Designing sterically demanding thiolate coated AuNPs for electrical characterization of BPDT in a NP–molecule–nanoelectrode platform‡
Francois
Calard†
a,
Ishtiaq Hassan
Wani†
b,
Aqib
Hayat
b,
Thibaut
Jarrosson
a,
Jean-Pierre
Lère-Porte
a,
S. Hassan M.
Jafri
 bc,
Françoise
Serein-Spirau
*a,
Klaus
Leifer
*b and
Andreas
Orthaber
bc,
Françoise
Serein-Spirau
*a,
Klaus
Leifer
*b and
Andreas
Orthaber
 *d
*d
aInstitut Charles Gerhardt de Montpellier, UMR CNRS 5253, Architectures Moléculaires et Matériaux Nanostructurés, Ecole Nationale Supérieure de Chimie de Montpellier, 8, rue de l'Ecole Normale, 34296 Montpellier Cedex, France. E-mail: francoise.spirau@enscm.fr
bApplied Materials Science, Department of Engineering Science, Uppsala University, PO box 534, 75120 Uppsala, Sweden. E-mail: klaus.leifer@angstrom.uu.se
cDepartment of Electrical Engineering, Mirpur University of Science and Technology, Mirpur, Azad Kashmir, 10250, Pakistan
dDepartment of Chemistry, Ångström Laboratories, Uppsala University, PO box 523, 75120 Uppsala, Sweden. E-mail: andreas.orthaber@kemi.uu.se
First published on 2nd February 2017
Abstract
Molecular electronics with single or few molecules requires a stable metal–molecule nanojunction platform. Herein, we report the design and synthesis of gold nanoparticles coated with sterically demanding thiol ligands that are essential to fabricate a versatile and stable nanoelectrode–molecule–nanoparticle platform suitable for electrical characterization of small organic molecules. By combining ω-tetraphenylmethane ether functionalized alkyl thioacetate and alkyl thiols, we prepared highly stable gold nanoparticles in a one-phase reaction providing simple and efficient purification. This robust preparation gives highly pure nanoparticles in very high yields (up to 90%) with long-time shelf stability. The synthesis in this work has superior reproducibility compared to previous synthesis processes that are currently being used for such molecular electronics platforms. Electron microscopy confirms the formation of uniform and small nanoparticles in the range of 5 to 7 nm. These nanoparticles with different ligand surface coverages were placed in a 20 nm nanoelectrode setup using dielectrophoretic forces. This setup was utilized to characterize the conductivity of the molecular wire 4,4′-biphenyldithiol introduced via ligand place-exchange under ambient conditions.
Design, System, ApplicationWe have designed a sterically demanding and dynamic ligand shell of gold nanoparticles combining two types of thiol-based ligand. Simple, short alkyl thiols cover the direct surface of the nanoparticles; the ligand shell is complemented with ω-functionalized alkyl thiols that provide a bulky terminus through a hydrolytically stable tetraphenylmethane ether moiety. Our ligand design provides superior behaviour in the nanoparticles' preparation and their long term storage. Nevertheless, the ligands provide a dynamic behaviour of the ligands. Such behaviour is essential for the application of the presented nanogap–nanoparticle platform. Stable nanoparticle separation is required to avoid short circuiting, e.g. during the trapping experiment, but also to provide appropriate interparticle distances. At the same time, the dynamic behaviour is essential to allow for the observed place exchange with organic dithiols. This platform ultimately allows us to perform the electrical characterization of conjugated organic linkers and could provide the basis for future “molecular electronics” and sensing systems operating under ambient conditions. |
Introduction
