for example, TiO2) with other functional nanomaterials and engineering their shapes and sizes. Designing highly efficient photocatalyst systems with enhanced optical, electron transport and charge separation properties is a major challenge in the effort to obtain high photocatalytic efficiencies. Designing a molecular photocatalyst system using graphene or its derivatives and noble metal nanostructures coupled with wide-bad-gap metal oxide photocatalysts represents a unique strategy for improving charge separation and electron transport properties.
As shown in Fig. 2, the coupling of metal oxide photocatalysts with noble metal nanostructures results in the formation of a Schottky barrier at their interface, which acts as an electron trapping site and prevents charge recombination under UV light irradiation. On the other hand, this combination gives rise to a visible light-activated photocatalyst system with plasmonic effect (discussed in the next section), which improves the optoelectronic properties and thus enhances photocatalytic activity. Furthermore, the coupling of this bifunctional photocatalyst with molecular graphene or its derivatives enhances the electron transport properties of the system because of the excellent electronic properties of molecular graphene. In addition, tuning the characteristics of these functional nanostructures by controlling their shapes and sizes can further improve the photocatalytic properties, including charge separation and electron transport.
This review covers the recent development of such novel high-performance photocatalytic nanocomposite systems based on the assembly of functional nanomaterials with metal oxide photocatalysts. These multicomponent photocatalytic systems have the potential to overcome the challenges of other functional photocatalysts studied extensively in the literature, such as metal oxide–graphene and metal oxide–noble metal systems.
There is extensive research interest in designing high-performance photocatalysts using graphene- and noble metal-based nanomaterials. Various strategies have been implemented to engineer their properties with the aim of providing a photocatalyst system with high electron transfer and visible light activity. Furthermore, nanomaterials such as graphene and its derivatives, TiO2/ZnO and noble metal NPs are of high importance and have special relevance as next-generation photocatalysts due to their unique properties such as high electrical conductivity, large band gap, and SPR properties, respectively (Fig. 2). The various characteristics and mechanisms of action of these nanomaterials responsible for the high electron transport, high charge separation and band gap reduction (enable visible light absorption) for designing high-performance photocatalyst materials are briefly discussed below.
1.1 Metal oxides (TiO2/ZnO)
As discussed above, several metal oxides have been used for photocatalytic applications. TiO2 and ZnO are semiconductors with wide band gaps of 3.0–3.2 and 3.3 eV, respectively.2,12 The energy band gap of the materials is generally calculated using the following expression:| (F(R) × hν) ∝ (hν − Eg)n, | (1) |
These nanomaterials play a vital role in the fields of energy and the environment owing to their nontoxicity, biocompatibility and sustainability. In addition, these materials are very resistant to corrosion, have low cost, and can be synthesized by simple methods such as hydrothermal treatment, electrospinning and sol–gel methods.31,34–37 The largest problem in the utilization of metal oxides in the field of photocatalysis is their wide band gaps, which restrict their application to the UV light region, which present small percentage only in solar light.8 Engineering the band gaps of these nanomaterials can reduce their band gap, enables visible light absorption. Furthermore, doping (e.g., with cations or anions),38,39 surface modifications (designing the porosity or functional groups)40–42 and the formation of nanocomposites with other functional nanomaterials (noble metals, quantum dots, graphene, other metal oxides, etc.)43–45 are possible ways to maximize their applications in photocatalysis and a variety of other fields (e.g., solar cells, water splitting, sensors). Designing metal oxide-based nanocomposite systems with functional nanomaterials has generated excellent results in the field of photocatalysis. This strategy provides visible light-activated photocatalysts with high surface reactivity and high electron transfer properties for high-performance photocatalysis and is discussed in more detail in the next sections.
1.2 Graphene and its derivatives
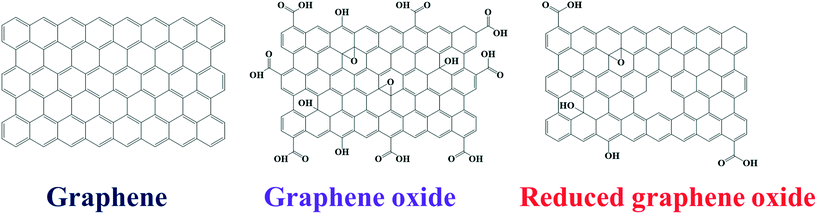
Graphene, a single layer of graphite, possesses a two-dimensional sheet-type structure containing sp2-hybridized carbon atoms arranged in a hexagonal manner.46,47 The research community has given graphene much consideration in the last decade owing to its excellent electronic properties.48 Graphene also has excellent mechanical properties, flexibility, and transparency, making it very useful in biomedical applications, flexible electronics, and many other fields. Derivatives of graphene such as graphene oxide (GO) and reduced graphene oxide (rGO) have also been used extensively due to their functional characteristics (Fig. 3). GO contains additional oxygen and hydrogen atoms. The oxygen atoms are attached to the carbon atoms that show sp3 and sp2 hybridization due to the aliphatic chains and aromatic rings, respectively. Due to this additional functionality, GO is hydrophilic in nature, while graphene shows hydrophobic behavior.Moreover, the different functionalities (in aromatic and aliphatic regions) offer different types of interactions on the surface of GO. On the other hand, rGO has few reduced sites compared to GO. Since rGO is prepared from the reduction of GO, some defects along with some oxygen functional groups remain within the structure or on the rGO surface, as shown in Fig. 3 (right side). Both GO and rGO have excellent properties, and rGO shows tunable optical properties due to its variable functionality.49–52 Graphene and its derivatives have been successfully used to design molecular nanocomposite systems and to engineer the properties of other nanomaterials, resulting in excellent performance in various fields including photocatalysis. These functional nanomaterials have been used to design photocatalyst systems because of their excellent electron transfer properties, which decrease the recombination of photoexcited charge carriers in metal oxides. Graphene and its derivatives also help in establishing good interaction of target molecules and the surfaces of the metal oxide nanomaterials due to their adsorption behavior. The functionalities of graphene and its derivatives allow the formation of various types of composite structures. For example, Saleh et al. prepared polyimide-modified graphene via interfacial polymerization and used it for Sb(III) adsorption.53 Similarly, graphene was modified with polyamidoamine dendrimer followed by decoration with Ag NPs and utilized for surface-enhanced Raman scattering (SERS).54
Due to their excellent electronic properties, graphene and its derivatives play major roles as electron sinkers and in suppressing charge recombination in nanocomposite materials with metal oxide photocatalysts. In addition, these functional carbon nanomaterials play important roles in transferring electrons to molecules adsorbed on photocatalytic surfaces.55,56 In particular, when the photocatalytic surface is covered with photocatalysis reaction products, hindering surface interaction with incoming pollutant molecules, functional carbon nanomaterials are useful for enhancing the photocatalytic activity. Graphene derivatives (GO and rGO) can behave like semiconductors depending on their functionality and can degrade water pollutants photo-catalytically.57 Graphene derivatives can also narrow the band gap and act as charge transfer media. Thus, these derivatives can prevent nanoparticle agglomeration by providing good dispersion of metal oxides or metal NPs within the composites and enhance the photocatalytic efficiency.58
CNTs, another allotrope of carbon with a similar atomic (single-wall CNTs) structure to graphene, have received considerable attention in the field of photocatalysis due to their electronic properties.1,26 The only difference between graphene and CNTs is that CNTs have a rolled (tube-like) structure that may result in different properties and areas of application. However, studies reveal that graphene nanostructures are far better at transferring their exceptional strength, electronic and mechanical properties to the host materials in nanocomposites as compared to CNTs.59–61 Therefore, even though graphene and CNTs are almost identical in their chemical structures and most of their physical properties, graphene is better at lending its attributes to host materials. In this review, we discuss the role of graphene and its derivatives in metal oxide–noble metal-based photocatalyst nanocomposites for high-performance photocatalytic activities.
