Open Access Article
Open Access ArticleFast catalytic conversion of recalcitrant cellulose into alkyl levulinates and levulinic acid in the presence of soluble and recoverable sulfonated hyperbranched poly(arylene oxindole)s†
Feng
Yu
ab,
Ruyi
Zhong
b,
Hui
Chong
a,
Mario
Smet
*a,
Wim
Dehaen
*a and
Bert F.
Sels
*b
aDepartment of Chemistry, KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: mario.smet@kuleuven.be; wim.dehaen@kuleuven.be
bCentre for Surface Chemistry and Catalysis, KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: bert.sels@kuleuven.be; Fax: +32 1632 1998; Tel: +32 1632 1593
First published on 27th October 2016
Abstract
Sulfonated hyperbranched polymers were recently reported to efficiently mimic cellulase activity, producing large quantities of glucose from cellulose. The polymer structure allows tuning of the acid properties in terms of active site confinement and acid strength, while being sufficiently flexible to strongly interact with a solid carbohydrate. Whereas previous research focussed on catalysis in water, herein the sulfonated hyperbranched poly(arylene oxindole)s (SHPAOs) were used in alcoholic media, converting cellulose into alkyl glucosides and alkyl levulinates. Interestingly high reaction rates were noticed in the alcoholic solvent, ethanol being the solvent of choice. Unlike most previous reports, low reaction temperature, high cellulose concentrations and no external pressure were employed. A chlorinated SHPAO, denoted as 5-Cl-SHPAO, due to its high acid strength, exhibits the best catalytic efficiency, yielding 79% ethyl glucoside (EG) in 1 h and 60% ethyl levulinate (EL) in 6 h, the latter value being considerably higher than those of the reference sulfuric acid (29%) and 2-naphthalenesulfonic acid (42.5%) under similar reaction conditions. Worth mentioning is a combined ethyl glucosides and ethyl levulinate (levulinic acid) yield of >90% from microcrystalline cellulose at complete conversion. The cellulose reaction runs in a chemical regime in the temperature range of 150 to 190 °C, 160 °C being the most optimal with regard to the reaction speed and product yields. Time profiles and analysis of the product distributions reveal fast formation of alkylglucosides, while their conversion is the slowest step in the cascade to alkyl levulinate. Besides being very fast, reaction rates in an alcoholic solvent appear less affected by the properties of the cellulose. Therefore, even large particles of highly crystalline cellulose are easily converted to high alkyl levulinate yields. Obtaining a high levulinic acid (LA) yield directly from cellulose appears difficult, also in the presence of a hyperbranched polymer. Therefore, a two-stage catalytic strategy that uses the facile formation of alkyl levulinate from cellulose in alcohol in the presence of catalytic amounts of 5-Cl-SHPAO is proposed. After alcoholic evaporation of the alkyl levulinate product solution, aliquots of water are added to hydrolyse the product into LA. As this reaction in the presence of the remaining soluble catalyst is complete, a 60% LA yield from microcrystalline cellulose is demonstrated. Catalyst recovery is demonstrated through nanofiltration. Due to the soluble character of the hyperbranched catalyst in the alcoholic solvent, it is easily separated from the solid humins, and recovered from the solution over a commercial low molecular weight cut-off filter. The recovered catalyst showed comparable catalytic activity (per catalyst weight) and product selectivity.
Introduction
With the worldwide rising demand for a decreased dependence on fossil resources, the conversion of abundant and renewable biomass resources into chemicals and fuels has attracted much attention in recent years.1–9 The biomass-based chemical production is considered as an environmentally benign process, due to its ‘carbon neutrality’, since released CO2 through the use of biomass carbohydrates is consumed in the growth of biomass through photosynthesis.10,11 As the world's largest resource of inedible biomass, cellulose acts as the most promising alternative for the sustainable production of chemicals and fuels, while it will be available through future lignocellulosic biorefineries.2,7,9,12,13 The selective transformation of cellulose into chemicals under mild conditions is still a major challenge because of its robust crystalline structure formed by inter- and intramolecular hydrogen bonds.14–17 So far, processes for the pyrolysis or gasification of cellulose to bio-oils or syngas at high temperatures have been developed, but all these processes suffer from low product selectivity and high energy input.1,5In view of sustainable chemistry, the selective transformation of cellulose to platform molecules, which can be easily converted into a variety of valuable chemicals or fuels under mild conditions, is highly desirable.17,18 Extensive research work has been devoted to the production of glucose and levulinic acid (LA) through hydrolysis of cellulose in aqueous medium. For instance, in the presence of carbon catalysts the hydrolysis of cellulose to glucose is well studied by Fukuoka's group.19–22 Glucose, a monosaccharide of cellulose, can be transformed among others into chemicals such as ethanol,23 5-hydroxymethyl furfural (HMF),24 sorbitol,25 gluconic acid,26 alkyl glucosides,27 ethylene glycol,28–30 and LA.31–35
LA is also a versatile building block with high chemical reactivity,36 which can be catalytically converted to a variety of valuable chemicals and fuels, like levulinate esters,37,38 α-angelicalactone,39,40 γ-valerolactone (GVL),40–44 5-aminolevulinate,40,45 diphenolic acid40,46–48 and so on.49
Mineral liquid acids, especially sulfuric acid, have long been utilized as homogeneous catalysts for the conversion of cellulose to glucose and LA in aqueous medium.50–54 The use of a concentrated liquid acid is necessary to obtain a satisfactory yield of glucose or LA, which, unfortunately makes this process very costly considering the requirement of corrosive-resistant reactors, and the neutralization of waste acid besides the complicated separation of the products from the acid.3 As a promising alternative, the application of solid acids in the catalytic conversion of cellulose to glucose or LA has attracted much interest in recent years.49,55,56 However, the transfer barrier between the solid catalyst and water-insoluble cellulose dramatically suppresses its catalytic efficiency. Intense ball-milling of cellulose with a solid catalyst, e.g., together with acidic carbons, is required to improve such contact.21 The utilization of ionic liquids (ILs) as reaction media, which are able to dissolve cellulose, has brought a significant enhancement in the catalytic efficiency for the hydrolysis of cellulose to glucose and LA in the presence of an acid catalyst.57,58 Very recently, functionalized ILs with acidic functionalities have also been studied for the degradation of cellulose to LA.59,60 Although ILs could evidently promote the degradation of cellulose, the use of ILs is still expensive due to the high cost of preparation and the arduous procedures for the product separation and dry IL regeneration, which greatly limits their practical integration in today's large-scale production schemes.