In order to advance molecular electronics, two-terminal electron transport has been extensively studied with highly specialized laboratory setups such as scanning tunnelling microscopes (STM), conductive atomic force microscopes (AFM), and mechanically controlled break junctions.1 These laboratory setups are excellent tools to determine fundamental physical properties through single or few molecule networks; however, it is still a challenge to fabricate reliable molecular electronic devices with single or few molecules. The understanding of the electrical properties of molecular systems under ambient conditions in a device like our setup could be an important step for molecular electronics. Being able to prepare systems that determine the electrical properties of molecular systems under ambient conditions shows a new direction for molecular electronics devices.2 In this context, very recently, interesting optoelectronic applications of nanoparticles have been reported,3 together with detailed reflective EXAFS studies.4 The fabrication of large scale devices is possible by electrodeposition and nanolithography5 but displays a large variation in electrical properties. One of the first robust platforms that sustain both fundamental experiments and application oriented developments is the nanoelectrode6 and/or nanoparticle–nanoelectrode7 platform where functionalised gold nanoparticles (AuNPs) are trapped by dielectrophoretic forces in between these nanoelectrodes.8 Measurements on molecules in such bridged junctions often demonstrate poor reproducibility in physical properties due the large variability of the established metal–molecule interactions.9 A rational approach to reproducible NP–nanogap setups was achieved using sterically shielded nanoparticles, i.e. having a robust though flexible organic ligand shell, where stable AuNP separation is achieved by placing sterically shielded AuNPs in nanogaps.10 The physical properties of these devices are dependent on the molecules linking the nanoparticles and nanoelectrodes. The dynamic behaviour of the organic ligand shell can be further exploited to introduce bridging dithiolate molecules using a place-exchange procedure to establish conjugated junctions.11Fundamental to all these applications are precise nano-objects with well-defined ligand shells. The exact control in bottom-up approaches provides well-defined particle sizes and shapes.12 Nanoparticles soluble in organic solvents can be obtained from citrate covered AuNPs through (partial) ligand exchange reactions with polymers or hydrophobic (e.g. thiol-based) ligands. However, the predominant preparation of gold nanoparticles in organic media relies on the Brust–Schiffrin method, which is a 2-phase preparation (liquid–liquid).13 The necessity of phase transfer reagents in these syntheses often results in ill-defined ligand shells and tedious purifications. Despite the widespread use of the Brust/Schiffrin method for thiol capped AuNPs, several studies have quite recently investigated methodologically simpler one-phase methods for the preparation of organic soluble gold nanoparticles. Several works described the use of silanes as mild and efficient reducing agents in a one-phase tetrahydrofuran (THF) system for thiolate stabilized gold nanoparticles.10a,14 These procedures provide excellent means for bottom-up synthesis of small, i.e. <10 nm sized, pseudo-spherical AuNPs. Highly pure materials are obtained by alcohol induced precipitation, however atom efficiencies, i.e. nanoparticle yields, have been poor thus far. Herein, we have synthesized monodisperse nanoparticles having sterically demanding ligands. These particular ligands are essential in the practical application in a nanoelectrode–nanoparticle platform in which such a dynamically protective ligand shell is required. The nanoparticles trapped in a nanoelectrode are used as the template for place exchange with conductive molecules in stable nanoelectrodes as schematically shown in Fig. 1, bottom.
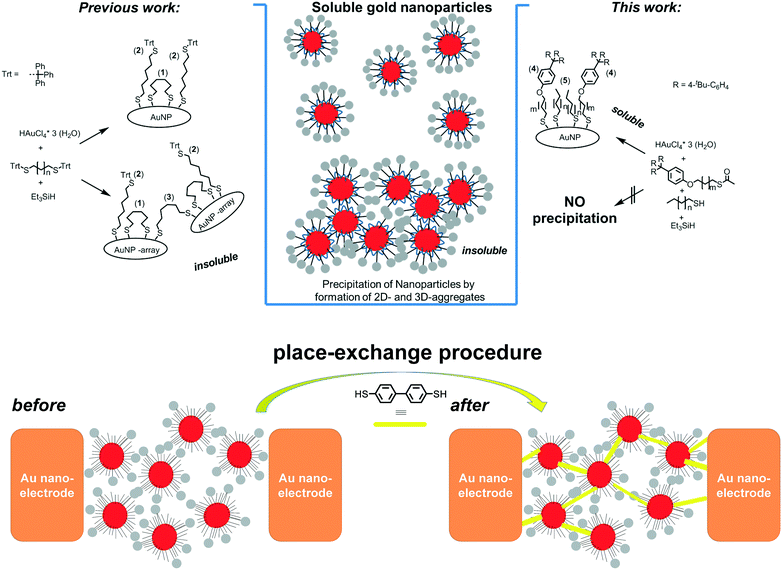 | ||