1.3 Noble metal nanostructures
Noble metal NPs (mainly Ag and Au NPs) exhibit unique optical properties that distinguish them from other metals.62 This uniqueness originates from their localized SPR properties, which result from the collective oscillation of free electrons on their surfaces during interaction with incoming electromagnetic radiation, as shown in Fig. 4.63,64 These nanostructures have received great interest in fundamental studies and for potential applications in various fields because of their physico-chemical and SPR properties. The plasmonic properties of these noble metal NPs can be tuned by engineering and modifying their shapes and sizes as well as the dielectric constant of the surrounding environment.65–69 More details on the tuning of the optical properties, the influencing factors and theoretical interpretations have been reported in recent review articles.63,64 In the field of photocatalysis, noble metal nanostructures have been extensively studied to enhance the light absorption capability (SPR absorption) of metal oxide–noble metal photocatalysts to the visible region of solar light by forming nanocomposite materials known as plasmonic photocatalysts.70 In addition, the dielectric behavior of metal oxide nanomaterials also affects the plasmonic properties of noble metal NPs, which enhance the photocatalytic properties of the nanocomposites. Moreover, noble metal NPs form Schottky junctions at their interfaces with metal oxides. These junctions act as electron trapping centers and prevent the recombination of photoexcited charge carriers, as discussed in the next sections.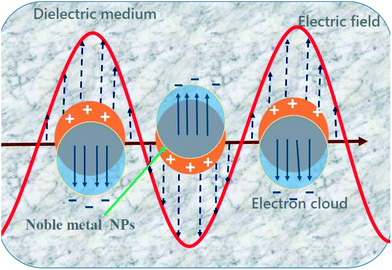 | ||
| Fig. 4 Schematic representation of the collective oscillations of surface electrons in response to incoming electromagnetic irradiation for noble metal NPs. | ||
2. Designing and engineering high-performance photocatalyst systems based on metal oxide–graphene–noble metal nanocomposites
Several metal oxide-based photocatalyst systems have been functionalized and proposed to enhance photocatalytic activity by modifying and engineering their electrical and optical properties. This section discusses the progress in designing and engineering high-performance photocatalytic systems from basic metal oxide (TiO2/ZnO) photocatalysts to nanocomposite photocatalyst systems comprising metal oxides with graphene and noble metal nanostructures. Here, the main focus is to understand the molecular behavior of graphene (and its derivatives) related to the photocatalytic properties and their interactions with metal oxides and noble metal nanostructures leading to the better materials assembly and functionality. In addition, emphasis is placed on the engineering of these nanocomposite materials via tailoring their shapes/sizes, core–shell structures and functionalities to enhance charge transfer and optical properties with the goal of designing novel photocatalytic systems. To better understand the design and engineering of these high-performance photocatalysts, brief introductions to extensively studied nanocomposite photocatalyst systems (e.g., metal oxide–noble metal and metal oxide–graphene systems) are first given. This is followed by a detailed review on progress in designing multicomponent metal oxide–graphene–noble metal nanocomposite systems. Special emphasis is placed on engineering the properties of these systems to enhance their photocatalytic activities.2.1 Metal oxide–noble metal-based photocatalyst systems
As shown in Fig. 2, noble metal nanostructures deposited on the surfaces of metal oxide photocatalysts enhance the absorption capability of plasmonic photocatalysts a form Schottky barriers/junctions at the interfaces. These interactions between noble metal nanostructures and photocatalysts extend the absorption in the visible region through the plasmonic effect and prevent the recombination of photoexcited charge carriers through Schottky barriers. A recent review article summarizes the various approaches to designing and functionalizing plasmonic photocatalysts based on metal oxides (TiO2, ZnO, and CeO2) and noble metals (Au, Ag, Pt, Pd and their alloys) along with their applications in fields of photocatalysis, photovoltaics, biomedicine and SERS.71 Noble metals and metal oxide nanocomposites have been designed in different forms including core–shell nanostructures, composite thin films, noble metal nanoparticles attached to the surfaces of metal oxide nanoparticles and one-dimensional nanostructures (nanotubes, nanofibers etc.).71,72 These various nanostructures and morphologies provide additional improvements in optical and electronic properties. Furthermore, their surface area and attraction to target molecules are significantly enhanced, facilitating charge transport. Here, we discuss the most significant advances in the field of photocatalytic applications using these plasmonic photocatalysts in recent years.Extensive studies have been conducted on plasmonic photocatalysts. However, there is always scope to enhance the functionality of nanomaterials through modifications and engineering their properties for better response in terms of applications in various fields. This can be achieved by controlling the parameters or intrinsic properties of the materials. For instance, Marchal et al. synthesized Au–TiO2 photocatalysts and studied the effects of Au content and calcination temperature on photocatalytic water splitting.73 Au/TiO2 and Pt/TiO2 photocatalysts also showed that the optimum loading of noble metal NPs on the surface of TiO2 can provide high hydrogen production.74 Similarly, the effects of Ag and Pt contents were studied to improve the photocatalytic degradation of dyes.75,76 Furthermore, the Ag content and phase of TiO2 were also shown to contribute to the photocatalytic performance of plasmonic photocatalysts.77–79 Recently, Yu et al. developed a Au–TiO2 nanoforest photocatalyst with both anatase and rutile phases and studied its UV/visible photocatalytic response in the photodegradation of Rhodamine B (RhB).79 Ag@TiO2 photocatalysts also showed enhanced antimicrobial activity compared to pure TiO2, demonstrating the multifunctional applications resulting from the presence of two kinds of nanostructures.80 Similarly, Ag-loaded ZnO photocatalyst systems were also examined for the photocatalytic degradation of dye molecules. The effects of Ag loading on different ZnO materials were studied towards the degradation of phenol.81
As discussed above, composition plays an important role in the photocatalytic performance of metal oxide–noble metal nanocomposite photocatalysts. Similarly, the shapes and sizes of components also affect the optical properties of the resulting materials and their photocatalytic performance. For instance, by optimizing the Pt concentration and calcination temperature, Yang et al. showed that Pt/TiO2 hollow nanofibers are excellent photocatalysts for the degradation of orange II compared to nanofibers.76 Very recently, She et al. studied the effect of Au NP size (ranging from 20–80 nm) on the photocatalytic performance of photocatalyst systems based on Au–ZnO nanorods.82 They demonstrated that the 40 nm Au NPs on ZnO nanorods exhibited the highest photocatalytic activity among the tested Au NPs. Fig. 5 (left) shows the effect of Au NP size on the photocatalytic activity of the Au–ZnO nanorod photocatalysts for the photodegradation of RhB molecules. Fig. 5 also shows possible mechanisms of photodegradation. The small Au NPs exhibit more photoexcited charge separation than the large Au NPs, whereas the large Au NPs have a stronger electric field generated by LSPR. The optimum Au NP size represents a balance between these factors, and a trade-off mechanism has been proposed to explain the highest observed photocatalytic activity, as shown in Fig. 5 (right).
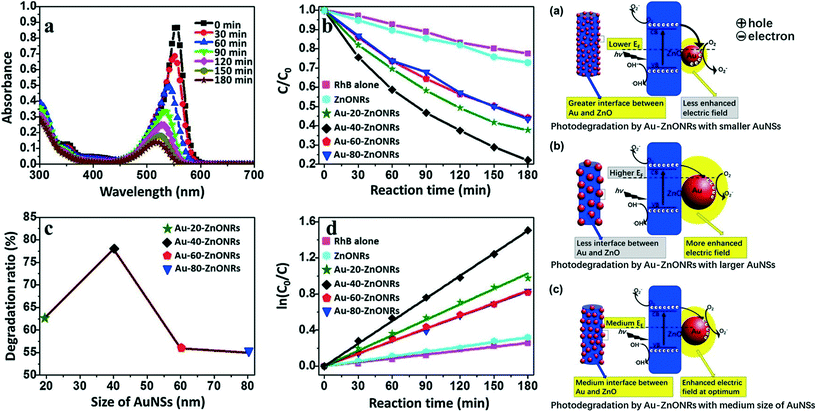 | ||
| Fig. 5 Left: Photocatalytic performances of photocatalysts. (a) UV–Vis absorption spectra of RhB under 200–780 nm irradiation for different periods of time to track RhB photodegradation in the presence of Au–40–ZnONRs. (b) Kinetic traces of RhB photodegradation in the absence (purple squares) and presence of various photocatalysts: ZnONRs (cyan hexagons), Au–20–ZnONRs (green stars), Au–40–ZnONRs (black diamonds), Au–60–ZnONRs (red pentagons) and Au–80–ZnONRs (blue triangles). (c) Degradation ratio of RhB by Au–ZnONRs with AuNSs of different sizes for 180 min. (d) Fitted curves of the kinetic traces in (b) according to the Langmuir–Hinshelwood model. Light source = 300 W Xe lamp, initial concentration = 5 ppm RhB, photocatalyst loading = 1 mg mL−1. Right: Schematic illustration of the photodegradation mechanism by Au–ZnONRs with AuNSs of different sizes [small (a), large (b) and optimal size (c)] showing the size-dependent effects on photocatalytic activity. The right part in schematic image is the enlargement of the box on the left schematic image. Reprinted with permission from ref. 82. Copyright© 2017 Elsevier Inc. | ||
Similarly, several plasmonic photocatalyst systems have been designed, and their structural and morphological properties have been engineered for photocatalysis applications. These include systems based on ZnO NPs/Ag nanowires,83 Au/ZnO nanorods,84 mesoporous Ag/ZnO nanocrystals,85 Ag/TiO2 NPs,86 and Ag NPs/TiO2 inverse opals.87
Bimetallic NPs in the form of alloys or core–shell nanostructures contribute to a wider range of visible light absorption compared to metal NPs and play a significant role in charge separation because of their dual SPR properties; therefore, bimetallic NPs enhance the photocatalytic activity of nanocomposite photocatalysts.35,88–92 For example, Tahir et al. demonstrated that Au–Ag bimetallic NPs coupled with TiO2 nanowires exhibited greater photocatalytic reduction of CO2 compared to Au–TiO2 systems, Ag–TiO2 systems, TiO2 nanowires and TiO2 NPs (the performance was enhanced by 1.72, 1.84, 72.52 and 201.00 times, respectively). This enhancement was attributed to the synergic plasmonic effect of Ag and Au, which facilitated charge transfer during photocatalytic reduction.88 An Au–Cu alloy deposited on TiO2 (P25) exhibited high CO2 conversion to CH4.92 Similarly, Misra et al. recently immobilized Au@Ag core–shell bimetallic NPs on TiO2 nanofibers and utilized them for the photocatalytic degradation of MB.35 Interestingly, Chehadi et al. designed Au-based plasmonic photocatalyst systems coupled with different metal oxides (Au/TiO2, Au/ZnO and Au/Al2O3) and investigated the photocatalytic degradation of bisphenol A under visible light. Au/TiO2 exhibited the best photocatalytic degradation due to the efficient electron transfer from the Au NPs to TiO2.93
As discussed above, other metal oxide photocatalysts have also been reported to exhibit enhanced photocatalytic activities in combination with noble metals. For example, Cao et al. prepared an Au–BiVO4 heterogeneous nanostructure and utilized it for the photocatalytic degradation of dye and water oxidation. Au–BiVO4 showed a higher photocatalytic activity for the degradation of MO under visible light than BiVO4 owing to SPR absorption by Au and effective charge separation.94 WO3 nanorods were synthesized via a combined ion exchange process and hydrothermal treatment. Au NPs were deposited on these WO3 nanorods, and the WO3 nanorods/Au NP composites showed enhanced photocatalytic activity towards RhB degradation under simulated solar light compared to the WO3 nanorods.95
Some studies have shown that the use of linker molecules help establish a good contact between metal NPs and metal oxide surfaces, which enhances the photocatalytic activity.35,96 For example, Ding et al. prepared an Au/TiO2 photocatalyst via linker-assisted direct deposition. The linker-assisted Au/TiO2 offered close contact between Au and TiO2 after mild annealing. The prepared linker-assisted Au/TiO2 photocatalyst showed better activity for dye degradation than the conventional Au/TiO2 photocatalyst under visible light.96 These studies show that tailoring surface functionality with functional materials can improve the surface properties of metal oxide photocatalysts and enhance their photocatalytic activities. In this way, coupling plasmonic photocatalysts with molecular graphene nanostructures (as discussed in section 1.2) could improve photocatalytic performance through the synergic effect of noble metals (plasmonic effect) and graphene (excellent electronic properties). This is discussed further in section 2.3.