Recently, research on the use of alcohols instead of water as reaction media for catalytic conversion of cellulose to chemicals has attracted attention as to improve product work-up and to minimize waste water. Alkyl glucosides and alkyl levulinates are the main products from such acid-catalyzed alcoholysis of cellulose. Alkyl glucosides are non-ionic compounds with excellent surfactant properties, good biodegradability and low toxicity, which make them widely useful in the preparation of detergents, cosmetics, food emulsifiers, pharmaceutical dispersing agents and agrochemicals.61,62 Alkyl levulinates are valuable chemicals and intermediates with numerous potential industrial applications either in the flavoring and fragrance industry or as additives in diesel and biodiesel transportation fuels.37,63,64
The generally accepted reaction pathway for the direct conversion of cellulose to alkyl levulinate in alcohol is schematically shown in Scheme 1. It proceeds through solubilization of cellulose into alkyl glucoside, followed by the formation of 5-(alkoxymethyl)furfural and its conversion into alkyl levulinate in an acidic environment.65–68 To date, several types of acid catalysts, including mineral acids, organic acids, heteropolyacids and solid acids, have been successfully utilized in the acid-catalyzed alcoholysis of cellulose to alkyl glucosides and alkyl levulinates.67,69–78 For instance, Wang et al. examined several kinds of acid catalysts, such as diluted H2SO4, heteropolyacids and some solid acids bearing SO3H groups, in the catalytic alcoholysis of cellulose in methanol media at around 200 °C, and found that heteropolyacid H3PW12O40 exhibited the best catalytic performance, giving 53–57% yield of methyl glucoside when using 3% cellulose loading.69,70 In the presence of a solid catalyst Amberlyst 15DRY, the alcoholysis of cellulose into alkyl glucosides was studied in ILs under mild reaction conditions by de Vos et al. and Corma et al. respectively, and a very satisfactory yield of the alkyl glucoside (up to 86%) was achieved after around 24 h of reaction.71,72 In addition, Tominaga and coworkers employed an efficient mixed-acid catalyst system consisting of In(OTf)3 and 2-naphthalenesulfonic acid as Lewis and Brønsted acids respectively for the conversion of cellulose in methanol at 180 °C and 0.5 MPa pressure of N2, achieving the highest yield of methyl levulinate (75%) after 5 h at the initial cellulose concentration of ∼3 wt%.74 Despite their excellent catalytic outcome, these catalytic approaches suffer from their own drawbacks such as high temperature and pressure required, the difficulty in separating and reusing the acid catalyst, or a low initial cellulose loading.
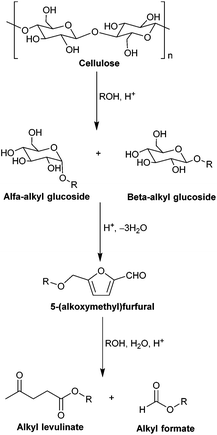 | ||
| Scheme 1 Proposed reaction pathway for the acid-catalyzed alcoholysis of cellulose to alkyl levulinate. | ||
The development of an efficient acid catalyst for the direct degradation of cellulose, especially the high crystalline ones, to valuable chemicals under mild conditions therefore remains a pertinent challenge. We previously reported the preparation of sulfonated hyperbranched poly(arylene oxindole)s (SHPAOs) through a facile and attractive strategy, consisting of a one-step A2 + B3 polycondensation via superelectrophilic arylation of isatin and the subsequent controlled sulfonation in oleum. The structures of SHPAOs are shown in Fig. 1. This new class of acid catalysts, combining the advantages of homogeneous and heterogeneous catalysis, has been proven a highly efficient and recyclable catalyst for the degradation of cellulose and other biomass-derived carbohydrates in aqueous medium.79 By further studying the molecular design of this polymer catalyst, it was found that the presence of a 5-chloro-substituent in the isatin residue substantially facilitated the hydrolysis of the glycoside bonds of the cellulose polymer, consequently providing up to 50% LA.80 Additionally, this polymer acid catalyst also acts as a functional cellulase bio-mimic for glucose production from cellulose,81 and can be successfully used in the production of diphenolic acid via condensation of phenol with LA.47,48 Herein, we demonstrate the first catalytic application of acidic hyperbranched polymer catalysts in the degradation of cellulose in alcoholic media under mild reaction conditions, with the aim of exploring new efficient approaches to produce a manifold of valuable bio-derived chemicals.
Results and discussion
Alcoholysis of cellulose to alkyl glucoside in methanol and ethanol
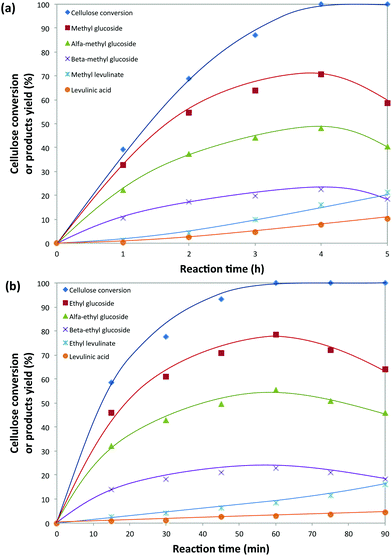
Cellulose alcoholysis was initially conducted in methanol (2 mL) using 20 mg of 5-Cl-SHPAO as the acid catalyst and 40 mg of microcrystalline cellulose (Avicel® PH-101) at 160 °C. The time evolution of cellulose consumption is shown in Fig. 2a. With time, both α-methyl glucoside and β-methyl glucoside are progressively formed with gradual consumption of cellulose. Complete conversion of cellulose is accomplished in about 4 hours, accompanied by a maximum 71% yield of methyl glucoside, which includes 48% yield of α-methyl glucoside and 23% yield of β-methyl glucoside. Further prolonging of time increases the yields of methyl levulinate and LA at the expense of the alkyl glucosides, pointing to their consecutive conversion. Such a result in an alcoholic solvent in the presence of 5-Cl-SHPAO is better than that earlier reported for reactions in water, in which 24% glucose and 6.6% LA were obtained.Cellulose conversion in methanol was then compared with that in ethanol under identical conditions. A similar tendency in the product evolution is observed in ethanol. To our surprise, the reaction time to attain both full cellulose conversion and maximum ethyl glucoside yield was greatly reduced in ethanol. As shown in Fig. 2b, complete consumption of cellulose is accomplished in only 1 hour in ethanol, along with a superior 79% ethyl glucoside yield, whereas 4 h was required in methanol. These results suggest that ethanol is a more suitable solvent than methanol for catalytic cellulose conversion in the presence of 5-Cl-SHPAO. The main side-products in ethanol are the useful ethyl levulinate and levulinic acid, formed in 11.5% combined yield.
Exploring cellulose to alkyl levulinate conversion in alcoholic media
In order to fully and efficiently convert the intermediate ethyl glucoside into ethyl levulinate (EL), the reaction time was substantially extended in the following experiments. The first catalyst screening involved a series of cellulose reactions in the presence of various acid reference catalysts including H2SO4, 2-naphthalenesulfonic acid, Amberlyst 15, and SHPAOs (with 0.073 mmol of H+) in ethanol at 160 °C. The evaluation is shown in Table 1.| Entry | Catalyst | Acid density (mmol H+ per g) | Time (h) | Conversion (%) | EL yield (%) |
|---|---|---|---|---|---|
| a Alcoholysis conditions: 40 mg of microcrystalline cellulose, 0.073 mmol H+ in the added catalyst based on acid–base titration, 2 mL of ethanol in a 10 mL autoclave at 160 °C. b 10 mg of 5-Cl-SHPAO was used. c 40 mg of 5-Cl-SHPAO was used. Humin as the side product accounts for the major carbon loss in the reaction. | |||||
| 1 | No catalyst | — | 6 | — | — |
| 2 | Amberlyst 15 | 2.27 | 6 | 38 | 6 |
| 3 | H2SO4 | — | 6 | 77 | 29 |
| 4 | 2-Naphthalenesulfonic acid | — | 6 | >99 | 43 |
| 5 | SHPAO | 3.66 | 6 | >99 | 52 |
| 6 | 5-Cl-SHPAO | 3.63 | 6 | >99 | 60 |
| 7 | 5-MeO-SHPAO | 3.62 | 6 | >99 | 48 |
| 8b | 5-Cl-SHPAO | 3.63 | 10 | >99 | 48 |
| 9c | 5-Cl-SHPAO | 3.63 | 6 | >99 | 56 |
Even after 6 h, no conversion of cellulose was observed without any acid catalyst (Table 1, entry 1). Very poor catalytic performance was obtained in the presence of Amberlyst 15, a typical solid acid, showing only 6% EL yield at 38% cellulose conversion (Table 1, entry 2). The benchmark H2SO4 gave 29% EL yield at 77% cellulose conversion within 6 h (Table 1, entry 3). The use of 2-naphthalenesulfonic acid provided an improvement in catalytic performance, yielding 43% EL at full cellulose conversion (Table 1, entry 4).