| Fig. 1 left) Synthesis of AuNPs with ω-trityl substituted alkanedithiols. Controlled conditions of the mixing time yield partial and full deprotection of the trityl thioether moiety giving two types of thiolate ligand: backbiting (1) and non-backbiting ligands (2).10a Fully de-protected though non-backbiting ligands could cause significant crosslinking of free thiols (3) and concomitant formation of insoluble 3D arrays of AuNPs. right) Schematic representation of the synthesis of AuNPs with ω-tetraphenyl ether substituted monothiols 4 (m = 10) and linear alkyl thiols 5a–d (where, n = 7, 9). bottom) Schematic representation (top view) of the place-exchange procedure used to introduce biphenyl dithiol. | ||
Results and discussion
In our previous study we have described alkyl-dithiols with sterically demanding trityl thioether protecting groups as sterically demanding ligands for AuNPs.10a Challenged by the low yields of isolated AuNPs due to significant losses during the purification steps, we hypothesized that fully de-protected though non-backbiting ligands cause crosslinking of AuNPs, concomitantly resulting in insoluble 3D-AuNP arrays. In order to circumvent these AuNP losses, different thiol ligands have been introduced in the AuNP synthesis. Detailed investigations of ω-trityl stabilized AuNPs reveal that two types of ligand are essential: short alkyl (di)thiols that occupy a certain surface area and trityl-based alkyl thiols that protrude from the bottom ligand layer and give rise to steric shielding, i.e. ensuring a reproducible nanoparticle separation even in aggregates (Fig. 1 left). In our new approach we have designed the nanoparticle surface by combining two alkylthiols; one with a hydrolytically stable and sterically demanding terminal substituent (4) and complemented the ligand shell with unbranched alkyl thiols (5) (Fig. 1 right).Synthesis
A very bulky tris-[(para-tert-butyl)phenyl] methyl phenol was alkylated with 11-bromoundecan-1-ol in a Mitsunobu reaction in the presence of diisopropyl azodicarboxylate (DIAD) and triphenylphosphine in ca. 65% yield. In the second step, the terminal bromine was substituted with a thioacetate group by treatment with potassium thioacetate in DMF giving 4 in 58% yield after chromatographic purification as a stable white solid (Scheme 1). The acetate group precludes oxidation which is a common problem for free thiols, but can be easily cleaved under acidic or basic conditions to afford gold thiolates.15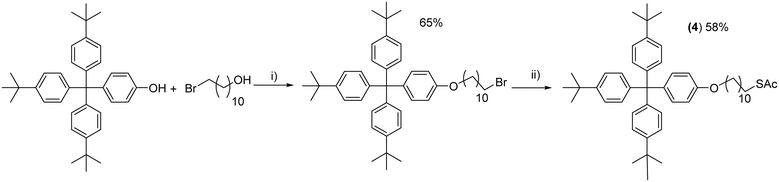 | ||
| Scheme 1 Synthesis of the alkyl thioacetate with the bulky tris-[(para-tert-butyl)phenyl] methyl phenyl end group (4). i) DIAD, PPh3, THF, 0 °C – r.t., overnight. ii) KSAc, DMF. | ||
Indeed, long alkyl chain substituted monothiols are key compounds in the single phase synthesis of AuNPs. The AuNP synthesis is initiated by the addition of HAuCl4 to a vigorously stirred THF solution of alkane thiol (5a–d) and 2–30 molar percent of thioacetate 4. The conditioning of the reaction presents an important part since the acidic conditions lead to the cleavage of the thioacetate protecting group and formation of presumably a variety of gold thiolate species.16 Importantly, aggregation due to double deprotection as observed by Wallner et al. is not a concern during this conditioning period. After approximately 3 hours, one equivalent of triethylsilane diluted in THF is added dropwise to this bright yellow solution. Formation of gold nanoparticles is accompanied by an immediate color change to dark purple which is indicative of the distinct surface plasmon resonance (SPR) at 524 nm of small AuNPs. The reaction mixture is stirred for more than 5 hours in the dark to ensure a uniform size distribution of the nanoparticles. The absence of a solid precipitate confirms the clean formation of thiolate stabilized AuNPs. Purification of the AuNPs is enabled by filtration of the AuNP solution in excess of ethanol which leads to precipitation.