2.2 Metal oxide–graphene based photocatalyst systems
As shown in the Fig. 2, when graphene nanostructures are coupled with metal oxide photocatalysts, due to the electron transport properties, the graphene nanostructures extract the photoexcited electrons from the metal oxide surfaces, making them available for photocatalysis reactions. This property of graphene nanomaterials also prevents the recombination of electrons with the holes in the metal oxides and thus enhances the photocatalytic efficiency of the metal oxide–graphene photocatalyst systems. A few reviews on graphene-based metal oxide photocatalysts published in recent years provide a complete view of their mechanisms of photocatalytic action.97–99 However, the present review includes the most recent results and progress in the photocatalytic applications of metal oxide–graphene photocatalyst systems. Metal oxide–graphene nanocomposite photocatalyst systems can be prepared by several synthesis methods, including sol–gel methods,100,101 two-step oxidation,102 hydrothermal methods,103–106 and solvothermal methods.107,108 Using these techniques, several graphene–TiO2 nanocomposites with enhanced optical and photocatalytic properties have been reported.109–111As discussed in section 1.2, graphene nanostructures have also been used in many other functional forms such as GO and rGO. The oxygen functionalities in GO and rGO play an important role in the enhancement of the optical and photocatalytic properties of graphene-based TiO2 photocatalysts. For example, molecular GO–TiO2 and rGO–TiO2 nanocomposite systems were designed for the photocatalytic degradation of diphenhydramine (2-(diphenylmethoxy)-N,N-dimethylethylamine)hydrochloride.112 The GO–TiO2 nanocomposite demonstrated higher photocatalytic activity than the rGO–TiO2 nanocomposite, which was explained as follows. The low oxygenated surface functionality of rGO likely resulted in the less uniform deposition of TiO2 onto the graphene sheets and led to aggregation in aqueous solution.112 Similarly, TiO2 NPs on GO showed excellent photocatalytic degradation for MB.113,114
Preparation method also affects the molecular design of graphene-based photocatalytic systems and hence their photocatalytic properties. For instance, Fan et al. synthesized rGO–TiO2 nanocomposites using hydrazine reduction, UV-assisted photocatalytic reduction, and hydrothermal routes to investigate hydrogen production from alcohol. These rGO–TiO2 photocatalysts exhibited better hydrogen evolution rates than pure TiO2. However, the hydrothermally synthesized rGO–TiO2 photocatalysts showed the most efficient photocatalytic activity due to the close contact between TiO2 and rGO.115 Kim et al. synthesized nano-GO, which self-assembled with TiO2 NPs, resulting in photocatalysts based on core–shell nanostructures. These core–shell nano-GO–TiO2 photocatalysts were reduced by photo-irradiation to form nanographene–TiO2 core–shell nanostructures. The photocatalytic activity of this core–shell structure towards hydrogen evolution was evaluated. The sample loaded with 0.70% reduced nano-GO showed the maximum efficiency; further increasing the loading decreased the efficiency owing to light shielding by reduced nano-GO on TiO2. This assembly showed a higher activity for hydrogen evolution than TiO2 deposited on graphene sheets. This was attributed to the direct coverage of the TiO2 surface by reduced nano-GO, whereas a small fraction of TiO2 was in contact with the large graphene sheets.102 In another report, GO encapsulated on TiO2 microspheres exhibited enhanced photocatalytic activity compared to TiO2 for the degradation of RhB.116 This indicates that the surface coverage by graphene nanostructures, which improves contact with the metal oxide surface, is an important factor in electron transfer and photocatalytic efficiency. Jo et al. observed a shift in the absorption spectrum of a GO–TiO2 nanocomposite attributed to the interaction between GO and TiO2; this nanocomposite also showed a higher photocatalytic degradation efficiency for toxic organic vapors of toluene compared to a TiO2 photocatalyst.117 Similarly, several studies have reported rGO–TiO2-based photocatalysts prepared using different synthesis techniques. These photocatalysts showed excellent photocatalytic activity for the photodegradation of MB, photoreduction of Cr(VI) and photocatalytic hydrogen production owing to the large surface area, strong adsorption ability and electron transfer capability resulting from the incorporation of molecular rGO.100,118 Interestingly, a comparable study was carried out on the photocatalytic degradation of MB using rGO/TiO2, rGO/ZnO and rGO/Ta2O5 photocatalysts; the results showed that rGO/TiO2 exhibited high photocatalytic efficiency under visible light irradiation.119
Several graphene-based metal oxide nanomaterials have been studied. For example, Ng et al. prepared a BiVO4–rGO composite for water splitting reactions. The activity of BiVO4–rGO for water splitting was 10 times greater than that of BiVO4 alone under visible light due to enhanced charge separation at interfaces.120 Yan et al. prepared a rGO–BiVO4 composite using a microwave-assisted method; 2% rGO–BiVO4 achieved 68.2% ciprofloxacin degradation under visible light in 60 min, three times greater than the degradation achieved by BiVO4 (22.7%) alone. The enhancement in photocatalytic activity was attributed to enhanced charge separation.121 BiVO4–graphene photocatalysts were synthesized via a one-step hydrothermal route, and the composite structure showed higher photocatalytic activity than BiVO4 for the degradation of model pollutants (MB, MO, and RhB).122 An et al. synthesized a composite of WO3 nanorods and graphene using an in situ hydrothermal route. The composite showed high photocatalytic efficiency towards the degradation of RhB under visible light owing to enhanced charge separation and transfer, enhanced visible light absorption and adsorption of organic compounds.123 Chai et al. prepared rGO/WO3 composites via a one-pot hydrothermal route. The rGO concentration of 15% was found to be optimum for the photocatalytic degradation of MB due to the excellent electron accepting and transport properties of rGO.124 Similarly, a WO3/graphene nanocomposite was synthesized using electrochemical exfoliation with the help of tungsten phosphate acid. The photocatalytic activity of the nanocomposite was 2 and 2.2 times higher than those of graphene and WO3, respectively, for the degradation of MB under UV light. The enhanced activity was attributed to increased charge separation and light absorption.125 A WO3@graphene composite was synthesized using the sonochemical method and used for oxygen evolution from water.126
GO- and rGO-based nanocomposite photocatalysts with one-dimensional TiO2 nanofibers,127 nanorods,128 and nanotubes103 have also been studied extensively, resulting in enhanced photocatalytic efficiencies for hydrogen production and the degradation of organic pollutants. Furthermore, one-dimensional TiO2 nanostructures increase the surface area of the nanocomposite, providing additional benefits over graphene-based composites and enhancing the photocatalytic activity on the surface.
In a recent report, a three-dimensional rGO–TiO2 aerogel was prepared by a hydrothermal method and utilized for the photodegradation of carbamazepine (CBZ) under UV light. The mechanism of carbamazepine photodegradation is shown in Fig. 6.129
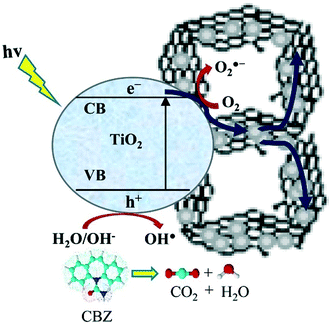 | ||
| Fig. 6 Schematic diagram showing the proposed mechanism of CBZ photodegradation. Reprinted with permission from ref. 129. Copyright© 2016 Elsevier B. V. | ||
Similarly, photocatalysts based on ZnO and graphene nanostructures have also been designed and engineered to improve functionality by tailoring their morphologies and applied in photocatalytic applications. For example, graphene–ZnO nanocomposites have been extensively designed for the photodegradation of organic dye molecules.130–135 Recent studies have reported graphene–ZnO rod-like structures,135,136 GO–ZnO,137 and rGO–ZnO138 nanocomposites. These studies reveal that nanocomposites based on graphene (or its derivatives) and ZnO provide excellent photocatalytic activities under UV light irradiation and act as visible light-active photocatalysts, unlike pristine ZnO nanomaterials. The advantage of graphene nanostructures is that they strongly adsorb target molecules near the metal oxide photocatalytic surface and accelerate photoexcited electron transfer by suppressing electron recombination with photogenerated holes, facilitating the photocatalytic reactions. As discussed in the last section, the coupling of noble metal NPs to these graphene-based photocatalysts could enhance the absorption of visible light and electron transfer, resulting in nanocomposite materials with high photocatalytic activities.
2.3 Photocatalyst systems based on metal oxide–graphene–noble metal nanocomposites
As discussed above, the design of efficient photocatalyst systems based on metal oxides coupled with noble metals or graphene (or its derivatives) nanostructures has attracted considerable interest from researchers. Multifunctional metal oxide-based photocatalysts with graphene and noble metal nanostructures are important for designing high-performance photocatalytic systems. The integration of metal oxide photocatalysts with graphene and noble metal nanostructures provides synergic contribution to the enhanced photocatalytic efficiency. It occurs due to the individual interaction of graphene and metal nanostructures to the photocatalyst surface resulting in high electrons transport and plasmonic effects, respectively. Designing such multifunctional systems provides several pathways for electron transport arising from noble metal–metal oxide, metal oxide–graphene, and noble metal–graphene interfaces. This is the key factor in obtaining high photocatalytic activities in these systems.The interest in designing metal oxide–graphene–noble metal heterostructures has grown in the last few years. The composites have the properties of three functional materials (metal oxides, noble metals and graphene and its derivatives), which play a role in their high-performance photocatalytic activity. These heterostructures can be designed, and their properties could be manipulated by engineering the shapes, sizes, and functionalities of any of the components. As discussed above, metal oxides such as TiO2 and ZnO are wide-band-gap semiconductors, and the heterostructures involving these metal oxides utilize UV light. In contrast, noble metal NPs and graphene utilize a wide range of solar light depending on their functionalities, shapes, and sizes, etc. Therefore, coupled, multifunctional heterostructures can provide high charge separation and electron transport for photocatalytic reactions.