All sulfonated hyperbranched polymers, including SHPAO, 5-Cl-SHPAO and 5-MeO-SHPAO show superior catalytic performance (Table 1, entries 5–7). Among these sulfonated hyperbranched polymer catalysts, 5-Cl-SHPAO affords the highest EL yield, viz. 60% at complete cellulose conversion in 6 h (Table 1, entry 6). The other polymer catalysts, SHPAO and 5-MeO-SHPAO, showed 52% and 48% EL yield, respectively.
Catalytic conversion of cellulose is more efficient in ethanol than in water, showing a higher overall product yield and higher selectivity to ethyl levulinate. Yet, humin formation remains inevitable, accounting for the loss of about 35 mol%.
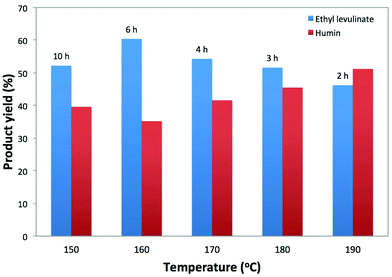
The temperature effect on the conversion of cellulose to EL with 5-Cl-SHPAO was studied in the range of 150 to 190 °C. The reactions were carried out in ethanol with cellulose and catalyst concentrations of 2.5 wt% and 1.3 wt%, respectively. The experimental results, shown in Fig. 3, demonstrate that the reaction temperature has a significant effect on the EL production efficiency at full cellulose conversion. With increasing reaction temperature, the time to complete the reaction reduced with a factor of 5, i.e. from 10 to 2 h. These kinetics correspond to an apparent activation energy of around 54 kJ mol−1 (Fig. S1, ESI†), which is much lower than ∼162 kJ mol−1, from a water medium even with using ball-milled cellulose according to our previous study,80 as well as an estimated value of 123 kJ mol−1 from sulfuric acid catalyzed ethanolysis of cellulose to EL according to a recent report.82 The low activation energy value in the presence of 5-Cl-SHPAO and ethanol nevertheless agrees with a chemical regime, as the reaction rate is not affected by the agitation speed (in the range of 200 to 1000 rpm). The maximum EL yield is obtained at 160 °C. The presence of such optimum agrees with different activation barriers for the individual reactions of the cascade, as presented in Scheme 1. At lower temperature, while cellulose is completely converted, there is still a lot of ethyl glucoside to convert. Although the reaction rate of humin formation is very slow at low temperature, the long reaction time leads to a substantial carbon loss. At higher temperature, by-products like humins are already formed at incomplete cellulose conversion. The trend of humin yields (based on carbon) at different reaction temperatures is shown in Fig. 3. The optimal reaction temperature of 160 °C was kept for the remaining experiments.
To gain more insight into the reaction evolution of cellulose to EL in the presence of 5-Cl-SHPAO, the product distribution was monitored as a function of reaction time (Fig. 4). The catalytic reaction was conducted in ethanol at 160 °C for 9 h in the presence of 80 mg of cellulose (5.1 wt%) and 20 mg of the catalyst 5-Cl-SHPAO (1.3 wt%). Rapid cellulose conversion is noted in the initial stage of the reaction, cellulose being completely consumed already after 2 h. The yield of EL monotonically increases to 58% with time, while ethyl glucoside yield presents a volcano trend, showing its immediate formation at a very short reaction time, followed by a progressive decline after delivering the maximum ethyl glucoside yield of around 75% at 1 h. Besides that, other products, such as glucose, levoglucosan, HMF and 5-ethoxymethylfurfural (EMF), were also detected with a very low concentration during cellulose conversion. These results reveal that the alcoholysis of cellulose to EL proceeds through a reaction pathway with ethyl glucoside as the intermediate, while the conversion of ethyl glucoside to EMF, the precursor of EL, seems to be rate-determining, considering the rapid consumption of cellulose to ethyl glucoside and the appearance of low concentrations of other intermediates in the time-dependent product distribution.
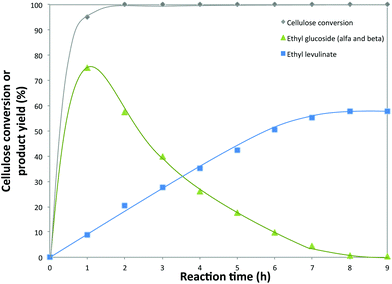 | ||
| Fig. 4 Conversion of cellulose into ethyl levulinate using 5-Cl-SHPAO as the catalyst in ethanol. Reaction conditions: microcrystalline cellulose (80 mg), 5-Cl-SHPAO (20 mg), ethanol (2 mL), 160 °C. | ||
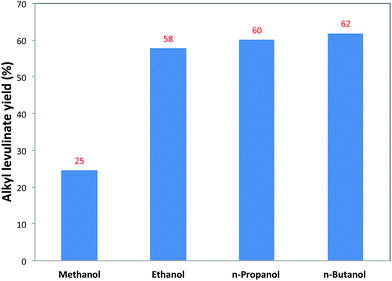
Comparison of different solvents for the conversion of cellulose to alkyl levulinate was conducted at 160 °C with the loading of 5.1 wt% cellulose and 1.3 wt% 5-Cl-SHPAO (see Fig. 5). Changing the solvent from methanol to higher alcohols, such as ethanol, n-propanol and n-butanol, led to the formation of the corresponding alkyl levulinate via the same pathway as described for ethanol. Interestingly, the yield of alkyl levulinates after 8 h progressively enhances from 25 to 62% with an increasing chain length of the alcohol. It is worth noting that the yield of ML obtained in methanol is extraordinarily low compared with the results from other alcohols, probably resulting from the much slower reaction rates for the formation of the corresponding alkyl glucoside in methanol when compared to the higher alcohols, as already suggested by the data in Fig. 2. Prolonging the reaction time to 18 h is required to obtain the maximum 52% yield of ML, as a result of further conversion of methyl glucoside.