The precipitate, i.e. the thiol-stabilized AuNPs (AuNP-c,d), is collected as a red-brown powder after repetitive washing and centrifugation steps with ethanol (51 mg, based on 115 mg of HAuCl4·3H2O). The yields of the described AuNP synthesis were confirmed by multiple measurements and gave extraordinary atom efficiency with respect to the gold precursor. By minimizing undesired gold–thiolate aggregation, we obtained yields of up to 90% while similar approaches with silane reducing agents have yields between ca. 7%10a and ca. 30%.14,17
We have investigated different samples of (AuNP-c,d) for their stability in solution and the solid state. These samples have been repetitively dried under vacuum and re-dissolved in THF or CHCl3 and no visible signs of decomposition or an insoluble precipitate were observed indicating that no compromising aggregation occurred during the isolation and the drying steps. In order to assess the robustness of our syntheses and the influence of different reaction parameters, we have modified the reaction temperature, used different alkyl thiols, and employed different ratios of alkyl thiol 5 to thioacetate 4. We observe that neither thiols with a hexyl (5a) nor octyl (5b) alkyl chain could be used in our experimental procedure. The dropwise addition of triethylsilane to the reaction mixture leads to the formation of a brown suspension of aggregated and non-stabilized gold species in less than one minute, which might be attributed to fast reaction kinetics or formation of insoluble gold clusters. Conversely, longer linear alkylthiols (>C8), such as decyl (5c) or dodecyl thiol (5d) seem to be relevant to stabilize reduced metallic gold in nanospherical objects and to elaborate nanoparticles later on called AuNP-c,d. These thiols with linear decyl or dodecyl chain lengths are in good accordance with the length of the alkyl linker in compound 4, supporting our initial assumption of the surface situation. Our findings are in contrast with previous studies that have shown flow preparations of stable pure octane- or hexane thiol-stabilized gold nanoparticles.17 In order to substantiate the role of the sterically demanding ligand, we have investigated different ratios of alkyl thiols (both 5c and 5d) to thioacetate 4. We have tested ratios between 2 and 30 mol% of 4 (with respect to the alkyl thiol). Due to the highly acidic conditions, we assume full cleavage of the acetate protecting group during the conditioning time (3 h) and similar reactivity towards the gold ions to the unprotected alkyl thiol. Hence, we assume that the initial thiol ratios are representative of the final ratio of molecules on the nanoparticle surface. As an additional parameter, we performed our experiments at two temperature conditions (r.t. and 0–5 °C). Interestingly, we did not find a significant influence of the temperature on the gold nanoparticle formation. Although these results are unexpected, from a synthetic point of view, the robustness of our synthetic procedure is highly encouraging since precise temperature control is not needed in order to ensure reliable and high yield AuNP formation (vide infra). The impact of different ratios of 4 to 5 and the alkyl chain lengths will be discussed in the next section.
Nanoparticle characterization
We have investigated the formed nanoparticles by means of UV spectroscopy, SEM and TEM analysis. Furthermore we have used dynamic light scattering (DLS) in order to estimate hydrodynamic particle sizes and the degree of aggregation of these nanoparticles in solution. The very simple and efficient analysis of gold nanoparticles by means of UV/vis spectroscopy provides a rough means of determining particle sizes and shapes. Spherical gold nanoparticles give rise to one absorption feature in the visible region, while e.g. nanorods show two distinct bands.13b,18 The absorption maximum is mainly dependent on the particle size and the dielectric constant of the surrounding medium. Typically, small spherical AuNPs in a non-polar solvent exhibit an absorption band between 520–530 nm. We observe in our experiments that reduction with triethyl silane gives a single absorption band centered at 524 nm which is in agreement with previous observations10a and indicates the formation of small, spherical AuNPs as shown in Fig. S1.