Jafari et al. designed ternary-structure photocatalytic systems via a hydrothermal route by loading Ag/TiO2 on graphene sheets. The photocatalytic activity was found to be highly dependent on the Ag/TiO2 coverage on the graphene sheets, and the plasmonic effect of the Ag NPs enhanced visible light absorption. Interestingly, the photocatalytic efficiency of 1-wt% graphene-hybridized Ag/TiO2 towards RhB degradation was found to be the highest among the different tested combinations; this was attributed to the separation and transport of photogenerated excitons resulting from the high conductivity of graphene.139 Vasilaki et al.140 deposited Ag NPs (1–4 wt%) on TiO2 (P25) via a chemical reduction method followed by hydrothermal deposition on rGO sheets. The MB degradation kinetics were investigated for different Ag loadings. The maximum efficiency was obtained by the Ag/TiO2 system with 3 wt% Ag (Fig. 7 a and b). A decrease in photocatalytic efficiency was observed at higher Ag loading due to the limiting of light absorption on the surface of TiO2. An efficient photocatalyst with high photocatalytic activity could be designed by individually optimizing the loadings of Ag NPs and rGO on the TiO2 surface.140 As shown in Fig. 7c, rGO mainly transports electrons from the conduction band of TiO2, including those generated via the plasmonic effect of Ag NPs, preventing charge recombination and resulting in higher photocatalytic efficiency.
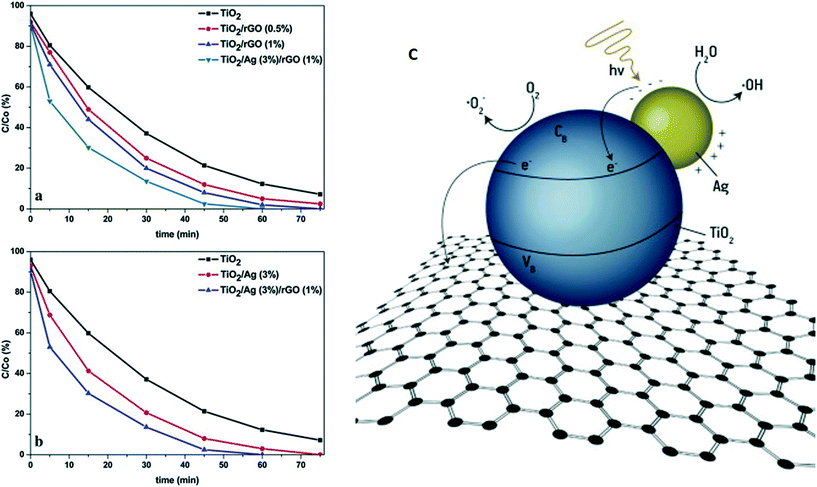 | ||
| Fig. 7 Photocatalytic decolorization of MB aqueous solutions by (a) TiO2, TiO2/rGO with various rGO loadings and TiO2/Ag (3 wt%) deposited on rGO and (b) TiO2, TiO2/Ag (3 wt%) and TiO2/Ag (3 wt%) on rGO (1 wt%). Light source = 150 W, high-pressure mercury lamp with filter (λ < 390 nm); initial concentration = 20 mg L−1; photocatalyst loading = 200 ppm. (c) Proposed mechanism showing the mobility of charge carriers in the case of TiO2/Ag coupled with rGO. Reprinted with permission from ref. 140. Copyright© 2015 Elsevier B.V. | ||
Ag/TiO2/rGO nanocomposites showed increased superficial areas and enhanced photocatalytic properties towards MB under visible light compared to P25, Ag/TiO2 and TiO2/rGO.141 Similarly, Ag NPs assembled in a TiO2–graphene ternary nanocomposite photocatalyst exhibited higher photocatalytic activity than the pure TiO2 and TiO2–graphene nanocomposites.142 These results were attributed to the faster charge transfer and longer exciton lifetimes resulting from the incorporation of graphene and Ag NPs. In addition, the Ag NPs showed the SPR effect and enhanced light absorption in the photocatalyst system. The Ag/TiO2/graphene nanocomposite prepared by Ag deposition via the reduction of AgNO3 over TiO2/graphene showed high photocatalytic activity due to the strong adsorption of aromatic dye molecules by graphene along with the charge separation and transport attributable to the Ag NPs and graphene sheets.32
Similarly, Au–TiO2–graphene nanocomposite photocatalyst systems were designed with smaller crystal size and higher surface area compared to TiO2.143 These systems were investigated for the degradation of acid blue 92 under UV and visible light irradiation. The incorporation of Au NPs not only increased visible light absorption, but also helped reduce the energy band gaps of the nanocomposite photocatalysts. Furthermore, the systems exhibited enhanced photocatalytic efficiency due to the synergic effects discussed above. Similar to Ag and Au NPs, photocatalytic systems incorporating Pt or Pd NPs in TiO2–graphene nanocomposites also exhibit high photocatalytic activity. TiO2–Pt/Pd–graphene nanocomposite photocatalysts were investigated for the degradation of pollutants such as 2,4-dichlorophenoxyacetic acid and Reactive Red 195 under UV and visible light.144 Pt-based photocatalysts showed higher activity than Pd-based systems due to the high photonic efficiency of Pt. The photonic efficiency of metal is dependent on the work function and electron affinity of the metal having a favorable contact with TiO2.144
Interestingly, magnetic nanocomposites were designed using rGO loaded with TiO2 NPs, γ-Fe2O3 and varying amounts of Ag. The magnetic nanocomposites showed enhanced visible light-driven photocatalytic activity towards crystal violet. The highest efficiency was achieved in the composite containing 11.5 wt% Ag NPs. The nanocomposites offered effective charge separation due to the strongly coupled Ag/γ–Fe2O3/rGO co-catalyst and graphene nanosheets. The nanocomposites showed excellent stability and recovery via magnetic action.145
As discussed in previous sections, the modifications and engineering of nanostructures affect the surface functionality and contribute to the resulting photocatalytic efficiency of the nanomaterial. Several studies have focused on the modifications and engineering of functional nanomaterials to improve surface properties and enhance photocatalytic activity. Accordingly, high-performance photocatalyst systems have been designed and studied as functions of the concentration of functional nanomaterials, size, shape and functionality. The photocatalytic activities of Ag–rGO–TiO2 nanorods assembled by the sequential deposition of Ag NPs and rGO on TiO2 were studied that exhibited the highest efficiency towards MB degradation, owing to transfer of the SPR generated hot electrons on metal to the graphene layer deposited on TiO2.14 In another report, TiO2 nanorod–Ag NPs–GO nanocomposite photocatalyst systems were designed, and the effects of Ag loading on the degradation of acid orange 7 (AO-7) and phenol were investigated. With increasing Ag content, the solar light-driven photocatalytic properties of the GO–TiO2–Ag nanocomposites towards the degradation of AO-7 increased, while optimum loading was required in case of phenol degradation. These nanocomposites also showed multifunctional antibacterial properties towards E. coli. The participation of active radicals [such as ˙O2−, hydroxyl radical (˙OH)] towards AO-7, and e−/h+ towards E.coli, were found to be responsible for the observed behavior of multifunctionality of these nanocomposite photocatalysts. In addition, AO-7 can act as an excited dye.146 In a study on the degradation of AO-7 by a Pt–TiO2/graphene-based photocatalyst under solar light irradiation, the high photocatalytic activity towards AO-7 degradation was attributed mainly to charge separation by graphene and the Pt–TiO2 interface.147 Similarly, nanocomposite photocatalysts based on TiO2 nanotubes (NTs) were designed via anodization followed by Ag NP and rGO deposition via electrochemical reduction. The photocatalytic activity of the nanocomposites for the degradation of 2,4-dichlorophenoxyacetic acid was found to be higher.148 In a similar report by Sim et al., TiO2 NTs were deposited by Pt NPs and rGO to form TiO2–Pt–rGO nanocomposite photocatalytic systems that exhibited enhanced photocatalytic conversion of CO2 to CH4 in the presence of water vapor under visible light irradiation. The resultant yield was highly efficient compared to the use of TiO2 and TiO2 NTs–Pt NPs; this was attributed to the synergic effect of SPR absorption by Pt NPs and charge-carrier separation and transport by rGO.149
Interestingly, Wang et al.150 designed and engineered rGO/Au/TiO2 hybrid shells for the degradation of RhB using a silica template, as shown in Fig. 8. GO was first deposited on the silica spheres followed by the deposition of Au NPs and then coating with TiO2. Silica was removed via NaOH, and calcination was carried out under N2 to obtain crystalline TiO2 and rGO. The energy band gap of this nanohybrid system was found to be 2.76 eV, whereas those of TiO2 and GO/TiO2 were 3.16 and 2.90 eV, respectively. The observed decrease in the band gap of this nanohybrid system was attributed to interactions among rGO, TiO2, and Au NPs. The sandwich-like hollow nanohybrid shells showed high-performance photocatalytic activity for the degradation of RhB under UV and visible light due to the porous nature of the nanohybrid shells whereas the enhanced absorption in visible region caused by the presence of both rGO and Au NPs (Fig. 8). Additional rGO hybridized with TiO2, increasing the photocatalytic activity by enhancing electrical conductivity and visible light absorption. On the other hand, excessive rGO did not hybridize with TiO2, leading to a decrease in photocatalytic efficiency. Similarly, excess Au NPs acted as recombination centers for photoexcited charge carriers. Authors also studied the effect of TiO2 shell thickness on the photocatalytic behavior of the designed rGO/Au/TiO2 nanohybrid photocatalyst shells.150
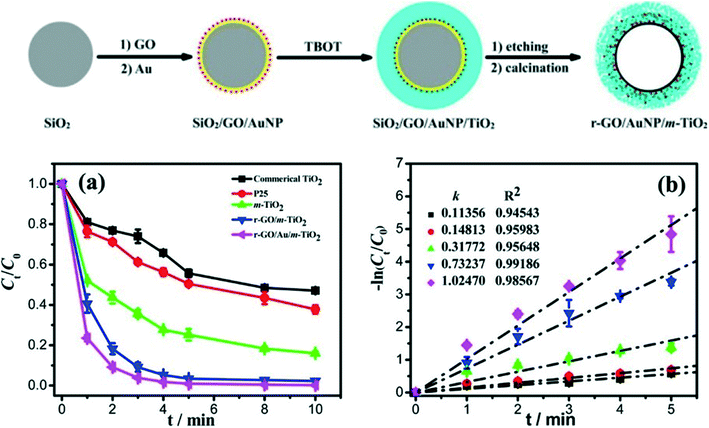 | ||
| Fig. 8 Top: Schematic illustration of the synthesis of r-GO/AuNP/m-TiO2 sandwich-like hollow spheres. Bottom: (a) Evolution of RhB concentration with reaction time under UV irradiation and (b) apparent reaction rate constant versus UV irradiation time in the presence of different photocatalysts. Light source = UV lamp (300 W Hg) with a 365 nm filter; initial concentration = 2 × 10−5 mol L−1; photocatalyst loading = 0.2 mg mL−1. Reprinted with permission from ref. 150. Copyright © 2015, American Chemical Society. | ||
Similarly, ZnO nanomaterials have been used as to design high-performance photocatalysts and engineer their properties in combination with functional nanomaterials, as discussed above. Yang et al.151 designed ZnO nanorod-based ZnO–Ag–graphene nanocomposites in the form of vertically aligned arrays attached with Ag NPs and grown on graphene-based solid substrates (Fig. 9). These heterostructures showed higher photocatalytic activity for the degradation of RhB dye (13.80 times higher) than bare ZnO photocatalysts along with higher stability and reusability. This excellent performance was attributed to the combined effect of the electronic and plasmonic properties of graphene and Ag NPs,151 as discussed in Fig. 2.
