Sulfonated polyaniline as a solid organocatalyst for dehydration of fructose into 5-hydroxymethylfurfural†
Abstract
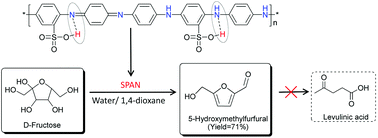
The rehydration of 5-hydroxymethylfurfural (HMF), an important bio-based chemical building block, to levulinic acid (LA) and formic acid (FA) over Brønsted acid catalysts is the key block to the effective production of HMF from hexose. In this work, we develop a novel acidic solid organocatalyst, sulfonated polyaniline (SPAN), for the effective dehydration of fructose into HMF in the low-boiling water/1,4-dioxane cosolvent. The highest HMF yield of 71% is obtained from fructose with complete restriction of HMF rehydration to LA. We demonstrate that hydrogen bonds form between the ring-attached sulfonic acid group and the quinoid imine nitrogen as a result of internal doping, which confines the Brønsted acidity of the SPAN catalyst. The H-bonded sulfonic acid species is active for fructose-to-HMF dehydration and complete suppression on HMF rehydration. The chemical bonding of sulfonic acid groups on the backbone of the PAN chain allows stable recyclability of the polymer catalyst. This work highlights the potential importance of confining Brønsted acidity on a solid organocatalyst via H-bonding for transforming renewable carbohydrates into fine chemicals.


 Please wait while we load your content...
Please wait while we load your content...