‡ Structural analysis of nanoparticles is carried out by electron microscopy (Fig. 2). Scanning electron microscopy (SEM) with a resolution of ca. 2 nm has been carried out by drop-casting the solution of nanoparticles on Si/SiO2 wafer. Interestingly, we see individual nanoparticles but also significant amounts of monolayered small agglomerates of nanoparticles (Fig. 2a). The formation of these agglomerates occurs during the sample preparation due to drop-casting and the drying process. The hexagonally packed agglomerates show highly ordered 2D layers of nanoparticles as shown in Fig. 2b. We have performed high resolution transmission electron microscopy (HR-TEM) imaging for size analysis as shown in Fig. 2c. The TEM imaging of gold nanoparticles with 0%, 5%, 15% and 30% of bulky thiol 4 confirms the formation of small nanoparticles between 5 and 7 nm (Fig. 2d) with an average size of ∼6 nm (see Table S4‡) which is slightly larger than previously reported gold nanoparticle preparations.10a One observes that the gold nanoparticles in the small agglomerates are evenly spaced with an interparticle distance of 1.75 nm ± 0.35 nm, an average from at least 70 individual distances. Both size and particle spacing show only small variations with the addition of the bulky thiol 4. Interestingly, the measured interparticle distances are significantly shorter as compared to a fully extended thiol 4, which lets us assume that significant folding and secondary interactions of the ligand shell occur during the self-assembly process of the individual nanoparticles.19 Most likely, the packing leads also to an interlacing of neighboring ligand shells, thus distances between neighboring gold surfaces made them ideal for place exchange with short organic dithiol molecules.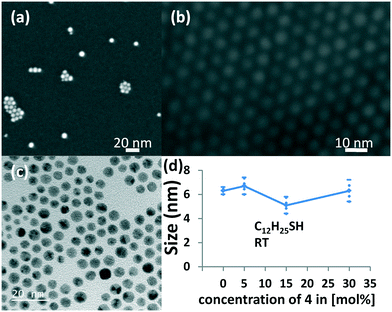 | ||
Fig. 2 a) SEM images of AuNP-c(2%) prepared at 0–5 °C with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 ratio of 4 98 ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C10H21SH (Table S1,‡ entry 2), b) 2D arrays of AuNP-c(5%) formed by drop-casting on SiO2, c) TEM image of AuNP-d(15%) prepared at r.t. with a 15 C10H21SH (Table S1,‡ entry 2), b) 2D arrays of AuNP-c(5%) formed by drop-casting on SiO2, c) TEM image of AuNP-d(15%) prepared at r.t. with a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 ratio of 4 85 ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5d (= C12H21SH) (Table S4,‡ entry 13), and d) size distribution of different nanoparticle preparations from TEM measurements with standard deviation. 5d (= C12H21SH) (Table S4,‡ entry 13), and d) size distribution of different nanoparticle preparations from TEM measurements with standard deviation. | ||
In order to assess the degree of agglomeration of these nanoparticles in solution we have performed DLS measurements at room temperature in toluene which is the same solvent used for the deposition of AuNPs on TEM and SEM grids. The DLS measurements indicate that only a small fraction of the AuNPs is aggregated in solution, which underlines the hypothesis that the extensive aggregation and formation of hexagonal single layers observed during the TEM/SEM analysis is caused by the slow evaporation of the solvent. Generally, the size determination by means of DLS includes the organic ligand shell, which is subjected to swelling phenomena.20 The intensity weighted size distributions calculated from DLS measurements are very sensitive to larger aggregates, but also significantly overestimated their fraction of total particles. Hence, we have used this method of analysis to be able to detect even the smallest fractions of agglomerated AuNPs. Throughout our investigation of different AuNP preparations we find larger agglomerates of a few hundreds of nanometers but in negligible quantities (<1%).
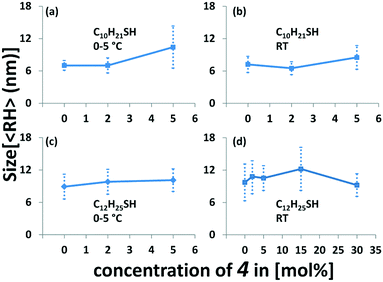
A comparison of the different reaction conditions indicates that only small variations are obtained by changing the thiol ratios (Fig. 3 and Tables S1 and S4‡). Our results indicate that an increasing amount of thioacetate for low concentrations of 4 leads to a slight increase of the particle size. The series of AuNP-c prepared at 5 °C and room temperature show very similar sizes. Using no or small amounts of thioacetate 4, i.e. 2% leads to virtually identical particle sizes, whereas a slight increase is observed when 5% thioacetate 4 is employed in the preparation. The series of preparations of AuNP-d (at 0 °C) with 0, 2, and 5% of 4 give an increasing average particle size from 8.9, 9.8, and 10.1 nm (Fig. 3c). Similar trends are observed for the same particles (AuNP-d) prepared at room temperature (Fig. 3d).21 A further increase of thioacetate 4 to 15% shows again an increase of size i.e. 12.2 nm (Fig. 3d) while the highest concentration (30%) gives the smallest AuNPs i.e. 9.2 nm (Fig. 3d) in this series, which might indicate that optimal conditions are met in a range between 5 and 15% of thioacetate 4, in agreement with previously observed ligand distributions.10a