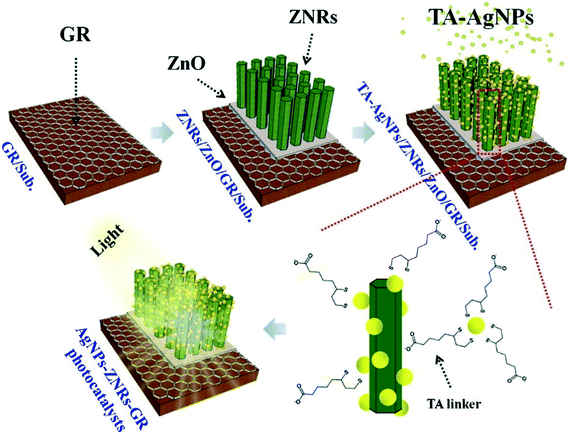 | ||
| Fig. 9 Schematic illustration of the synthesis of AgNPs–ZNRs–GR photocatalysts. Reprinted with permission from ref. 151. Copyright © 2015 Elsevier Inc. | ||
Using Au nanorods deposited on graphene–ZnO nanospheres, Roy et al. synthesized graphene–ZnO–Au nanocomposites that showed higher photocatalytic activity for the reduction of nitrobenzene to aniline compared to commercial ZnO nanospheres.33 Similarly, Khao et al. synthesized Au NPs decorated rGO wrapped on ZnO hollow spheres. The design of this system is shown in Fig. 10. The Au/rGO/ZnO nanocomposite photocatalyst demonstrated excellent photocatalytic activity towards MB degradation due the photoexcited charge separation and high electron transfer rate of the multi-component system.152 The same group further designed ZnO nanosheets aggregated hollow spheres were further modified with the Au–rGO with excellent photoluminescence properties, indicating the high suppression of photoexcited charge recombination, which is favorable for the photocatalytic degradation of MB molecules.153 Similarly, the ZnO–graphene nanostructures decorated with Pd NPs synthesized by Zhang et al. showed better photocatalytic activity than ZnO–graphene nanostructures due to the excellent charge separation at the Pd–ZnO metal–semiconductor interface.154
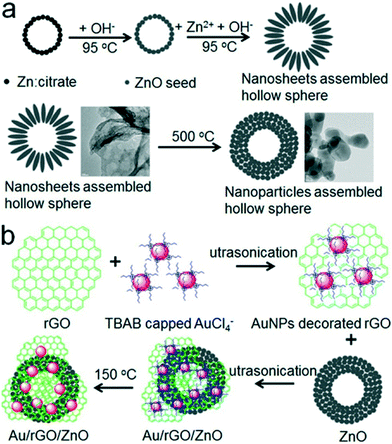 | ||
| Fig. 10 Schematic illustration of the synthesis of a ZnO hollow hierarchical structure (a) and the synthesis of an Au/rGO/ZnO hybrid (b). Reprinted with permission from ref. 152. Copyright © 2015, American Chemical Society. | ||
Metal oxide–graphene–noble metal nanocomposite-based photocatalytic systems have also been designed, and their properties have been engineered to obtain high photocatalytic activity for hydrogen production. Chun-Ren et al.155 developed a TiO2 bead/Ag/graphene-based photoanode and configured a photoelectrochemical device for hydrogen production. Compared to the commercially available P25 photoanode, the designed heterostructural photoanode achieved 47% more hydrogen production.155 A similar study was carried out using Ag–TiO2/graphene heterostructures.156 An Ag–TiO2/graphene photocatalytic system exhibited high photocatalytic activity for hydrogen production compared to TiO2, Ag–TiO2 and TiO2/graphene systems. It was carried out in presence of visible light. The presence of Ag NPs and graphene brings the plasmonic effect and high specific surface area, respectively. An Ag concentration of 0.09% in the TiO2–graphene (ATG9) composite resulted in the highest rate of hydrogen evolution (225 μ mole h−1 g−1). But, with an increase in Ag concentration (ATG12 and ATG15), lower hydrogen production rates were obtained, which attributed to the formation of recombination centers for the photoexcited charge carriers and thus, slow down the photocatalytic performance of the nanocomposites as shown in Fig. 11. This study highlights possible applications of metal oxide-based photocatalysts in the energy field.156
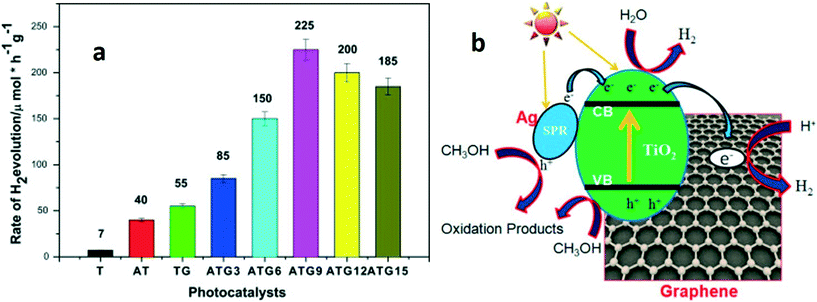 | ||
| Fig. 11 (a) Photocatalytic hydrogen production in the presence of different catalysts under visible light irradiation (λ ≥ 420 nm): pure TiO2 (T), Ag–TiO2 (AT), TiO2–graphene (TG) and AT–graphene (ATG) with variable concentrations of Ag. Light source = 300 W Xe lamp with filter (λ > 420 nm); initial solution = 60 mL water and 20 mL methanol; catalyst loading = 0.625 mg mL−1. (b) Schematic representation of the mechanism of hydrogen production over the Ag–TiO2/graphene composite under visible light. Reprinted with permission from ref. 156. Copyright © 2016 Elsevier Ltd and Techna Group S.r.l. | ||
Wang et al. obtained similar results for the use of Au–TiO2–graphene nanocomposite photocatalysts for hydrogen production from aqueous methanolic solution.157 In another similar study, graphene and Cu were co-deposited on TiO2 and used for hydrogen generation from water splitting in the presence of methanol. With the optimum Cu loading (1.5%), the hydrogen generation achieved by the graphene–TiO2 photocatalyst was five times higher than that of the graphene–TiO2 photocatalyst without Cu. A few defects favor the charge carrier transfer, while a high number of defects leads to the trapping of carriers.158
Other metal oxide photocatalysts have shown efficient photocatalytic activities when coupled with noble metal- and graphene-based nanostructures. For example, Xu et al. synthesized an Ag/graphene nanosheets/BiVO4 ternary composite via a one-pot solvothermal route. The composite with composition 3% Ag/5% graphene nanosheets/BiVO4 showed the best photocatalytic activity towards the degradation of RhB due to the enhanced exciton separation.159 Similarly, the Ag@BiVO4@rGO composite was prepared by a stepwise chemical method with photo-deposition. The Ag@BiVO4@rGO composite showed a higher photocatalytic activity for the photoreduction of BrO3− under visible light than the Ag@BiVO4 and rGO@BiVO4 composites and pure BiVO4. The enhancement in photocatalytic activity was attributed to the effective charge separation caused by Ag and rGO along with the adsorption sites for BrO3− provided by rGO.160
These results indicate that every system has different properties that depend on its composition along with the structure, shape, functionality, etc. of the individual components. It should be noted that noble metals enhance visible light absorption but also can also act as electron reservoirs. Graphene and its derivatives exhibit electron separation (photogenerated excitons) and electron transfer properties due to their high conductivity. They can also exhibit light absorption properties depending on their structures.