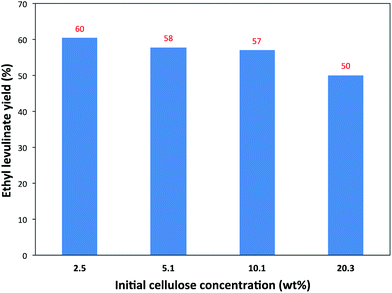
Another important aspect, that should be researched more seriously, is the influence of the cellulose concentration. Usually, very low cellulose loadings, as low as 1–3 wt%, are tested, likely for practical reasons, but such low amounts aren't very relevant for future applications. Experiments with different initial cellulose concentrations were therefore carried out in the presence of a constant 1.3 wt% of the 5-Cl-SHPAO catalyst. As such reactions tested with 2.5 wt%, 5.1 wt%, 10.1 wt% and 20.3 wt% cellulose loadings were employed in the catalytic reaction in ethanol at 160 °C. The results are summarized in Fig. 6.
There is a gradual but slight decrease in the EL yield from 60% to 50% with an increasing cellulose concentration from 2.5 wt% to 20.3 wt%. For instance, a satisfactory EL yield of 57% is achieved with 10.1 wt% cellulose in ethanol at 160 °C.
Conversion of other carbohydrates into alkyl levulinates
In a following set of experiments, the catalytic conversion of a series of biomass-derived carbohydrates and hydrolytic products, most of which are generally considered as reaction intermediates during cellulose alcoholysis, such as LA, HMF, fructose, glucose and methyl α-D-glucopyranoside, to alkyl levulinates was explored at 160 °C in the presence of the 1.3 wt% 5-Cl-SHPAO catalyst and 5.1 wt% carbohydrate substrate. The experimental results obtained in both methanol and ethanol are summarized in Table 2.| Entry | Substrate | Alcohol | Time (h) | Alkyl levulinate yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 80 mg of substrate, 20 mg of 5-Cl-SHPAO, 2 mL of alcohol in a 10 mL autoclave at 160 °C. >99% conversion was achieved in all cases. | ||||
| 1 | LA | Methanol | 1 | 99 |
| 2 | LA | Ethanol | 1 | 96 |
| 3 | HMF | Methanol | 1 | 80 |
| 4 | HMF | Ethanol | 1 | 89 |
| 5 | Fructose | Methanol | 1 | 79 |
| 6 | Fructose | Ethanol | 1 | 68 |
| 7 | Glucose | Methanol | 12 | 60 |
| 8 | Glucose | Ethanol | 8 | 61 |
| 9 | Methyl α-D-glucopyranoside | Methanol | 10 | 62 |
| 10 | Sucrose | Ethanol | 6 | 62 |
| 11 | Cellobiose | Ethanol | 8 | 60 |
| 12 | Ball-milled cellulose | Ethanol | 8 | 57 |
| 12 | α-Cellulose | Ethanol | 8 | 57 |
| 13 | Sigmacell type 20 | Ethanol | 8 | 57 |
| 14 | Inulin | Methanol | 8 | 71 |
In both alcohols, the reaction with LA, HMF and fructose is completed within 1 h. A quantitative conversion is obtained with LA as the substrate, affording 99.0% ML and 96.0% EL yield in methanol and ethanol respectively (Table 2, entries 1 and 2). This result suggests that the reaction in the conversion of cellulose to alkyl levulinate is easily accomplished. Switching from LA to HMF, the yield of the corresponding alkyl levulinate decreased in both alcohols, especially in methanol, yielding 80% ML and 89% in ethanol (Table 2, entries 3 and 4). As humins are the main side-products, ethanol seems to play a better protecting role, likely by forming more stable 5-(ethoxymethylfurfural) (and its acetals). Though HMF is more selectively converted to the corresponding alkyl levulinate in ethanol, fructose conversion shows better yields in methanol. The ML yield obtained from fructose (79%) in methanol was very close to that from HMF (80%), whereas a significant drop in the EL yield to 68% from fructose was observed in ethanol (Table 2, entries 5 and 6). These results insinuate that humin formation from fructose is a dominant pathway in ethanol, while being less favourable in methanol.
Reactions with glucose are substantially slower. The reaction time required for completion in methanol and ethanol is 12 h and 8 h respectively, in contrast to one hour for the previous substrates. Yields of EG and MG are comparable and amount to around 60% (Table 2, entries 7 and 8). As the kinetic profile in Fig. 4 shows very fast formation of methyl glucoside isomers, its further conversion (likely to the corresponding fructose analogue) in the cascade to ML is rate-limiting. The reaction with methyl α-D-glucopyranoside in methanol is comparably slow, yielding about 60% ML (Table 2, entry 9).
Disaccharides, such as sucrose which consists of glucose and fructose, and cellobiose consisting of two glucose monomers, were also reacted in the presence of 5-Cl-SHPAO in ethanol at 160 °C. Comparable EL yields were obtained. The reaction time for the maximum EL yield is comparable for cellobiose and glucose, indicating that glucoside ethanolysis is faster. Sucrose conversion is slower than that of fructose, showing that ethanolysis of the non-reducing sugar disaccharide is slower.
Considering the high ML yield observed in the conversion of fructose in methanol, we further studied the reactivity of inulin, a non-digestible fructose polysaccharide, in methanol in the presence of 5-Cl-SHPAO. The direct conversion of inulin gave a yield of 71% ML with complete conversion in 8 hours, demonstrating the wide application of this catalyst system for the production of alkyl levulinate from diverse biomass-derived polysaccharides.
Finally, reactions with various cellulose types, including ball-milled cellulose, α-cellulose, and Sigmacell Type 20 cellulose, as substrates were carried out in ethanol. Despite the substantially different properties of the cellulose types with regard to the crystallinity, degree of polymerisation and particle size (see ref. 83 and 84 overview in Table S2, ESI†), similar EL yields around 57% at full conversion were obtained. This result is unexpected taking into account the general perception (usually based on reactions in water)16,54,79 of such properties for cellulose conversion. Apparently, solubilization of cellulose into alkyl glucoside, even the large crystalline cellulose particles, seems surprisingly efficient in the presence of a hyperbranched catalyst.