The differences between the gold core and the hydrodynamic diameter are between 3 and 7 nm. Interestingly, for AuNP-d(0) the ligand shell is calculated to be ca. 1.7 nm, hence slightly larger compared to the AuNP with the highest concentration of 4, i.e.AuNP-d(30) where the ligand shell is found to be 1.5 nm. An increase of thiolate concentration to 5% [AuNP-d(5)] results in ligand shells of 1.9 nm. Interestingly, the calculated ligand shell for AuNP-d(15) is significantly larger (3.6 nm) than the estimated size of the individual linker molecule (2.3 nm), from MM2 calculations starting from a fully stretched geometry. A comparison with interparticle distances from the TEM measurements clearly indicates that the ligand shell is significantly expanded in solution and these aggregation effects lead to a compression of the ligand shell (1.6 to 2.0 nm). Our results are in agreement with previous comparisons of “sizes” obtained by electron microscopy and DLS measurements.19,20,22
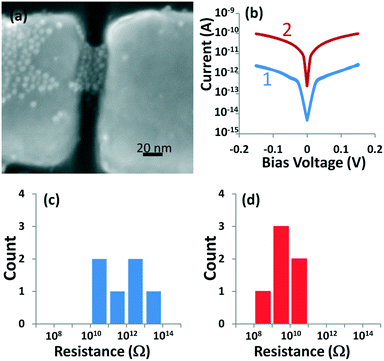
For the application in the field of molecular electronics, these synthesized nanoparticles are trapped by dielectrophoretic forces7,9,10b,c between nanoelectrodes as shown in Fig. 4a. These trapped nanoparticles form a 3D-network in between the electrodes separating adjacent gold surfaces by the organic ligand shells. Selected current–voltage response curves as shown in Fig. 4b demonstrate a very low electron conduction at room temperature which is dominated by electron tunneling (Fig. 4b-1). Introduction of 4,4′-biphenyldithiol (10 mM in toluene) through place exchange significantly alters the current–voltage characteristics. The π-conjugated backbone of the BPDT linkers gives rise to a conductivity increase by approx. two orders of magnitude as shown in (Fig. 4b-2). The low bias resistance histogram of the six devices where we have trapped AuNP-d(15%) showed high resistance ranging from 17 GΩ to 65 TΩ (Fig. 4c) with a geometric mean resistance of 880 GΩ directly after dielectrophoretic trapping of the nanoparticles. There is a statistically significant increase in conductivity of devices after place exchange (10 mM BPDT for 24 h). The geometric mean resistance of these devices decreased by two orders of magnitude to 7.9 GΩ (Fig. 4d). We have performed these experiments with nanoparticles AuNP-d that have 5% and 30% surface coverage. The average resistances are 0.3 GΩ (2 devices) and 6.2 GΩ (4 devices) after place exchange with BPDT for AuNPs with 5% and 30% thiol 4, respectively, whereas the change in conduction is 2–3 orders of magnitude as well. In summary, such an increased conductivity can only be explained by conductivity via a discrete hopping mechanism associated with new states introduced by the conjugated BPDT linkers and is an excellent measure of molecular conductivity determined at room temperature. Similarly, a 2–3 order of magnitude change in conductivity has been observed for dithiol terminated oligophenylethynylene (OPE) molecules in 2D-AuNP arrays.23 However, recent reports indicate that charge transport is also dependent on the dimensionality of the nanoparticle arrays.24
Conclusions
In this work we have presented a straightforward synthesis of an alkyl thioacetate with sterically demanding tris-[(para-tert-butyl)phenyl] methyl phenyl ether as a stable thiol precursor. Mixtures of alkyl thiols with thioacetate and HAuCl4 are converted into thiol stabilized gold nanoparticles in a single phase reaction with triethylsilane as a mild reducing agent. Facile purification of the thiolate stabilized AuNPs is achieved by ethanol induced precipitation and consecutive washing–centrifugation steps giving high yields of purified AuNPs. High stability of the nanoparticles both in solution and the solid state was observed and no significant aggregation or decomposition was noticed over a period of weeks to months. The aggregation behavior of these AuNPs was investigated using dynamic light scattering and electron microscopy in order to assess their behavior in solution and upon evaporation of the solvent on a solid substrate, respectively. On a solid substrate, AuNPs arrange in arrays of predominantly hexagonal closest packing with varying amounts of individual particles. DLS measurements reveal that in solution the individual particles build the majority and less than 1% shows agglomerates of a few hundred nanometers. These nanoparticles are electrophoretically trapped between nanoelectrodes and used to introduce and assess the molecular conductivity of 4,4′-biphenyldithiol linkers. The electrical characterization of these ω-conjugated molecules presents a new and unique fabrication of molecular electronic devices at the scale of 10–20 nm for applications under standard atmospheric conditions such as transistors or sensors.Experimental section