3. Summary and future aspects
High-performance nanocomposite photocatalysts comprising multi-functional elements such as metal oxide photocatalysts, graphene (and its derivatives) and noble metal nanoparticles have been reviewed. The progress in designing these nanomaterials and engineering their properties for enhanced photocatalytic properties has been summarized along with their individual properties. We hope that this review will encourage researchers to carry out more investigations to improve the properties of these photocatalysts by engineering their morphologies, functionalities and interfaces and hence the photocatalytic properties. Further developments to design these photocatalysts with better reproducibility and higher stability are also desired. These nanomaterials are also promising for other fields such as antibacterial, sensing and photovoltaic applications due to the multifunctional components.Conflicts of interest
There are no conflicts to declare.Acknowledgements
RKG acknowledges financial assistance from the Department of Science and Technology (DST), India, through the INSPIRE Faculty Award (Project No. IFA-13 ENG-57) and Grant No. DST/TM/WTI/2K16/23(G). JP acknowledges DST, India for the prestigious INSPIRE faculty award (INSPIRE/04/2015/002452) and National Research Foundation (NRF)/UFS, South Africa for rating-2017 (Y1)/incentive awards. DST support to the Center for Nanosciences is acknowledged.References
- T. A. Saleh and V. K. Gupta, J. Colloid Interface Sci., 2012, 371, 101–106 CrossRef CAS PubMed.
- S.-Y. Lee and S.-J. Park, J. Ind. Eng. Chem., 2013, 19, 1761–1769 CrossRef CAS.
- M. Ahmaruzzaman, Adv. Colloid Interface Sci., 2011, 166, 36–59 CrossRef CAS PubMed.
- V. K. Gupta, R. Jain, A. Mittal, T. A. Saleh, A. Nayak, S. Agarwal and S. Sikarwar, Mater. Sci. Eng., C, 2012, 32, 12–17 CrossRef CAS PubMed.
- P. V. A. Padmanabhan, K. P. Sreekumar, T. K. Thiyagarajan, R. U. Satpute, K. Bhanumurthy, P. Sengupta, G. K. Dey and K. G. K. Warrier, Vacuum, 2006, 80, 1252–1255 CrossRef CAS.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- D. Sudha and P. Sivakumar, Chem. Eng. Process., 2015, 97, 112–133 CrossRef CAS.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- C. Yu, W. Zhou, H. Liu, Y. Liu and D. D. Dionysiou, Chem. Eng. J., 2016, 287, 117–129 CrossRef CAS.
- X. Zhang, Y. Wang, B. Liu, Y. Sang and H. Liu, Appl. Catal., B, 2017, 202, 620–641 CrossRef CAS.
- S. Dong, J. Feng, M. Fan, Y. Pi, L. Hu, X. Han, M. Liu, J. Sun and J. Sun, RSC Adv., 2015, 5, 14610–14630 RSC.
- K. M. Lee, C. W. Lai, K. S. Ngai and J. C. Juan, Water Res., 2016, 88, 428–448 CrossRef CAS PubMed.
- J. Bogdan, A. Jackowska-Tracz, J. Zarzyńska and J. Pławińska-Czarnak, Nanoscale Res. Lett., 2015, 10, 57 CrossRef PubMed.
- H. J. Huang, S.-Y. Zhen, P.-Y. Li, S.-D. Tzeng and H.-P. Chiang, Opt. Express, 2016, 24, 15603–15608 CrossRef PubMed.
- Y. Tang, R. Wang, Y. Yang, D. Yan and X. Xiang, ACS Appl. Mater. Interfaces, 2016, 8, 19446–19455 CAS.
- W. He, R. Wang, C. Zhou, J. Yang, F. Li and X. Xiang, Ind. Eng. Chem. Res., 2015, 54, 10723–10730 CrossRef CAS.
- X. Xiang, L. Xie, Z. Li and F. Li, Chem. Eng. J., 2013, 221, 222–229 CrossRef CAS.
- Y. Li, L. Zhang, X. Xiang, D. Yan and F. Li, J. Mater. Chem. A, 2014, 2, 13250–13258 CAS.
- W. He, Y. Yang, L. Wang, J. Yang, X. Xiang, D. Yan and F. Li, ChemSusChem, 2015, 8, 1568–1576 CrossRef CAS PubMed.
- W. He, R. Wang, L. Zhang, J. Zhu, X. Xiang and F. Li, J. Mater. Chem. A, 2015, 3, 17977–17982 CAS.
- R. Wang, K. Pan, D. Han, J. Jiang, C. Xiang, Z. Huang, L. Zhang and X. Xiang, ChemSusChem, 2016, 9, 2470–2479 CrossRef CAS PubMed.
- X. Xiang, J. Fielden, W. Rodríguez-Córdoba, Z. Huang, N. Zhang, Z. Luo, D. G. Musaev, T. Lian and C. L. Hill, J. Phys. Chem. C, 2013, 117, 918–926 CAS.
- L. Baia, E. Orbán, S. Fodor, B. Hampel, E. Z. Kedves, K. Saszet, I. Székely, É. Karácsonyi, B. Réti, P. Berki, A. Vulpoi, K. Magyari, A. Csavdári, C. Bolla, V. Coşoveanu, K. Hernádi, M. Baia, A. Dombi, V. Danciu, G. Kovács and Z. Pap, Mater. Sci. Semicond. Process., 2016, 42, 66–71 CrossRef CAS.
- B. Grbić, N. Radić, S. Stojadinović, R. Vasilić, Z. Dohčević-Mitrović, Z. Šaponjić and P. Stefanov, Surf. Coat. Technol., 2014, 258, 763–771 CrossRef.
- C. Shifu, C. Lei, G. Shen and C. Gengyu, Powder Technol., 2005, 160, 198–202 CrossRef.
- T. A. Saleh and V. K. Gupta, J. Colloid Interface Sci., 2011, 362, 337–344 CrossRef CAS PubMed.
- I. M. Szilágyi, B. Fórizs, O. Rosseler, Á. Szegedi, P. Németh, P. Király, G. Tárkányi, B. Vajna, K. Varga-Josepovits, K. László, A. L. Tóth, P. Baranyai and M. Leskelä, J. Catal., 2012, 294, 119–127 CrossRef.
- P. Dong, B. Yang, C. Liu, F. Xu, X. Xi, G. Hou and R. Shao, RSC Adv., 2017, 7, 947–956 RSC.
- S. G. Kumar and K. S. R. K. Rao, Appl. Surf. Sci., 2017, 391, 124–148 CrossRef CAS.
- G. Xiuquan, L. Cuiyan, Y. Shuai, M. Mingguo, Q. Yinghuai and Z. Jiefang, Nanotechnology, 2016, 27, 402001 CrossRef PubMed.
- J. Chen, F. Qiu, W. Xu, S. Cao and H. Zhu, Appl. Catal., A, 2015, 495, 131–140 CrossRef CAS.
- Y. Wen, H. Ding and Y. Shan, Nanoscale, 2011, 3, 4411–4417 RSC.
- P. Roy, A. P. Periasamy, C.-T. Liang and H.-T. Chang, Environ. Sci. Technol., 2013, 47, 6688–6695 CrossRef CAS PubMed.
- N. Singh, K. Mondal, M. Misra, A. Sharma and R. K. Gupta, RSC Adv., 2016, 6, 48109–48119 RSC.
- M. Misra, N. Singh and R. K. Gupta, Catal. Sci. Technol., 2017, 7, 570–580 CAS.
- D. Li, Q. Qin, X. Duan, J. Yang, W. Guo and W. Zheng, ACS Appl. Mater. Interfaces, 2013, 5, 9095–9100 CAS.
- N. Liu, X. Chen, J. Zhang and J. W. Schwank, Catal. Today, 2014, 225, 34–51 CrossRef CAS.
- B. E. Sernelius, K. F. Berggren, Z. C. Jin, I. Hamberg and C. G. Granqvist, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 10244–10248 CrossRef CAS.
- N. Singh, J. Prakash, M. Misra, A. Sharma and R. K. Gupta, ACS Appl. Mater. Interfaces, 2017 DOI:10.1021/acsami.7b07571.
- M. Nolan, Chem. Commun., 2011, 47, 8617–8619 RSC.
- H. Park, Y. Park, W. Kim and W. Choi, J. Photochem. Photobiol., C, 2013, 15, 1–20 CrossRef CAS.
- Q. Jin, H. Yamamoto, K. Yamamoto, M. Fujishima and H. Tada, Phys. Chem. Chem. Phys., 2013, 15, 20313–20319 RSC.
- A. Tyagi, K. M. Tripathi, N. Singh, S. Choudhary and R. K. Gupta, RSC Adv., 2016, 6, 72423–72432 RSC.
- X. Bian, K. Hong, X. Ge, R. Song, L. Liu and M. Xu, J. Phys. Chem. C, 2015, 119, 1700–1705 CAS.
- V. Kumar, J. Prakash, J. P. Singh, K. H. Chae, C. Swart, O. M. Ntwaeaborwa, H. C. Swart and V. Dutta, Colloids Surf., B, 2017, 159, 191–199 CrossRef CAS PubMed.
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Coord. Chem. Rev., 2016, 312, 99–148 CrossRef CAS.
- C. Han, N. Zhang and Y.-J. Xu, Nano Today, 2016, 11, 351–372 CrossRef CAS.
- A. H. Castro Neto, F. Guinea, N. M. R. Peres, K. S. Novoselov and A. K. Geim, Rev. Mod. Phys., 2009, 81, 109–162 CrossRef CAS.
- H. Md Tanvir, J. S. Brian, M. Price, R. Conor, D. Hung, G. Zygmunt and V. N. Anton, Nanotechnology, 2017, 28, 065705 CrossRef PubMed.