A novel one-pot strategy for direct conversion of microcrystalline cellulose to LA via alkyl levulinate
Although a number of novel acid catalysts and reaction routes have been developed over the past few years to improve the catalytic performance for the production of levulinic acid (LA) (instead of alkyl levulinate) from microcrystalline cellulose,79,85–93 so far LA yield from cellulose is still quite low, especially under mild reaction conditions. For example, Zhang et al. developed a new sold acid catalyst comprising of acid sites and cellulose-binding sites, namely sulfonated chloromethyl polystyrene resin (CP-SO3H-1.69), for the conversion of cellulose to LA. A LA yield of 33.1% was obtained at 170 °C after 10 h in aqueous medium in the presence of 5 wt% cellulose.91 Harsh acidic reaction conditions to break down cellulose in water and the inevitable competitive humin formation in such circumstances, preclude high LA yields. To overcome this, various cellulose pre-treatment approaches have been developed and widely employed in the production of LA from cellulose. The methods of cellulose pretreatment included ball-milling treatment,79,94 non-catalytic hydrothermal treatment of cellulose in water,90 pretreating cellulose with acids at an appropriate temperature,54 ultrasound and plasma treatment,95,96 and so on.97,98 For instance, in 2012 Huber et al. introduced a two-step process, including non-catalytic hydrothermal decomposition of cellulose at 190–270 °C and a subsequent acid-catalyzed hydrolysis process at 160 °C by using the Amberlyst 70 catalyst, for converting cellulose into LA, affording a LA yield of 28% from a high initial cellulose concentration of 20.8 wt%.90 These additional processes significantly improved the efficiency for the transformation of cellulose to LA, but they also brought extra issues like higher energy input and complicated recovery of pretreated cellulose from the processes. Besides the pretreatment approach, other strategies, such as a catalytic partial oxidation,86 introducing a biphasic reaction system87,91 and the use of microwave irradiation instead of conventional heating,53,99,100 have also been demonstrated to promote the transformation of cellulose to LA. For instance, Lin and co-workers developed a one-pot catalytic aqueous phase partial oxidation process to produce LA from cellulose over a ZrO2 catalyst, a high LA yield of 52% was obtained at 240 °C in 25 min under 24 bar pressure consisting of 97.2% N2 and 2.8% O2.86 However, in view of the requirement of harsh reaction conditions, the cumbersome separation of pretreated cellulose from the reaction medium, the low cellulose concentration and the high catalyst loading, it still remains challenges in practically direct production of LA from cellulose feedstock.The high alkyl levulinate yield (of around 60%), obtained directly from (the most recalcitrant) cellulose in ethanol in the presence of the hyperbranched 5-Cl-SHPAO catalyst in alcoholic media, inspired us to propose a new route to produce LA. A new facile one-pot synthesis strategy was therefore developed, following a two-step batch mode procedure in alcoholic and aqueous media. This novel reaction pathway to produce LA from microcrystalline cellulose is illustrated in Scheme 2.
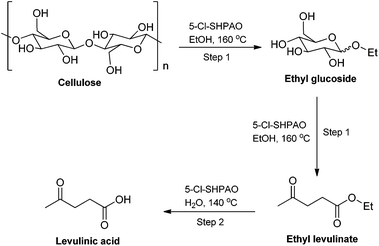 | ||
| Scheme 2 Reaction pathway for the production of levulinic acid from cellulose in alcoholic and aqueous media. | ||
In the first reaction step, cellulose is rapidly degraded to ethyl glucoside in ethanol at 160 °C, and further converted to EL. After ethanol removal, water is added to hydrolyze EL at 140 °C to obtain LA. The time for reaction completion depends on the cellulose concentration and acid catalyst content. For instance, in the presence of 5-Cl-SHPAO (20 mg) and ethanol (2 mL), the reaction for the conversion of 5.1 wt% cellulose into EL is completed in 8 h, while the hydrolysis to LA is complete within 1 h after ethanol evaporation and (1 mL) H2O addition. The two-step approach yields 60% of LA at complete cellulose conversion. Similarly, by using 12 h to finish the first step, 20.3 wt% microcrystalline cellulose gives 52% LA yield, corresponding to a 0.26 M aqueous LA solution. To the best of our knowledge, the LA yield obtained by our two-step approach is among the very few highest yields reported so far even employing a much higher cellulose loading (20.3 wt%) and a lower catalyst loading (1.3 wt%) under the mildest reaction conditions (≤160 °C) reported ever. For comparison, direct (5.1 wt%) cellulose-to-LA conversion in water at 160 °C in the presence of 5-Cl-SHPAO, only yielded 7% LA at 17% cellulose conversion. The first reaction in methanol ultimately shows similar LA yields, but it requires a longer reaction time in the first step. Recovery of methanol though is easier due to its lower boiling point and no azeotrope with water.
This result indicates that the approach of first utilizing alcohol provides a much more efficient pathway to convert microcrystalline cellulose to LA. Relatively mild temperatures are applied in order to convert highly concentrated (untreated) cellulose suspensions in the presence of low catalyst loadings.
Catalyst recovery
Catalyst recovery is an important factor to study, especially if the catalyst cost is considerable. It is therefore worth mentioning that alcohol soluble hyperbranched polymers like 5-Cl-SHPAO can be recycled through ultrafiltration after reaction. In contrast to classic solid acid catalysts, 5-Cl-SHPAO is readily recycled in this proposed concept case due to its solubility, which makes this catalyst easily separated from the solid humin by-product.The recycling efficiency of the hyperbranched polymer 5-Cl-SHPAO was examined for the reactions of cellulose with alkyl glucoside, alkyl levulinate and LA, respectively. Upon completion of the reaction, the mixture was subjected to a classic filtration to remove the remaining cellulose and/or the formed humin, and the solids were washed with water (or alcohol) to recuperate the polymer catalyst. Both filtrates were transferred together to the upper chamber of a Microsep 3 K Omega centrifuge filtering tube, which contains a semi-permeable membrane with a molecular weight cut-off of 3000 Da. The filtering tube was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 h, forcing the product solution through the nanofilter into the lower reservoir. The polymer catalyst was kept in the retentate placed in the upper sample reservoir. After an appropriate washing and a following drying step, the catalyst was fully recycled with complete retention of the catalytic activity and selectivity. The 1H NMR of the recycled polymer catalyst reveals its stability after 8 h of reaction at 160 °C in ethanol (Fig. S11 in ESI†). For instance, the recycled 5-Cl-SHPAO catalyst from the 8 h EL production reaction was reloaded into a new batch reactor with 80 mg fresh cellulose and 2 mL ethanol. The reaction was carried out at 160 °C for 8 h, providing a similar 58% EL yield at 100% cellulose conversion. The reusability of the 5-Cl-SHPAO catalyst was evaluated for 5 runs. After 5 runs under the same reaction conditions, the yield of EL was decreased by 11% (from 58% to 47%), while the conversion of cellulose was always complete (Fig. S2†). It is worth mentioning that the alcoholic media make the recycling conduct of the polymer catalyst much easier when compared with cumbersome recycling work in aqueous reaction medium. This is due to the excellent separation of solid humin and liquid solution in alcoholic reaction media.
000 rpm for 1 h, forcing the product solution through the nanofilter into the lower reservoir. The polymer catalyst was kept in the retentate placed in the upper sample reservoir. After an appropriate washing and a following drying step, the catalyst was fully recycled with complete retention of the catalytic activity and selectivity. The 1H NMR of the recycled polymer catalyst reveals its stability after 8 h of reaction at 160 °C in ethanol (Fig. S11 in ESI†). For instance, the recycled 5-Cl-SHPAO catalyst from the 8 h EL production reaction was reloaded into a new batch reactor with 80 mg fresh cellulose and 2 mL ethanol. The reaction was carried out at 160 °C for 8 h, providing a similar 58% EL yield at 100% cellulose conversion. The reusability of the 5-Cl-SHPAO catalyst was evaluated for 5 runs. After 5 runs under the same reaction conditions, the yield of EL was decreased by 11% (from 58% to 47%), while the conversion of cellulose was always complete (Fig. S2†). It is worth mentioning that the alcoholic media make the recycling conduct of the polymer catalyst much easier when compared with cumbersome recycling work in aqueous reaction medium. This is due to the excellent separation of solid humin and liquid solution in alcoholic reaction media.
To evaluate the potential leaching of sulfonic acid groups during the catalytic reaction, experiments of inductively coupled plasma (ICP) measurement were conducted and the result showed that less than 2.2% of the initial sulphur content had leached into the filtrate solution in each of the three consecutive 8 h reactions in ethanol.