General
Dynamic light scattering experiments were performed in toluene (spectroscopic grade) obtained from Sigma-Aldrich with a Nano ZS Zetasizer system (Malvern Instruments) at a 173° backscattering angle. For topographical analysis, a Merlin scanning electron microscope (Carl Zeiss Instruments) equipped with a field emission gun (FEG) and an in-lens detector was used. Samples were prepared with 5 μl of diluted AuNP solution blow dried under nitrogen on Si/SiO2 (∼10 × 10 mm) wafer. Prior to this, in order to remove the dust particles, the NP solutions were filtered through cotton. For TEM imaging, Tecnai F30 was used in bright field mode. The energy of the electron beam was 300 kV. For image analysis, Image J software was used and the samples were prepared on a 3 mm Cu grid.General procedure for thiol-stabilized gold nanoparticles
(AuNP-a–d) 40 mg of HAuCl4·3H2O (0.1 mmol) was dissolved in 10 mL of dry and oxygen-free THF and introduced in a round bottom flask equipped with a magnetic stirrer. Thioacetate 4 was added to the stirred reaction medium followed by the addition of alkane thiol (0.5 mL of a 0.2 M solution in THF). The resulting yellow solution was vigorously stirred for 3 hours. Dropwise addition of 0.5 mL of a 0.2 M solution of triethylsilane at the given temperature leads to a red-purple solution which was vigorously stirred for at least 6 hours at room temperature. Gold nanoparticles were then precipitated by adding 100 mL of ethanol. After centrifugation, thiol-stabilized AuNPs (2) were collected as a dark brown powder that can be dried under vacuum.Fabrication of nanoparticle–nanoelectrode platform
A large number of nanoelectrodes were fabricated by combining electron beam lithography and photolithography. The nanosized gaps were milled with a focused Ga+ ion beam at 30 keV. Thiol 4 coated nanoparticles are trapped by dielectrophoretic forces at 1 MHz with an applied AC voltage of 1.25 V. The two-terminal electrical characterization has been carried out by using a standard probe station connected with an Agilent B1500A semiconductor device analyzer through preamplifiers.Funding sources
The Swedish research council (project grant 2013-4763, A. O.); the Uppsala University Molecular Electronics Center (U3MEC); the Austrian Science Fund (FWF, project number J 3193-N17), and COST action CM1302 – Smart Inorganic Polymers (SIPs).Abbreviations
| AuNPs | Gold nanoparticles |
| DIAD | Diisopropyl azodicarboxylate |
| DLS | Dynamic light scattering |
| DMF | Dimethylformamide |
| SEM | Scanning electron microscopy |
| SPR | Surface plasmon resonance |
| TEM | Transmission electron microscopy |
| THF | Tetrahydrofuran |
Acknowledgements
The authors would like to thank the Swedish research council (project grant 2013-4763, A. O.), the Uppsala University Molecular Electronics Center (U3MEC), the Austrian Science Fund (FWF, project number J 3193-N17), and COST action CM1302 – Smart Inorganic Polymers (SIPs).References
- J. R. Heath, Annu. Rev. Mater. Res., 2009, 39, 1 CrossRef CAS.
- P. Nicholas and S. Kyung-Ah, J. Phys.: Condens. Matter, 2008, 20, 374116 CrossRef PubMed.
- (a) T. Lahtinen, E. Hulkko, K. Sokolowska, T.-R. Tero, V. Saarnio, J. Lindgren, M. Pettersson, H. Hakkinen and L. Lehtovaara, Nanoscale, 2016, 8, 18665 RSC; (b) L. Fontana, I. Fratoddi, I. Venditti, D. Ksenzov, M. V. Russo and S. Grigorian, Appl. Surf. Sci., 2016, 369, 115 CrossRef CAS; (c) R. Matassa, G. Familiari, E. Battaglione, C. Sibilia, G. Leahu, A. Belardini, I. Venditti, L. Fontana and I. Fratoddi, Nanoscale, 2016, 8, 18161 RSC.
- C. Battocchio, I. Fratoddi, I. Venditti, V. G. Yarzhemsky, Y. V. Norov, M. V. Russo and G. Polzonetti, Chem. Phys., 2011, 379, 92 CrossRef CAS.
- (a) A. Bezryadin, C. Dekker and G. Schmid, Appl. Phys. Lett., 1997, 71, 1273 CrossRef CAS; (b) E. D. Fabrizio, L. Grella, M. Gentili, M. Baciocchi, L. Mastrogiacomo and P. Morales, Jpn. J. Appl. Phys., 1997, 36, L70 CrossRef.
- (a) T. Blom, K. Welch, M. Stromme, E. Coronel and K. Leifer, Nanotechnology, 2007, 18, 285301 CrossRef; (b) H. Li, I. H. Wani, A. Hayat, S. H. M. Jafri and K. Leifer, Appl. Phys. Lett., 2015, 107, 103108 CrossRef.
- (a) S. H. M. Jafri, T. Blom, K. Leifer, M. Strømme, H. Löfås, A. Grigoriev, R. Ahuja and K. Welch, Nanotechnology, 2010, 21, 435204 CrossRef CAS PubMed; (b) S. H. M. Jafri, T. Blom, A. Wallner, K. Welch, M. Strømme, H. Ottosson and K. Leifer, Microelectron. Eng., 2011, 88, 2629 CrossRef CAS.
- (a) T. Dadosh, Y. Gordin, R. Krahne, I. Khivrich, D. Mahalu, V. Frydman, J. Sperling, A. Yacoby and I. Bar-Joseph, Nature, 2005, 436, 677 CrossRef CAS PubMed; (b) F. O. Hadeed and C. Durkan, Appl. Phys. Lett., 2007, 91, 123120 CrossRef.
