Enzyme catalysis in organic synthesis
Toshiyuki
Itoh
a and
Ulf
Hanefeld
b
aCentre for Research on Green Sustainable Chemistry, Graduate School of Engineering, Tottori University, 4-101 Koyama minami, Tottori 680-8552, Japan. E-mail: titoh@chem.tottori-u.ac.jp
bBiokatalyse, Afdeling Biotechnologie, Technische Universiteit Delft, van der Maasweg 9, 2629HZ Delft, The Netherlands. E-mail: u.hanefeld@tudelft.nl
A short glance into the past
Enzymes have always been a topic of chemical research. More than 180 years ago Wöhler and Liebig used an hydroxynitrile lyase to liberate HCN from a cyanohydrin. In 1908 the reverse reaction was the first enantioselective synthesis ever; the synthesis of (R)-mandelonitrile and mandelic acid. At this time, Fischer’s “lock and key” hypothesis was already standard knowledge and Buchner had just received the Nobel Prize for Chemistry for his discovery that fermentation could occur without a cell. Everything was thus set for enzymes to take a place at the heart of chemistry. Surprisingly this was however not the course of history. Many chemists disregarded enzymes in their work even though major scientific and industrial breakthroughs were made.More than 80 years ago the industrial production of ephedrine based on pyruvate decarboxylase was established. This process is still running strong today. At the same time as enzymatic ephedrine was industrialised it was also firmly demonstrated that enzymes can be applied in organic solvents. The proof of interfacial activation for lipases was published in the 1930s as well, yet it seemed that most chemists had lost touch with enzymes. Warburg in 1931 received the Nobel Prize in Physiology or Medicine for his work on redox enzymes, all of which are highly enantioselective and were long ignored by chemists. It took mainstream chemistry until the 1960s to (re-)discover enantioselective catalysis. Even then the perception that enzymes are “only good at catalysing the formation of one enantiomer since they are made of L-amino acids and the D-amino acid version does not exist” persisted. It took a younger and less biased generation to change this.
Today it is well established that Nature often provides a solution for the synthesis of both enantiomers (Fig. 1) and often both enzymes are used in the laboratory and in industry.
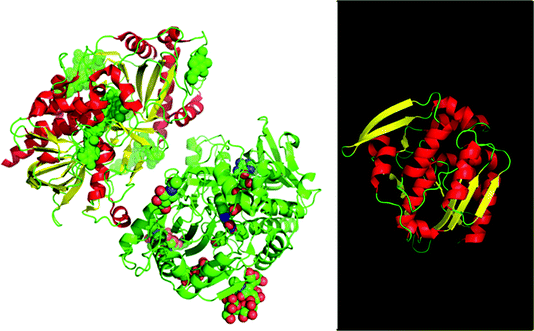 | ||
| Fig. 1 Hydroxynitrile lyases were already used in the 1830s by Wöhler and Liebig. The enzyme from almonds (left, PDB: 1JU2) is highly glycosylated and is R-selective. Structurally there is a difference like day and night with the enzyme from Hevea brasiliensis (right, PDB: 1QJ4) which is small, not glycosylated and is S-selective. This is one of the many examples for enantio-complimentary enzyme pairs available. | ||
Today
This issue of Green Chemistry presents the current state of the art in environmentally benign organic synthesis using enzymes. In all cases the enzyme is the key ingredient that enables the chemistry and helps to make it green. Long-standing topics of research, in particular lipases and other hydrolases, are still going strong. As this issue shows they are the catalyst of choice for complex substrates with low solubility in water. Applications in kinetic resolutions and dynamic kinetic resolutions as well as in ionic liquids are still very useful. More interesting though might be their application in polymer synthesis, in particular in combination with renewable starting materials.Contrasting with the lipases, a rather new development is halogenations with enzymes, which is almost counter intuitive, as halogenated compounds have a reputation of being “chemical”.
Several papers are directed towards the chemistry introduced by Warburg, demonstrating the industrial potential of enzymatic redox chemistry. Particularly enlightening is the application of light in co-factor recycling demonstrated for some of these redox enzymes.
Carbon–carbon bond synthesis was not only a strong point in the history of enzyme catalysed reactions, but also today is at the forefront of research. New substrates for aldolases and ThDP dependent enzymes move these enzymes into the future and ensure even wider industrial applications in environmentally benign syntheses.
A topic that shows the speed at which the field of biocatalysis is currently moving is the transaminases. Thoroughly reviewed and with many examples, a “hot” topic of less than a decade ago is now mature, and with amine dehydrogenase, the next “hot” enzyme in the amine chemistry is entering the stage. At the same time transaminase chemistry also offers fascinating stereo-inversion and application oriented reaction engineering. Continuous applications will be part of future enzyme applications. But most importantly, the application of transaminase in the conversion of waste to value, HMF to valuable amines, is described.
Indeed with this the real greenness of enzymes moves centre stage; enzymes for the conversion of renewable resources and in particular compounds that are otherwise considered waste. This issue contains a number of trail-blazing papers that demonstrate the power of enzymes in the conversion of bio-renewables and in particular of those parts that are currently disregarded. This waste to value conversion will lead the way into a sustainable future.
Future
As this issue amply demonstrates the future of (enantio)selective and environmental benign synthesis, is strongly based on enzymes. They help green chemistry and will allow in the future even more examples of sustainable and economically attractive green chemistry.| This journal is © The Royal Society of Chemistry 2017 |