The etherification of the alcoholic solvent during cellulose conversion
Like in all other acid-catalyzed reactions in alcoholic medium, the (unproductive) catalytic formation of dialkyl ether is unavoidable. Monitoring of diethyl ether by means of gas chromatography during the 5-Cl-SHPAO catalyzed ethanolysis of 5.1 wt% cellulose showed 5% and 29% of solvent conversion after 1 h and 8 h, respectively. For the conversion of 20.3 wt% cellulose, almost 35% of ethanol is converted to diethyl ether after 12 h. Except for the loss of the alcoholic solvent, the formation of diethyl ether does not bring significant hindrance to the practical application of the present catalytic system in the production of valuable chemicals from cellulose. In view of the low boiling point of diethyl ether, collecting the formed diethyl ether after reaction by simple distillation for other profitable applications may provide a means to valorise the alcohol loss. The same case counts for reactions in methanol.Experimental
The synthesis of the acidic catalyst 5-Cl-SHPAO and other sulfonated hyperbranched poly(arylene oxindole)s (Fig. 1) proceeded following the procedures reported in our previous work.80,81 The synthetic details and catalyst characterization can also be found in the ESI.†Catalytic experiments were performed in a stainless steel autoclave, equipped with a thermocouple and a magnetic stirrer. For typical runs, 80 mg of microcrystalline cellulose, 20 mg of polymer catalyst (based on 0.073 mmol H+) and 2 mL of alcohol were loaded into a batch reactor in air and mixed by magnetic stirring. The autoclave was then heated at 160 °C for a specific time under continuous stirring. After the reaction, the vessel was removed from heating and rapidly cooled in an ice bath. The product mixture was diluted, sampled, syringe filtered with a 0.45 μm PTFE membrane and subjected to chromatographic analysis.
Reaction products were analysed by high-pressure liquid chromatography (HPLC) in an Agilent 1200 Series system equipped with an isocratic pump and a refractive index (RI) detector on a Varian Metacarb 67H column (300 × 6.5 mm), using an aqueous solution of sulfuric acid (5 mM) at a flow rate of 0.65 mL min−1 and a column temperature of 35 °C. Quantification of each compound was based on calibration curves obtained by analysing standard solutions with known concentrations. Certain samples like alkyl levulinates and diethyl ether were analysed by GC (Agilent 6850 Series) equipped with a HP-1 column and an FID detector and quantitatively estimated using 1-methyl naphthalene as the internal standard.
Conclusions
This contribution has demonstrated the successful conversion of cellulose in the presence of catalytic amounts of sulfonated hyperbranched poly(arylene oxindole)s to form high yields of alkyl glucoside and alkyl levulinate under relatively mild reaction conditions, the strongly acidic 5-Cl-SHPAO being the catalyst of choice. The reactions proceed substantially faster in alcohol than in water, ethanol being better than methanol, while less humin side-products are formed in the alcoholic solvents. As such, a combined ethyl glucoside and ethyl levulinate yield close to 91% was achieved at short reaction times and full cellulose conversion, while ethyl levulinate was the preferred product, formed with 60% yield, at longer reaction times. These values were substantially higher than those obtained in the presence of sulfuric acid and 2-naphthalenesulfonic acid. In contrast to reactions in water, cellulose properties like the crystallinity, polymerization degree and particle size have no significant impact on the catalytic performance, which is likely due to the strong interaction of the soluble branched polymer with the solid cellulose in the alcoholic solvent. Large crystalline cellulose, even at high loadings up to 20 wt%, was rapidly converted in the presence of catalytic amounts of the hyperbranched sulfonated polymer. Reactions run in a chemical regime in the temperature range of 150 to 190 °C, 160 °C being the optimal temperature with respect to the reaction rate and selectivity. The reaction cascade proceeds through fast alkyl glucoside formation (cellulose(m)ethanolysis), followed by slow conversion to (the acetals of) 5-(alkoxymethylfurfural), which rapidly convert(s) into alkyl levulinate. Though reactions in ethanol and methanol show different kinetics of intermediate formation, the overall reaction selectivity towards alkyl levulinate from cellulose is similar. The main side reaction is humin formation, which is largely formed in the presence of fructose (in ethanol) and glucose (in methanol), and less in the presence of HMF (and its ether derivatives).The high yield of alkyl levulinate from recalcitrant cellulose was used to improve the production efficiency of levulinic acid (LA). While the direct conversion of cellulose in water shows very low LA yield at incomplete cellulose conversion, a two-step approach, comprising of alkyl levulinate formation from cellulose in the presence of catalytic amounts of 5-Cl-SHPAO followed by a catalytic hydrolysis (after alcohol removal and water addition), yields 60% (52%) of LA at complete 5.1 wt% (20.3 wt%) cellulose conversion. Recovery of the catalyst is carried out by ultrafiltration. In contrast to solid acids, the soluble hyperbranched polymer can be washed from the solid humins, and recovered on commercial low cut-off filters. The recovered catalyst is reusable in a subsequent catalytic experiment with conservation of catalytic activity and selectivity.
Acknowledgements
F. Y. acknowledges a DBOF doctoral fellowship from KU Leuven. BFS, WD and MS are grateful to the financial support of FWO through project G.0996.13. R. Z. acknowledges financial support from the China Scholarship Council (no. 201206210307).Notes and references
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- B. Kamm, P. R. Gruber and M. Kamm, Biorefineries - Industrial Processes and Products, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2006 Search PubMed.
- R. Rinaldi and F. Schueth, ChemSusChem, 2009, 2, 1096–1107 CrossRef CAS PubMed.
- S. Van de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82–94 CrossRef CAS.
- M. Dusselier, M. Mascal and B. F. Sels, in Selective Catalysis for Renewable Feedstocks and Chemicals, ed. K. M. Nicholas, Springer International Publishing, Cham, 2014, vol. 353, pp. 1–40 Search PubMed.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 709–719 CrossRef CAS PubMed.
- Introduction to Chemicals from Biomass, ed. J. Clark and F. Deswarte, John Wiley & Sons, Ltd, Chichester, UK, 2015 Search PubMed.
- B. Op de Beeck, M. Dusselier, J. Geboers, J. Holsbeek, E. Morré, S. Oswald, L. Giebeler and B. F. Sels, Energy Environ. Sci., 2015, 8, 230–240 CAS.
- S. Van den Bosch, W. Schutyser, R. Vanholme, T. Driessen, S. F. Koelewijn, T. Renders, B. De Meester, W. J. J. Huijgen, W. Dehaen, C. M. Courtin, B. Lagrain, W. Boerjan and B. F. Sels, Energy Environ. Sci., 2015, 8, 1748–1763 CAS.
- D. Tilman, J. Hill and C. Lehman, Science, 2006, 314, 1598–1600 CrossRef CAS PubMed.
- S. Liu, L. P. Abrahamson and G. M. Scott, Biomass Bioenergy, 2012, 39, 1–4 CrossRef.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51, 2564–2601 CrossRef CAS PubMed.
- S. Van de Vyver, J. Geboers, W. Schutyser, M. Dusselier, P. Eloy, E. Dornez, J. W. Seo, C. M. Courtin, E. M. Gaigneaux, P. A. Jacobs and B. F. Sels, ChemSusChem, 2012, 5, 1549–1558 CrossRef CAS PubMed.