- R. Maiti, A. Midya, C. Narayana and S. K. Ray, Nanotechnology, 2014, 25, 495704 CrossRef CAS PubMed.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed.
- V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Chem. Rev., 2016, 116, 5464–5519 CrossRef CAS PubMed.
- T. A. Saleh, A. Sarı and M. Tuzen, Chem. Eng. J., 2017, 307, 230–238 CrossRef CAS.
- T. A. Saleh, M. M. Al-Shalalfeh and A. A. Al-Saadi, Sci. Rep., 2016, 6, 32185 CrossRef CAS PubMed.
- X. An and J. C. Yu, RSC Adv., 2011, 1, 1426–1434 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- D. Chen, H. Zhang, Y. Liu and J. Li, Energy Environ. Sci., 2013, 6, 1362–1387 CAS.
- T.-F. Yeh, J. Cihlář, C.-Y. Chang, C. Cheng and H. Teng, Mater. Today, 2013, 16, 78–84 CrossRef CAS.
- M. A. Rafiee, J. Rafiee, I. Srivastava, Z. Wang, H. Song, Z.-Z. Yu and N. Koratkar, Small, 2010, 6, 179–183 CrossRef CAS PubMed.
- M. A. Rafiee, J. Rafiee, Z. Wang, H. Song, Z.-Z. Yu and N. Koratkar, ACS Nano, 2009, 3, 3884–3890 CrossRef CAS PubMed.
- M. A. Rafiee, J. Rafiee, Z.-Z. Yu and N. Koratkar, Appl. Phys. Lett., 2009, 95, 223103 CrossRef.
- J. Prakash, V. Kumar, R. E. Kroon, K. Asokan, V. Rigato, K. H. Chae, S. Gautam and H. C. Swart, Phys. Chem. Chem. Phys., 2016, 18, 2468–2480 RSC.
- J. Prakash, R. Harris and H. Swart, Int. Rev. Phys. Chem., 2016, 35, 353–398 CrossRef CAS.
- J. Prakash, J. C. Pivin and H. C. Swart, Adv. Colloid Interface Sci., 2015, 226(Part B), 187–202 CrossRef CAS PubMed.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS.
- P. Jai, A. Tripathi, V. Rigato, J. C. Pivin, T. Jalaj, C. Keun Hwa, G. Sanjeev, P. Kumar, K. Asokan and D. K. Avasthi, J. Phys. D: Appl. Phys., 2011, 44, 125302 CrossRef.
- J. Prakash, A. Tripathi, S. Gautam, K. H. Chae, J. Song, V. Rigato, J. Tripathi and K. Asokan, Mater. Chem. Phys., 2014, 147, 920–924 CrossRef CAS.
- M. C. Mathpal, P. Kumar, S. Kumar, A. K. Tripathi, M. K. Singh, J. Prakash and A. Agarwal, RSC Adv., 2015, 5, 12555–12562 RSC.
- P. Kumar, M. C. Mathpal, A. K. Tripathi, J. Prakash, A. Agarwal, M. M. Ahmad and H. C. Swart, Phys. Chem. Chem. Phys., 2015, 17, 8596–8603 RSC.
- Z. Xuming, C. Yu Lim, L. Ru-Shi and T. Din Ping, Rep. Prog. Phys., 2013, 76, 046401 CrossRef PubMed.
- X. Liu, J. Iocozzia, Y. Wang, X. Cui, Y. Chen, S. Zhao, Z. Li and Z. Lin, Energy Environ. Sci., 2017, 10, 402–434 CAS.
- P. Jai, K. Promod, R. A. Harris, S. Chantel, J. H. Neethling, A. J. V. Vuuren and H. C. Swart, Nanotechnology, 2016, 27, 355707 CrossRef PubMed.
- C. Marchal, M. Behr, F. Vigneron, V. Caps and V. Keller, New J. Chem., 2016, 40, 4428–4435 RSC.
- V. Jovic, Z. H. N. Al-Azri, W.-T. Chen, D. Sun-Waterhouse, H. Idriss and G. I. N. Waterhouse, Top. Catal., 2013, 56, 1139–1151 CrossRef CAS.
- L.-H. Chang and C.-P. Cho, Solid State Sci., 2016, 62, 112–120 CrossRef CAS.
- Z. Yang, J. Lu, W. Ye, C. Yu and Y. Chang, Appl. Surf. Sci., 2017, 392, 472–480 CrossRef CAS.
- S. Zhu, S. Liang, Q. Gu, L. Xie, J. Wang, Z. Ding and P. Liu, Appl. Catal., B, 2012, 119–120, 146–155 CrossRef CAS.
- Z. Cao, S. Zhu, H. Qu, D. Qi, U. Ziener, L. Yang, Y. Yan and H. Yang, J. Colloid Interface Sci., 2014, 435, 51–58 CrossRef CAS PubMed.
- Y. Yu, W. Wen, X.-Y. Qian, J.-B. Liu and J.-M. Wu, Sci. Rep., 2017, 7, 41253 CrossRef CAS PubMed.
- E. Kowalska, Z. Wei, B. Karabiyik, A. Herissan, M. Janczarek, M. Endo, A. Markowska-Szczupak, H. Remita and B. Ohtani, Catal. Today, 2015, 252, 136–142 CrossRef CAS.
- M. J. Sampaio, M. J. Lima, D. L. Baptista, A. M. T. Silva, C. G. Silva and J. L. Faria, Chem. Eng. J., 2017, 318, 95–102 CrossRef CAS.
- P. She, K. Xu, S. Zeng, Q. He, H. Sun and Z. Liu, J. Colloid Interface Sci., 2017, 499, 76–82 CrossRef CAS PubMed.
- M. Khademalrasool, M. Farbod and A. Iraji zad, J. Alloys Compd., 2016, 664, 707–714 CrossRef CAS.
- J. Lu, H. Wang, D. Peng, T. Chen, S. Dong and Y. Chang, Phys. E, 2016, 78, 41–48 CrossRef CAS.
- H. Bouzid, M. Faisal, F. A. Harraz, S. A. Al-Sayari and A. A. Ismail, Catal. Today, 2015, 252, 20–26 CrossRef CAS.
- K. H. Leong, B. L. Gan, S. Ibrahim and P. Saravanan, Appl. Surf. Sci., 2014, 319, 128–135 CrossRef CAS.
- Z. Chen, L. Fang, W. Dong, F. Zheng, M. Shen and J. Wang, J. Mater. Chem. A, 2014, 2, 824–832 CAS.
- M. Tahir, B. Tahir and N. A. S. Amin, Appl. Catal., B, 2017, 204, 548–560 CrossRef CAS.
- Q. Wang, X. Wang, M. Zhang, G. Li, S. Gao, M. Li and Y. Zhang, J. Colloid Interface Sci., 2016, 463, 308–316 CrossRef CAS PubMed.
- S. W. Verbruggen, M. Keulemans, M. Filippousi, D. Flahaut, G. Van Tendeloo, S. Lacombe, J. A. Martens and S. Lenaerts, Appl. Catal., B, 2014, 156–157, 116–121 CrossRef CAS.
- Y. Sato, S.-i. Naya and H. Tada, APL Mater., 2015, 3, 104502 CrossRef.
- Ş. Neaţu, J. A. Maciá-Agulló, P. Concepción and H. Garcia, J. Am. Chem. Soc., 2014, 136, 15969–15976 CrossRef PubMed.
- Z. Chehadi, N. Alkees, A. Bruyant, J. Toufaily, J.-S. Girardon, M. Capron, F. Dumeignil, T. Hamieh, R. Bachelot and S. Jradi, Mater. Sci. Semicond. Process., 2016, 42(Part 1), 81–84 CrossRef CAS.
- S.-W. Cao, Z. Yin, J. Barber, F. Y. C. Boey, S. C. J. Loo and C. Xue, ACS Appl. Mater. Interfaces, 2012, 4, 418–423 CAS.
- Q. Xiang, G. F. Meng, H. B. Zhao, Y. Zhang, H. Li, W. J. Ma and J. Q. Xu, J. Phys. Chem. C, 2010, 114, 2049–2055 CAS.
- D. Ding, K. Liu, S. He, C. Gao and Y. Yin, Nano Lett., 2014, 14, 6731–6736 CrossRef CAS PubMed.
- L.-L. Tan, S.-P. Chai and A. R. Mohamed, ChemSusChem, 2012, 5, 1868–1882 CrossRef CAS PubMed.
- X. Gong, G. Liu, Y. Li, D. Y. W. Yu and W. Y. Teoh, Chem. Mater., 2016, 28, 8082–8118 CrossRef CAS.
- X. Li, J. Yu, S. Wageh, A. A. Al-Ghamdi and J. Xie, Small, 2016, 12, 6640–6696 CrossRef CAS PubMed.
- Y. Haldorai, A. Rengaraj, C. H. Kwak, Y. S. Huh and Y.-K. Han, Synth. Met., 2014, 198, 10–18 CrossRef CAS.
- M. Wojtoniszak, B. Zielinska, X. Chen, R. J. Kalenczuk and E. Borowiak-Palen, J. Mater. Sci., 2012, 47, 3185–3190 CrossRef CAS.
- H.-i. Kim, G.-h. Moon, D. Monllor-Satoca, Y. Park and W. Choi, J. Phys. Chem. C, 2012, 116, 1535–1543 CAS.
- S. D. Perera, R. G. Mariano, K. Vu, N. Nour, O. Seitz, Y. Chabal and K. J. Balkus, ACS Catal., 2012, 2, 949–956 CrossRef CAS.
- Y. Zhang, Z. Zhou, T. Chen, H. Wang and W. Lu, J. Environ. Sci., 2014, 26, 2114–2122 CrossRef PubMed.
- H. Al-Kandari, A. M. Abdullah, A. M. Mohamed and S. Al-Kandari, J. Mater. Sci., 2016, 51, 8331–8345 CrossRef CAS.