- S. H. M. Jafri, T. Blom, A. Wallner, H. Ottosson and K. Leifer, J. Nanopart. Res., 2014, 16, 2811 CrossRef.
- (a) A. Wallner, S. H. M. Jafri, T. Blom, A. Gogoll, K. Leifer, J. Baumgartner and H. Ottosson, Langmuir, 2011, 27, 9057 CrossRef CAS PubMed; (b) S. H. M. Jafri, H. Löfås, T. Blom, A. Wallner, A. Grigoriev, R. Ahuja, H. Ottosson and K. Leifer, Sci. Rep., 2015, 5, 14431 CrossRef CAS PubMed; (c) S. H. M. Jafri, H. Lofas, J. Fransson, T. Blom, A. Grigoriev, A. Wallner, R. Ahuja, H. Ottosson and K. Leifer, Nanoscale, 2013, 5, 4673 RSC.
- J. Liao, J. S. Agustsson, S. Wu, C. Schonenberger, M. Calame, Y. Leroux, M. Mayor, O. Jeannin, Y.-F. Ran, S.-X. Liu and S. Decurtins, Nano Lett., 2010, 10, 759 CrossRef CAS PubMed.
- (a) M. Grzelczak, J. Perez-Juste, P. Mulvaney and L. M. Liz-Marzan, Chem. Soc. Rev., 2008, 37, 1783 RSC; (b) M. R. Langille, M. L. Personick, J. Zhang and C. A. Mirkin, J. Am. Chem. Soc., 2012, 134, 14542 CrossRef CAS PubMed.
- (a) M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801 RSC; (b) M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed; (c) S. Eustis and M. A. El-Sayed, Chem. Soc. Rev., 2006, 35, 209 RSC.
- A. Sugie, T. Somete, K. Kanie, A. Muramatsu and A. Mori, Chem. Commun., 2008, 3882 RSC.
- (a) M. C. Gurbhele-Tupkar, L. R. Perez, Y. Silva and W. J. Lees, Bioorg. Med. Chem., 2008, 16, 2579 CrossRef CAS PubMed; (b) A. K. Flatt, Y. Yao, F. Maya and J. M. Tour, J. Org. Chem., 2004, 69, 1752 CrossRef CAS PubMed.
- B. M. Barngrover and C. M. Aikens, J. Am. Chem. Soc., 2012, 134, 12590 CrossRef CAS PubMed.
- A. Sugie, H. Song, T. Horie, N. Ohmura, K. Kanie, A. Muramatsu and A. Mori, Tetrahedron Lett., 2012, 53, 4457 CrossRef CAS.
- V. Juvé, M. F. Cardinal, A. Lombardi, A. Crut, P. Maioli, J. Pérez-Juste, L. M. Liz-Marzán, N. Del Fatti and F. Vallée, Nano Lett., 2013, 13, 2234 CrossRef PubMed.
- R. D'Amato, I. Venditti, M. V. Russo and M. Falconieri, J. Appl. Polym. Sci., 2006, 102, 4493 CrossRef.
- A. Bootz, V. Vogel, D. Schubert and J. Kreuter, Eur. J. Pharm. Biopharm., 2004, 57, 369 CrossRef CAS PubMed.
- Particle sizes are obtained from intensity-weighted DLS analysis, which is known to be less sensitive to noise and give mathematically most stable results.
- (a) D. H. Lee, G. S. Cho, H. M. Lim, D. S. Kim, C. Kim and S. H. Lee, J. Ceram. Proc. Res., 2013, 14, 274 Search PubMed; (b) R. D'Amato, L. Medei, I. Venditti, M. V. Russo and M. Falconieri, Mater. Sci. Eng., C, 2003, 23, 861 CrossRef.
- (a) J. Liao, L. Bernard, M. Langer, C. Schönenberger and M. Calame, Adv. Mat., 2006, 18, 2444 CrossRef CAS; (b) J. Liao, S. Blok, S. J. van der Molen, S. Diefenbach, A. W. Holleitner, C. Schonenberger, A. Vladyka and M. Calame, Chem. Soc. Rev., 2015, 44, 999 RSC.
- Y. Wang, C. Duan, L. Peng and J. Liao, Sci. Rep., 2014, 4, 7565 CrossRef CAS PubMed.
Footnotes |
| † These authors contributed equally. |
| ‡ Electronic supplementary information (ESI) available: Synthetic and spectroscopic details. UV-vis spectra. SEM and TEM images of gold nanoparticles. DLS evaluations of AuNPs. Supporting Table: synthetic and spectroscopic details of the synthesized gold nanoparticles. See DOI: 10.1039/c6me00095a |
| This journal is © The Royal Society of Chemistry 2017 |