- C. E. Wyman, S. R. Decker, M. E. Himmel, J. W. Brady, C. E. Skopec and L. Viikari, in Polysaccharides: Structural Diversity and Functional Versatility, ed. S. Dumitriu, New York, USA, 2nd edn, 2005, pp. 995–1033 Search PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- M. Benoit, A. Rodrigues, K. De Oliveira Vigier, E. Fourré, J. Barrault, J.-M. Tatibouët and F. Jérôme, Green Chem., 2012, 14, 2212–2215 RSC.
- N. Meine, R. Rinaldi and F. Schüth, ChemSusChem, 2012, 5, 1449–1454 CrossRef CAS PubMed.
- B. Kamm, Angew. Chem., Int. Ed., 2007, 46, 5056–5058 CrossRef CAS PubMed.
- H. Kobayashi, T. Komanoya, K. Hara and A. Fukuoka, ChemSusChem, 2010, 3, 440–443 CrossRef CAS PubMed.
- H. Kobayashi, M. Yabushita, T. Komanoya, K. Hara, I. Fujita and A. Fukuoka, ACS Catal., 2013, 3, 581–587 CrossRef CAS.
- M. Yabushita, H. Kobayashi, K. Hara and A. Fukuoka, Catal. Sci. Technol., 2014, 4, 2312–2317 CAS.
- A. Shrotri, H. Kobayashi and A. Fukuoka, ChemSusChem, 2016, 9, 1299–1303 CrossRef CAS PubMed.
- Y. Lin and S. Tanaka, Appl. Microbiol. Biotechnol., 2006, 69, 627–642 CrossRef CAS PubMed.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- K. Van Gorp, E. Boerman, C. V. Cavenaghi and P. H. Berben, Catal. Today, 1999, 52, 349–361 CrossRef CAS.
- C. Baatz and U. Prüße, J. Catal., 2007, 249, 34–40 CrossRef CAS.
- M. Rather and S. Mishra, Sustainable Chem. Processes, 2013, 1, 7–21 CrossRef.
- A. Wang and T. Zhang, Acc. Chem. Res., 2013, 46, 1377–1386 CrossRef CAS PubMed.
- Z. Tai, J. Zhang, A. Wang, J. Pang, M. Zheng and T. Zhang, ChemSusChem, 2013, 6, 652–658 CrossRef CAS PubMed.
- R. Ooms, M. Dusselier, J. A. Geboers, B. Op de Beeck, R. Verhaeven, E. Gobechiya, J. A. Martens, A. Redl and B. F. Sels, Green Chem., 2014, 16, 695–707 RSC.
- B. Girisuta, L. Janssen and H. J. Heeres, Chem. Eng. Res. Des., 2006, 84, 339–349 CrossRef CAS.
- X. Hu, S. Wang, R. J. M. Westerhof, L. Wu, Y. Song, D. Dong and C.-Z. Li, Fuel, 2015, 141, 56–63 CrossRef CAS.
- N. A. S. Ramli and N. A. S. Amin, Energy Convers. Manage., 2015, 95, 10–19 CrossRef CAS.
- D. W. Rackemann, J. P. Bartley and W. O. S. Doherty, Ind. Crops Prod., 2014, 52, 46–57 CrossRef CAS.
- V. Choudhary, S. H. Mushrif, C. Ho, A. Anderko, V. Nikolakis, N. S. Marinkovic, A. I. Frenkel, S. I. Sandler and D. G. Vlachos, J. Am. Chem. Soc., 2013, 135, 3997–4006 CrossRef CAS PubMed.
- W. Deng, Q. Zhang and Y. Wang, Sci. China: Chem., 2015, 58, 29–46 CrossRef CAS.
- H. Joshi, B. R. Moser, J. Toler, W. F. Smith and T. Walker, Biomass Bioenergy, 2011, 35, 3262–3266 CrossRef CAS.
- E. Christensen, A. Williams, S. Paul, S. Burton and R. L. McCormick, Energy Fuels, 2011, 25, 5422–5428 CrossRef CAS.
- T.-N. Chen, T.-S. Deng and X.-L. Hou, Fine Chem., 2009, 26, 885–888 CAS.
- J. J. Bozell, L. Moens, D. C. Elliott, Y. Wang, G. G. Neuenscwander, S. W. Fitzpatrick, R. J. Bilski and J. L. Jarnefeld, Resour., Conserv. Recycl., 2000, 28, 227–239 CrossRef.
- I. T. Horváth, H. Mehdi, V. Fabos, L. Boda and L. T. Mika, Green Chem., 2008, 10, 238–242 RSC.
- W. R. H. Wright and R. Palkovits, ChemSusChem, 2012, 5, 1657–1667 CrossRef CAS PubMed.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Green Chem., 2013, 15, 584–595 RSC.
- W. Luo, M. Sankar, A. M. Beale, Q. He, C. J. Kiely, P. C. A. Bruijnincx and B. M. Weckhuysen, Nat. Commun., 2015, 6, 6540–6550 CrossRef PubMed.
- M. Mascal and S. Dutta, Green Chem., 2011, 13, 40–41 RSC.
- Y. Guo, K. Li, X. Yu and J. H. Clark, Appl. Catal., B, 2008, 81, 182–191 CrossRef CAS.
- S. Van de Vyver, J. Geboers, S. Helsen, F. Yu, J. Thomas, M. Smet, W. Dehaen and B. F. Sels, Chem. Commun., 2012, 48, 3497–3499 RSC.
- S. Van de Vyver, S. Helsen, J. Geboers, F. Yu, J. Thomas, M. Smet, W. Dehaen, Y. Román-Leshkov, I. Hermans and B. F. Sels, ACS Catal., 2012, 2, 2700–2704 CrossRef CAS.
- D. W. Rackemann and W. O. Doherty, Biofuels, Bioprod. Biorefin., 2011, 5, 198–214 CrossRef CAS.
- H. Zhao, J. H. Kwak, Y. Wang, J. A. Franz, J. M. White and J. E. Holladay, Energy Fuels, 2006, 20, 807–811 CrossRef CAS.
- J. Y. Cha and M. A. Hanna, Ind. Crops Prod., 2002, 16, 109–118 CrossRef CAS.
- J. Shen and C. E. Wyman, AIChE J., 2012, 58, 236–246 CrossRef CAS.
- A. Szabolcs, M. Molnar, G. Dibo and L. T. Mika, Green Chem., 2013, 15, 439–445 RSC.
- Y. Muranaka, T. Suzuki, H. Sawanishi, I. Hasegawa and K. Mae, Ind. Eng. Chem. Res., 2014, 53, 11611–11621 CrossRef CAS.
- J. Wang, J. Xi and Y. Wang, Green Chem., 2015, 17, 737–751 RSC.
- L. Hu, L. Lin, Z. Wu, S. Zhou and S. Liu, Appl. Catal., B, 2015, 174–175, 225–243 CrossRef CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- R. Rinaldi, R. Palkovits and F. Schueth, Angew. Chem., Int. Ed., 2008, 47, 8047–8050 CrossRef CAS PubMed.
- Z. Sun, M. Cheng, H. Li, T. Shi, M. Yuan, X. Wang and Z. Jiang, RSC Adv., 2012, 2, 9058–9065 RSC.
- H. Ren, Y. Zhou and L. Liu, Bioresour. Technol., 2013, 129, 616–619 CrossRef CAS PubMed.
- W. von Rybinski and K. Hill, Angew. Chem., Int. Ed., 1998, 37, 1328–1345 CrossRef CAS.
- F. A. Hughes and B. W. Lew, J. Am. Oil Chem. Soc., 1970, 47, 162–167 CrossRef CAS PubMed.
- B. C. Windom, T. M. Lovestead, M. Mascal, E. B. Nikitin and T. J. Bruno, Energy Fuels, 2011, 25, 1878–1890 CrossRef CAS.
- D. J. Hayes, Catal. Today, 2009, 145, 138–151 CrossRef CAS.
- L. Peng, L. Lin, H. Li and Q. Yang, Appl. Energy, 2011, 88, 4590–4596 CrossRef CAS.
- Y. Pierson, F. Bobbink and N. Yan, Chem. Eng. Process Technol., 2013, 1, 10–14 Search PubMed.
- D. Ding, J. Xi, J. Wang, X. Liu, G. Lu and Y. Wang, Green Chem., 2015, 17, 4037–4044 RSC.
- X. Hu and C.-Z. Li, Green Chem., 2011, 13, 1676–1679 RSC.
- W. Deng, M. Liu, Q. Zhang, X. Tan and Y. Wang, Chem. Commun., 2010, 46, 2668–2670 RSC.
- W. Deng, M. Liu, Q. Zhang and Y. Wang, Catal. Today, 2011, 164, 461–466 CrossRef CAS.
- I. A. Ignatyev, P. G. N. Mertens, C. Van Doorslaer, K. Binnemans and D. E. de Vos, Green Chem., 2010, 12, 1790–1795 RSC.
- N. Villandier and A. Corma, ChemSusChem, 2011, 4, 508–513 CrossRef CAS PubMed.
- M. Mascal and E. B. Nikitin, ChemSusChem, 2010, 3, 1349–1351 CrossRef CAS PubMed.
- K.-I. Tominaga, A. Mori, Y. Fukushima, S. Shimada and K. Sato, Green Chem., 2011, 13, 810–813 RSC.
- L. Zhou, H. Zou, J. Nan, L. Wu, X. Yang, Y. Su, T. Lu and J. Xu, Catal. Commun., 2014, 50, 13–16 CrossRef CAS.
- J. Zhang, S. Wu, B. Li and H. Zhang, ChemCatChem, 2012, 4, 1230–1237 CrossRef CAS.
- I. Delidovich, K. Leonhard and R. Palkovits, Energy Environ. Sci., 2014, 7, 2803–2830 CAS.
- E. Ahmad, M. I. Alam, K. K. Pant and M. A. Haider, Green Chem., 2016, 18, 4804–4823 RSC.
- S. Van de Vyver, J. Thomas, J. Geboers, S. Keyzer, M. Smet, W. Dehaen, P. A. Jacobs and B. F. Sels, Energy Environ. Sci., 2011, 4, 3601–3610 CAS.
- F. Yu, J. Thomas, M. Smet, W. Dehaen and B. F. Sels, Green Chem., 2016, 18, 1694–1705 RSC.
- F. Yu, M. Smet, W. Dehaen and B. F. Sels, Chem. Commun., 2016, 52, 2756–2759 RSC.
- G. Xu, C. Chang, S. Fang and X. Ma, Renewable Energy, 2015, 78, 583–589 CrossRef CAS.
- T. Ennaert, B. Op de Beeck, J. Vanneste, A. T. Smit, W. J. J. Huijgen, A. Vanhulsel, P. A. Jacobs and B. F. Sels, Green Chem., 2016, 18, 2095–2105 RSC.
- A. Deneyer, T. Ennaert, G. Cavents, J. Dijkmans, J. Vanneste, C. M. Courtin, M. Dusselier and B. F. Sels, Green Chem., 2016, 18, 5594–5606 RSC.
- J. Potvin, E. Sorlien, J. Hegner, B. DeBoef and B. L. Lucht, Tetrahedron Lett., 2011, 52, 5891–5893 CrossRef CAS.
- H. Lin, J. Strull, Y. Liu, Z. Karmiol, K. Plank, G. Miller, Z. Guo and L. Yang, Energy Environ. Sci., 2012, 5, 9773–9777 CAS.
- D. M. Alonso, J. M. R. Gallo, M. A. Mellmer, S. G. Wettstein and J. A. Dumesic, Catal. Sci. Technol., 2013, 3, 927–931 CAS.
- S. S. Joshi, A. D. Zodge, K. V. Pandare and B. D. Kulkarni, Ind. Eng. Chem. Res., 2014, 53, 18796–18805 CrossRef CAS.
- H. Yang, L. Wang, L. Jia, C. Qiu, Q. Pang and X. Pan, Ind. Eng. Chem. Res., 2014, 53, 6562–6568 CrossRef CAS.
- R. Weingarten, W. C. Conner Jr. and G. W. Huber, Energy Environ. Sci., 2012, 5, 7559–7574 CAS.
- Y. Zuo, Y. Zhang and Y. Fu, ChemCatChem, 2014, 6, 753–757 CrossRef CAS.
- D. Ding, J. Wang, J. Xi, X. Liu, G. Lu and Y. Wang, Green Chem., 2014, 16, 3846–3853 RSC.
- W. Liu, Y. Hou, W. Wu, Z. Liu, Q. Liu, S. Tian and K. N. Marsh, Ind. Eng. Chem. Res., 2012, 51, 15503–15508 CrossRef CAS.
- L. T. Fan, Y. H. Lee and D. R. Beardmore, Biotechnol. Bioeng., 1981, 23, 419–424 CrossRef CAS.
- Q. Zhang, M. Benoit, K. D. O. Vigier, J. Barrault, G. Jegou, M. Philippe and F. Jérôme, Green Chem., 2013, 15, 963–969 RSC.
- M. Benoit, A. Rodrigues, Q. Zhang, E. Fourré, K. De Oliveira Vigier, J.-M. Tatibouët and F. Jérôme, Angew. Chem., Int. Ed., 2011, 50, 8964–8967 CrossRef CAS PubMed.
- M. Kang, S. W. Kim, J.-W. Kim, T. H. Kim and J. S. Kim, Renewable Energy, 2013, 54, 173–179 CrossRef CAS.
- Q. Zhang, N. S. Oztekin, J. Barrault, K. D. O. Vigier and F. Jérôme, ChemSusChem, 2013, 6, 593–596 CrossRef CAS PubMed.
- F. Liu, F. Boissou, A. Vignault, L. Lemée, S. Marinkovic, B. Estrine, K. De Oliveira Vigier and F. Jérôme, RSC Adv., 2014, 4, 28836–28841 RSC.
- S. Tabasso, E. Montoneri, D. Carnaroglio, M. Caporaso and G. Gravotto, Green Chem., 2014, 16, 73–76 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of hyperbranched polymer catalysts (1H and 13C NMR, FTIR etc.), additional analytical information and the catalysis results. See DOI: 10.1039/c6gc02130a |
| This journal is © The Royal Society of Chemistry 2017 |