- X. Zhang, Y. Sun, X. Cui and Z. Jiang, Int. J. Hydrogen Energy, 2012, 37, 811–815 CrossRef CAS.
- H. M. Yadav and J.-S. Kim, J. Alloys Compd., 2016, 688(Part B), 123–129 CrossRef CAS.
- L.-L. Tan, W.-J. Ong, S.-P. Chai and A. R. Mohamed, Nanoscale Res. Lett., 2013, 8, 465 CrossRef PubMed.
- B. Cao, S. Cao, P. Dong, J. Gao and J. Wang, Mater. Lett., 2013, 93, 349–352 CrossRef CAS.
- X. Pan, M.-Q. Yang, Z.-R. Tang and Y.-J. Xu, J. Phys. Chem. C, 2014, 118, 27325–27335 CAS.
- S. Morales-Torres, L. M. Pastrana-Martínez, J. L. Figueiredo, J. L. Faria and A. M. T. Silva, Appl. Surf. Sci., 2013, 275, 361–368 CrossRef CAS.
- L. M. Pastrana-Martínez, S. Morales-Torres, V. Likodimos, P. Falaras, J. L. Figueiredo, J. L. Faria and A. M. T. Silva, Appl. Catal., B, 2014, 158–159, 329–340 CrossRef.
- S. P. Kim and H. C. Choi, Bull. Korean Chem. Soc., 2014, 35, 2661 Search PubMed.
- Y. Cong, M. Long, Z. Cui, X. Li, Z. Dong, G. Yuan and J. Zhang, Appl. Surf. Sci., 2013, 282, 400–407 CrossRef CAS.
- W. Fan, Q. Lai, Q. Zhang and Y. Wang, J. Phys. Chem. C, 2011, 115, 10694–10701 CAS.
- H. Liu, X. Dong, X. Wang, C. Sun, J. Li and Z. Zhu, Chem. Eng. J., 2013, 230, 279–285 CrossRef CAS.
- W.-K. Jo, Vacuum, 2014, 99, 22–25 CrossRef CAS.
- G. Jiang, Z. Lin, C. Chen, L. Zhu, Q. Chang, N. Wang, W. Wei and H. Tang, Carbon, 2011, 49, 2693–2701 CrossRef CAS.
- H. Sun, S. Liu, S. Liu and S. Wang, Appl. Catal., B, 2014, 146, 162–168 CrossRef CAS.
- Y. H. Ng, A. Iwase, A. Kudo and R. Amal, J. Phys. Chem. Lett., 2010, 1, 2607–2612 CrossRef CAS.
- Y. Yan, S. Sun, Y. Song, X. Yan, W. Guan, X. Liu and W. Shi, J. Hazard. Mater., 2013, 250, 106–114 CrossRef PubMed.
- Y. Fu, X. Sun and X. Wang, Mater. Chem. Phys., 2011, 131, 325–330 CrossRef CAS.
- X. An, J. C. Yu, Y. Wang, Y. Hu, X. Yu and G. Zhang, J. Mater. Chem., 2012, 22, 8525–8531 RSC.
- B. Chai, J. Li, Q. Xu and K. Dai, Mater. Lett., 2014, 120, 177–181 CrossRef CAS.
- Y. Zhao, X. Wei, Y. Wang and F. Luo, Chem. Phys. Lett., 2014, 607, 34–38 CrossRef CAS.
- J. Guo, Y. Li, S. Zhu, Z. Chen, Q. Liu, D. Zhang, W.-J. Moon and D.-M. Song, RSC Adv., 2012, 2, 1356–1363 RSC.
- H.-i. Kim, S. Kim, J.-K. Kang and W. Choi, J. Catal., 2014, 309, 49–57 CrossRef CAS.
- E. Lee, J.-Y. Hong, H. Kang and J. Jang, J. Hazard. Mater., 2012, 219–220, 13–18 CrossRef CAS PubMed.
- M. Nawaz, W. Miran, J. Jang and D. S. Lee, Appl. Catal., B, 2017, 203, 85–95 CrossRef CAS.
- R. Cai, J.-g. Wu, L. Sun, Y.-j. Liu, T. Fang, S. Zhu, S.-y. Li, Y. Wang, L.-f. Guo, C.-e. Zhao and A. Wei, Mater. Des., 2016, 90, 839–844 CrossRef CAS.
- A. R. Nanakkal and L. K. Alexander, J. Chem. Sci., 2017, 129, 95–102 CrossRef CAS.
- J. Xu, Y. Cui, Y. Han, M. Hao and X. Zhang, RSC Adv., 2016, 6, 96778–96784 RSC.
- V. R. Posa, V. Annavaram, J. R. Koduru, V. R. Ammireddy and A. R. Somala, Korean J. Chem. Eng., 2016, 33, 456–464 CrossRef CAS.
- S. Gayathri, P. Jayabal, M. Kottaisamy and V. Ramakrishnan, J. Appl. Phys., 2014, 115, 173504 CrossRef.
- R. Lv, X. Wang, W. Lv, Y. Xu, Y. Ge, H. He, G. Li, X. Wu, X. Li and Q. Li, J. Chem. Technol. Biotechnol., 2015, 90, 550–558 CrossRef CAS.
- J. Fan, T. Li, H. Heng, B. Markovic and I. Djerdj, Acta Chim. Slov., 2015, 62, 902–909 CrossRef CAS PubMed.
- S. A. Hosseini and S. Babaei, J. Braz. Chem. Soc., 2017, 28, 299–307 CAS.
- S. Liu, H. Sun, A. Suvorova and S. Wang, Chem. Eng. J., 2013, 229, 533–539 CrossRef CAS.
- Z. Jafari, N. Mokhtarian, G. Hosseinzadeh, M. Farhadian, A. Faghihi and F. Shojaie, J. Energy Chem., 2016, 25, 393–402 CrossRef.
- E. Vasilaki, I. Georgaki, D. Vernardou, M. Vamvakaki and N. Katsarakis, Appl. Surf. Sci., 2015, 353, 865–872 CrossRef CAS.
- W. Zhao, Z. Zhang, J. Zhang, H. Wu, L. Xi and C. Ruan, Mater. Lett., 2016, 171, 182–186 CrossRef CAS.
- Y. Yang, E. Liu, H. Dai, L. Kang, H. Wu, J. Fan, X. Hu and H. Liu, Int. J. Hydrogen Energy, 2014, 39, 7664–7671 CrossRef CAS.
- S. Ghasemi, S. J. Hashemian, A. A. Alamolhoda, I. Gocheva and S. Rahman Setayesh, Mater. Res. Bull., 2017, 87, 40–47 CrossRef CAS.
- S. Ghasemi, A. Esfandiar, S. Rahman Setayesh, A. Habibi-Yangjeh, A. Iraji zad and M. R. Gholami, Appl. Catal., A, 2013, 462–463, 82–90 CrossRef CAS.
- M. Ghavami, R. Mohammadi, M. Koohi and M. Z. Kassaee, Mater. Sci. Semicond. Process., 2014, 26, 69–78 CrossRef CAS.
- L. Liu, H. Bai, J. Liu and D. D. Sun, J. Hazard. Mater., 2013, 261, 214–223 CrossRef CAS PubMed.
- S. H. Hsieh, W. J. Chen and C. T. Wu, Appl. Surf. Sci., 2015, 340, 9–17 CrossRef CAS.
- Y. Wang, Y. Tang, Y. Chen, Y. Li, X. Liu, S. Luo and C. Liu, J. Mater. Sci., 2013, 48, 6203–6211 CrossRef CAS.
- L. C. Sim, K. H. Leong, P. Saravanan and S. Ibrahim, Appl. Surf. Sci., 2015, 358(Part A), 122–129 CrossRef CAS.
- M. Wang, J. Han, H. Xiong, R. Guo and Y. Yin, ACS Appl. Mater. Interfaces, 2015, 7, 6909–6918 CAS.
- T.-H. Yang, Y.-W. Harn, L.-D. Huang, M.-Y. Pan, W.-C. Yen, M.-C. Chen, C.-C. Lin, P.-K. Wei, Y.-L. Chueh and J.-M. Wu, J. Catal., 2015, 329, 167–176 CrossRef CAS.
- N. T. Khoa, S. W. Kim, D.-H. Yoo, S. Cho, E. J. Kim and S. H. Hahn, ACS Appl. Mater. Interfaces, 2015, 7, 3524–3531 CAS.
- N. T. Khoa, S. W. Kim, D. Van Thuan, H. N. Tien, S. H. Hur, E. J. Kim and S. H. Hahn, RSC Adv., 2015, 5, 63964–63969 RSC.
- L. Zhang, L. Du, X. Yu, S. Tan, X. Cai, P. Yang, Y. Gu and W. Mai, ACS Appl. Mater. Interfaces, 2014, 6, 3623–3629 CAS.
- K. Chun-Ren, G. Jyun-Sheng, S. Yen-Hsun and T. Jyh-Ming, Nanotechnology, 2016, 27, 435405 CrossRef PubMed.
- N. R. Khalid, E. Ahmed, M. Ahmad, N. A. Niaz, M. Ramzan, M. Shakil, T. Iqbal and A. Majid, Ceram. Int., 2016, 42, 18257–18263 CrossRef CAS.
- Y. Wang, J. Yu, W. Xiao and Q. Li, J. Mater. Chem. A, 2014, 2, 3847–3855 CAS.
- X.-J. Lv, S.-X. Zhou, C. Zhang, H.-X. Chang, Y. Chen and W.-F. Fu, J. Mater. Chem., 2012, 22, 18542–18549 RSC.
- L. Xu, Y. Wei, W. Guo, Y. Guo and Y. Guo, Appl. Surf. Sci., 2015, 332, 682–693 CrossRef CAS.
- F. Chen, Q. Yang, Y. Zhong, H. An, J. Zhao, T. Xie, Q. Xu, X. Li, D. Wang and G. Zeng, Water Res., 2016, 101, 555–563 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |